Relationship between Vitamin Deficiencies and Co-Occurring Symptoms in Autism Spectrum Disorder
Abstract
:1. Introduction
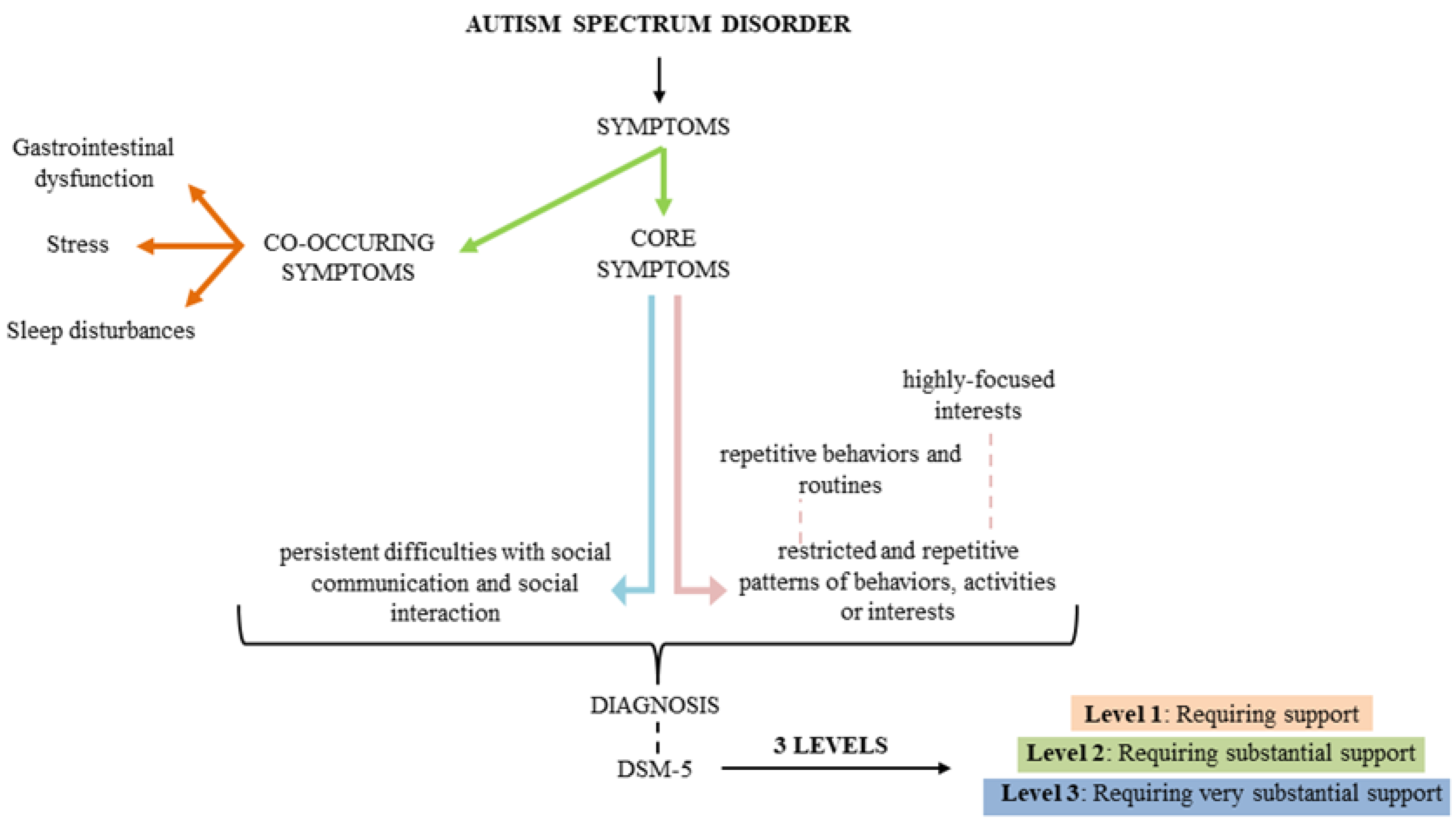
2. What is Autism Spectrum Disorder?
3. Feeding and Eating Problems in ASD Individuals
4. Perturbation of Micronutrients Function in ASD Children
4.1. Thiamine (Vitamin B1)
4.2. Pyridoxine (Vitamin B6)
4.3. Cobalamin (Vitamin B12)
4.4. Vitamin A
4.5. Vitamin D
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bjorklund, G.; Waly, M.I.; Al-Farsi, Y.; Saad, K.; Dadar, M.; Rahman, M.M.; Elhoufey, A.; Chirumbolo, S.; Jóźwik-Pruska, J.; Kałużna-Czaplińska, J. The Role of Vitamins in Autism Spectrum Disorder: What Do We Know? J. Mol. Neurosci. 2019, 67, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.P.; Zuckerman, K.E.; Fombonne, E. Obesity and Autism. Pediatrics 2015, 136, 1051–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreck, K.A.; Williams, K. Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Res. Dev. Disabil. 2006, 27, 353–363. [Google Scholar] [CrossRef]
- Martins, Y.; Young, R.L.; Robson, D.C. Feeding and eating behaviors in children with autism and typically developing children. J. Autism Dev. Disord. 2008, 38, 1878–1887. [Google Scholar] [CrossRef]
- Zhang, X.-C.; Shu, L.-Q.; Zhao, X.-S.; Li, X.-K. Autism spectrum disorders: Autistic phenotypes and complicated mechanisms. World J. Pediatr. 2019, 15, 17–25. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, J.M.; Moon, H.E.; Lee, D.S.; Kim, B.N.; Kim, J.; Kim, D.G.; Paek, S.H. A Short Review on the Current Understanding of Autism Spectrum Disorders. Exp. Neurobiol. 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Zhang, K.; Shi, L.; Zhang, Y.; Zhao, T.; Wang, L.; He, X.; Xia, K.; Liu, C.; et al. A comparative study of the genetic components of three subcategories of autism spectrum disorder. Mol. Psychiatry 2019, 24, 1720–1731. [Google Scholar] [CrossRef]
- American Psychiatric Association. DSM-5 Desk Reference; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar]
- Hyman, S.L.; Levy, S.E.; Myers, S.M. Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics 2020, 145, e20193447. [Google Scholar] [CrossRef] [Green Version]
- Bailey, A.; Le Couteur, A.; Gottesman, I.; Bolton, P.; Simonoff, E.; Yuzda, E.; Rutter, M. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol. Med. 1995, 25, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Hallmayer, J.; Cleveland, S.; Torres, A.; Phillips, J.; Cohen, B.; Torigoe, T.; Miller, J.; Fedele, A.; Collins, J.; Smith, K.; et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch. Gen. Psychiatry 2011, 68, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder. JAMA 2017, 318, 1182–1184. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, G.; Geschwind, D.H. Genetics of autism spectrum disorder. Handb. Clin. Neurol. 2018, 147, 321–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, T.; Morisaki, N.; Honda, Y.; Sampei, M.; Tani, Y. Chemicals, Nutrition, and Autism Spectrum Disorder: A Mini-Review. Front. Neurosci. 2016, 10, 174. [Google Scholar] [CrossRef] [Green Version]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Wu, F.; Ding, Y.; Hou, J.; Bi, J.; Zhang, Z. Advanced parental age and autism risk in children: A systematic review and meta-analysis. Acta Psychiatr. Scand. 2017, 135, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Fakhoury, M. Autistic spectrum disorders: A review of clinical features, theories and diagnosis. Int. J. Dev. Neurosci. 2015, 43, 70–77. [Google Scholar] [CrossRef]
- Marotta, R.; Risolei, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Malhi, P.; Venkatesh, L.; Bharti, B.; Singhi, P. Feeding Problems and Nutrient Intake in Children with and without Autism: A Comparative Study. Indian J. Pediatr. 2017, 84, 283–288. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vissoker, R.E.; Latzer, Y.; Gal, E. Eating and feeding problems and gastrointestinal dysfunction in Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2015, 12, 10–21. [Google Scholar] [CrossRef]
- Criado, K.K.; Sharp, W.G.; McCracken, C.E.; De Vinck-Baroody, O.; Dong, L.; Aman, M.G.; McDougle, C.J.; McCracken, J.T.; Eugene Arnold, L.; Weitzman, C.; et al. Overweight and obese status in children with autism spectrum disorder and disruptive behavior. Autism 2018, 22, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Kamal Nor, N.; Ghozali, A.H.; Ismail, J. Prevalence of Overweight and Obesity Among Children and Adolescents With Autism Spectrum Disorder and Associated Risk Factors. Front. Pediatr. 2019, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Buie, T.; Campbell, D.B.; Fuchs, G.J., 3rd; Furuta, G.T.; Levy, J.; Vandewater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, Diagnosis, and Treatment of Gastrointestinal Disorders in Individuals with ASDs: A Consensus Report. Pediatrics 2010, 125, S1–S18. [Google Scholar] [CrossRef] [Green Version]
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal Problems in Children with Autism, Developmental Delays or Typical Development. J. Autism Dev. Disord. 2014, 44, 1117–1127. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, B.J.; Dovgan, K.; Takahashi, N.; Beversdorf, D.Q. The Relationship Among Gastrointestinal Symptoms, Problem Behaviors, and Internalizing Symptoms in Children and Adolescents With Autism Spectrum Disorder. Frontiers in Psychiatry 2019, 10, 194. [Google Scholar] [CrossRef] [Green Version]
- Gorrindo, P.; Williams, K.C.; Lee, E.B.; Walker, L.S.; McGrew, S.G.; Levitt, P. Gastrointestinal Dysfunction in Autism: Parental Report, Clinical Evaluation, and Associated Factors. Autism Res. 2012, 5, 101–108. [Google Scholar] [CrossRef]
- Geraghty, M.E.; Bates-Wall, J.; Ratliff-Schaub, K.; Lane, A.E. Nutritional Interventions and Therapies in Autism: A Spectrum of What We Know: Part 2. ICAN Infant, Child, Adolesc. Nutr. 2010, 2, 120–133. [Google Scholar] [CrossRef] [Green Version]
- Linscheid, T.R. Behavioral treatments for pediatric feeding disorders. Behav. Modif. 2006, 30, 6–23. [Google Scholar] [CrossRef]
- Kodak, T.; Piazza, C.C. Assessment and behavioral treatment of feeding and sleeping disorders in children with autism spectrum disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Laud, R.B.; Girolami, P.A.; Boscoe, J.H.; Gulotta, C.S. Treatment outcomes for severe feeding problems in children with autism spectrum disorder. Behav. Modif. 2009, 33, 520–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.R.; Piazza, C.C.; Martinez, C.J.; Volkert, V.M.; Christine, M.S. An evaluation of two differential reinforcement procedures with escape extinction to treat food refusal. J. Appl. Behav. Anal. 2002, 35, 363–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazza, C.C.; Fisher, W.W.; Brown, K.A.; Shore, B.A.; Patel, M.R.; Katz, R.M.; Sevin, B.M.; Gulotta, C.S.; Blakely-Smith, A. Functional analysis of inappropriate mealtime behaviors. J. Appl. Behav. Anal. 2003, 36, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Shenkin, A. Micronutrients in health and disease. Postgrad. Med. J. 2006, 82, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, J.B.; George, F.; Audhya, T. Abnormally high plasma levels of vitamin B6 in children with autism not taking supplements compared to controls not taking supplements. J. Altern. Complement. Med. 2006, 12, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Sweetman, D.U.; O’Donnell, S.M.; Lalor, A.; Grant, T.; Greaney, H. Zinc and vitamin A deficiency in a cohort of children with autism spectrum disorder. Child Care Health Dev. 2019, 45, 380–386. [Google Scholar] [CrossRef]
- Mayor, S. Prenatal vitamins in early pregnancy may lower risk of autism in high risk families. BMJ 2019, 364, 1916. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Iosif, A.-M.; Guerrero Angel, E.; Ozonoff, S. Association of Maternal Prenatal Vitamin Use With Risk for Autism Spectrum Disorder Recurrence in Young Siblings. JAMA Psychiatry 2019, 76, 391–398. [Google Scholar] [CrossRef]
- Lonsdale, D.; Shamberger, R.J.; Audhya, T. Treatment of autism spectrum children with thiamine tetrahydrofurfuryl disulfide: A pilot study. Neuro Endocrinol. Lett. 2002, 23, 303–308. [Google Scholar]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 2011, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Mousain-Bosc, M.; Roche, M.; Polge, A.; Pradal-Prat, D.; Rapin, J.; Bali, J.P. Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin B6. I. Attention deficit hyperactivity disorders. Magnes. Res. 2006, 19, 46–52. [Google Scholar] [PubMed]
- Bertoglio, K.; Jill James, S.; Deprey, L.; Brule, N.; Hendren, R.L. Pilot study of the effect of methyl B12 treatment on behavioral and biomarker measures in children with autism. J. Altern. Complement. Med. 2010, 16, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Saad, K.; Eltayeb, A.A.; Mohamad, I.L.; Al-Atram, A.A.; Elserogy, Y.; Bjorklund, G.; El-Houfey, A.A.; Nicholson, B. A Randomized, Placebo-controlled Trial of Digestive Enzymes in Children with Autism Spectrum Disorders. Clin. Psychopharmacol. Neurosci. 2015, 13, 188–193. [Google Scholar] [CrossRef] [Green Version]
- Mazahery, H.; Conlon, C.A.; Beck, K.L.; Mugridge, O.; Kruger, M.C.; Stonehouse, W.; Camargo, C.A.J.; Meyer, B.J.; Jones, B.; von Hurst, P.R. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J. Steroid Biochem. Mol. Biol. 2019, 187, 9–16. [Google Scholar] [CrossRef]
- Carpenter, K.J. The discovery of thiamin. Ann. Nutr. Metab. 2012, 61, 219–223. [Google Scholar] [CrossRef]
- Obrenovich, M.E.; Shola, D.; Schroedel, K.; Agrahari, A.; Lonsdale, D. The role of trace elements, thiamin (e) and transketolase in autism and autistic spectrum disorder. Front. Biosci. 2015, 7, 229–241. [Google Scholar] [CrossRef]
- Dhir, S.; Tarasenko, M.; Napoli, E.; Giulivi, C. Neurological, Psychiatric, and Biochemical Aspects of Thiamine Deficiency in Children and Adults. Front. Psychiatry 2019, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yang, G.; Li, W.; Fan, Z.; Sun, A.; Luo, J.; Ke, Z.-J. Thiamine deficiency increases beta-secretase activity and accumulation of beta-amyloid peptides. Neurobiol. Aging 2011, 32, 42–53. [Google Scholar] [CrossRef]
- Vinh quốc Lương, K. The Role of Thiamine in Autism. Am. J. Psychiatry Neurosci. 2013, 1, 22. [Google Scholar] [CrossRef] [Green Version]
- Anwar, A.; Marini, M.; Abruzzo, P.M.; Bolotta, A.; Ghezzo, A.; Visconti, P.; Thornalley, P.J.; Rabbani, N. Quantitation of Plasma Thiamine, Related Metabolites and Plasma Protein Oxidative Damage Markers in Children with Autism Spectrum Disorder and Healthy Controls. Free Radic. Res. 2016, 50, S85–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B₆ and Its Role in Cell Metabolism and Physiology. Cells 2018, 7, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K. Why is vitamin B6 effective in alleviating the symptoms of autism? Med. Hypotheses 2018, 115, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Holloway, C. Pilot Study of a Moderate Dose Multivitamin/Mineral Supplement for Children with Autistic Spectrum Disorder. J. Altern. Complement. Med. 2004, 10, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B. Vitamin/Mineral Supplements for Children and Adults with Autism. Vitam. Miner. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Pouteau, E.; Kabir-Ahmadi, M.; Noah, L.; Mazur, A.; Dye, L.; Hellhammer, J.; Pickering, G.; Dubray, C. Superiority of Magnesium and Vitamin B6 over Magnesium Alone on Severe Stress in Healthy Adults with Low Magnesemia: A Randomized, Single-Blind Clinical Trial. PLoS ONE 2018, 13, e0208454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, F.; Samman, S. Vitamin B12 in health and disease. Nutrients 2010, 2, 299–316. [Google Scholar] [CrossRef]
- Briani, C.; Dalla Torre, C.; Citton, V.; Manara, R.; Pompanin, S.; Binotto, G.; Adami, F. Cobalamin deficiency: Clinical picture and radiological findings. Nutrients 2013, 5, 4521–4539. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148, 1995S–2027S. [Google Scholar] [CrossRef] [Green Version]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjorke-Monsen, A.-L.; Brito, A.; Gueant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Prim. 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Ou, J.-J.; Li, Y.-M.; Xiang, D.-X. Dietary Supplement for Core Symptoms of Autism Spectrum Disorder: Where Are We Now and Where Should We Go? Front. Psychiatry 2017, 8, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belardo, A.; Gevi, F.; Zolla, L. The concomitant lower concentrations of vitamins B6, B9 and B12 may cause methylation deficiency in autistic children. J. Nutr. Biochem. 2019, 70, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Hendren, R.L.; James, S.J.; Widjaja, F.; Lawton, B.; Rosenblatt, A.; Bent, S. Randomized, Placebo-Controlled Trial of Methyl B12 for Children with Autism. J. Child Adolesc. Psychopharmacol. 2016, 26, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Edem, D.O. Vitamin A: A Review. Asian J. Clin. Nutr. 2009, 1, 65–82. [Google Scholar] [CrossRef]
- Sommer, A. Vitamin a deficiency and clinical disease: An historical overview. J. Nutr. 2008, 138, 1835–1839. [Google Scholar] [CrossRef] [Green Version]
- Wiseman, E.M.; Bar-El Dadon, S.; Reifen, R. The vicious cycle of vitamin a deficiency: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3703–3714. [Google Scholar] [CrossRef]
- Bastos Maia, S.M.; de Costa Caminha, F.; Lins da Silva, S.; Rolland Souza, A.S.; Carvalho Dos Santos, C.; Batista Filho, M. The Prevalence of Vitamin A Deficiency and Associated Factors in Pregnant Women Receiving Prenatal Care at a Reference Maternity Hospital in Northeastern Brazil. Nutrients 2018, 10, 1271. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, J.; Xiong, X.; Yang, T.; Hou, N.; Liang, X.; Chen, J.; Cheng, Q.; Li, T. Correlation between Nutrition and Symptoms: Nutritional Survey of Children with Autism Spectrum Disorder in Chongqing, China. Nutrients 2016, 8, 294. [Google Scholar] [CrossRef] [Green Version]
- Megson, M.N. Is autism a G-alpha protein defect reversible with natural vitamin A? Med. Hypotheses 2000, 54, 979–983. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Zhu, J.; Yang, T.; Lai, X.; Liu, X.; Liu, J.; Chen, J.; Li, T. Vitamin A improves the symptoms of autism spectrum disorders and decreases 5-hydroxytryptamine (5-HT): A pilot study. Brain Res. Bull. 2018, 137, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhu, J.; Yang, T.; Lai, X.; Lei, Y.; Chen, J.; Li, T. Vitamin A and Vitamin D Deficiencies Exacerbate Symptoms in Children with Autism Spectrum Disorders. Nutr. Neurosci. 2019, 22, 637–647. [Google Scholar] [CrossRef]
- Kulie, T.; Groff, A.; Redmer, J.; Hounshell, J.; Schrager, S. Vitamin D: An evidence-based review. J. Am. Board Fam. Med. 2009, 22, 698–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehlayel, M.S.; Bener, A.; Sabbah, A. Is high prevalence of vitamin D deficiency evidence for asthma and allergy risks? Eur. Ann. Allergy Clin. Immunol. 2011, 43, 81–88. [Google Scholar] [PubMed]
- Cannell, J.J. Vitamin D and autism, what’s new? Rev. Endocr. Metab. Disord. 2017, 18, 183–193. [Google Scholar] [CrossRef]
- Vinkhuyzen, A.A.E.; Eyles, D.W.; Burne, T.H.J.; Blanken, L.M.E.; Kruithof, C.J.; Verhulst, F.; White, T.; Jaddoe, V.W.; Tiemeier, H.; McGrath, J.J. Gestational vitamin D deficiency and autism spectrum disorder. BJPsych Open 2017, 3, 85–90. [Google Scholar] [CrossRef]
- Jia, F.; Wang, B.; Shan, L.; Xu, Z.; Staal, W.G.; Du, L. Core symptoms of autism improved after vitamin D supplementation. Pediatrics 2015, 135, e196-8. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Shan, L.; Du, L.; Wang, B.; Li, H.; Wang, W.; Wang, T.; Dong, H.; Yue, X.; Xu, Z.; et al. Clinical improvement following vitamin D3 supplementation in Autism Spectrum Disorder. Nutr. Neurosci. 2017, 20, 284–290. [Google Scholar] [CrossRef]

| Vitamin | Participants | Dosage | Time of Administration | Type of the Study | Effects | Side Effects | Observations | References |
|---|---|---|---|---|---|---|---|---|
| B1 | 10 children | 50 mg TTFD | Twice a day / 2 months | PS | P.E. | - | ↑clinical symptoms ↑Pb, Hg, Cd, As levels | [41] |
| 141 children | 20 mg | Days 1–4: 1/6 of fd Days 5–8: 2/6 of fd Days 9–12: 3/6 of fd Days 13–16: 4/6 of fd Days 17–20: 5/6 of fd Days 21 and later: fd | RCT, DB, P | P.E. | diarrhea, constipation | ↑ATP, sulfation, NADH, NADPH, GSH ↑clinical symptoms ↓ OS | [42] | |
| B6 | 33 children | 6 mg/kg Mg and 0.6 mg/kg vit. B6 | 6 months | RCT | P.E. | - | ↑social interactions, communication, abnormal functioning, stereotyped restricted behavior ↑Erc-Mg values | [43] |
| 141 children | 40 mg | Days 1–4: 1/6 of fd Days 5–8: 2/6 of fd Days 9–12: 3/6 of fd Days 13–16: 4/6 of fd Days 17–20: 5/6 of fd Days 21 and later: fd | RCT, DB, P | P.E. | diarrhea, constipation | ↑ATP, sulfation, NADH, NADPH ↑clinical symptoms ↓ OS | [42] | |
| B12 | 30 children | 0.06 mg/kg | 12 weeks | PS, DB | N.E. | - | ↓ OS ↑GSH no behavioral improvements | [44] |
| D | 122 children | 0.0075 mg/kg | 3 months | RCT | P.E. | skin rashes, diarrhea and itching | ↑core symptoms | [45] |
| 73 children | 0.049 mg/kg | 1 year | RCT | P.E. | - | ↓ irritability and hyperactivity | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robea, M.-A.; Luca, A.-C.; Ciobica, A. Relationship between Vitamin Deficiencies and Co-Occurring Symptoms in Autism Spectrum Disorder. Medicina 2020, 56, 245. https://doi.org/10.3390/medicina56050245
Robea M-A, Luca A-C, Ciobica A. Relationship between Vitamin Deficiencies and Co-Occurring Symptoms in Autism Spectrum Disorder. Medicina. 2020; 56(5):245. https://doi.org/10.3390/medicina56050245
Chicago/Turabian StyleRobea, Madalina-Andreea, Alina-Costina Luca, and Alin Ciobica. 2020. "Relationship between Vitamin Deficiencies and Co-Occurring Symptoms in Autism Spectrum Disorder" Medicina 56, no. 5: 245. https://doi.org/10.3390/medicina56050245
APA StyleRobea, M. -A., Luca, A. -C., & Ciobica, A. (2020). Relationship between Vitamin Deficiencies and Co-Occurring Symptoms in Autism Spectrum Disorder. Medicina, 56(5), 245. https://doi.org/10.3390/medicina56050245





