Signs of Dehydration after Hip Fracture Surgery: An Observational Descriptive Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Ethical Statement
2.2. Data Collection and Analyses
2.3. Statistics
3. Results
3.1. Demographics
3.2. General Observations
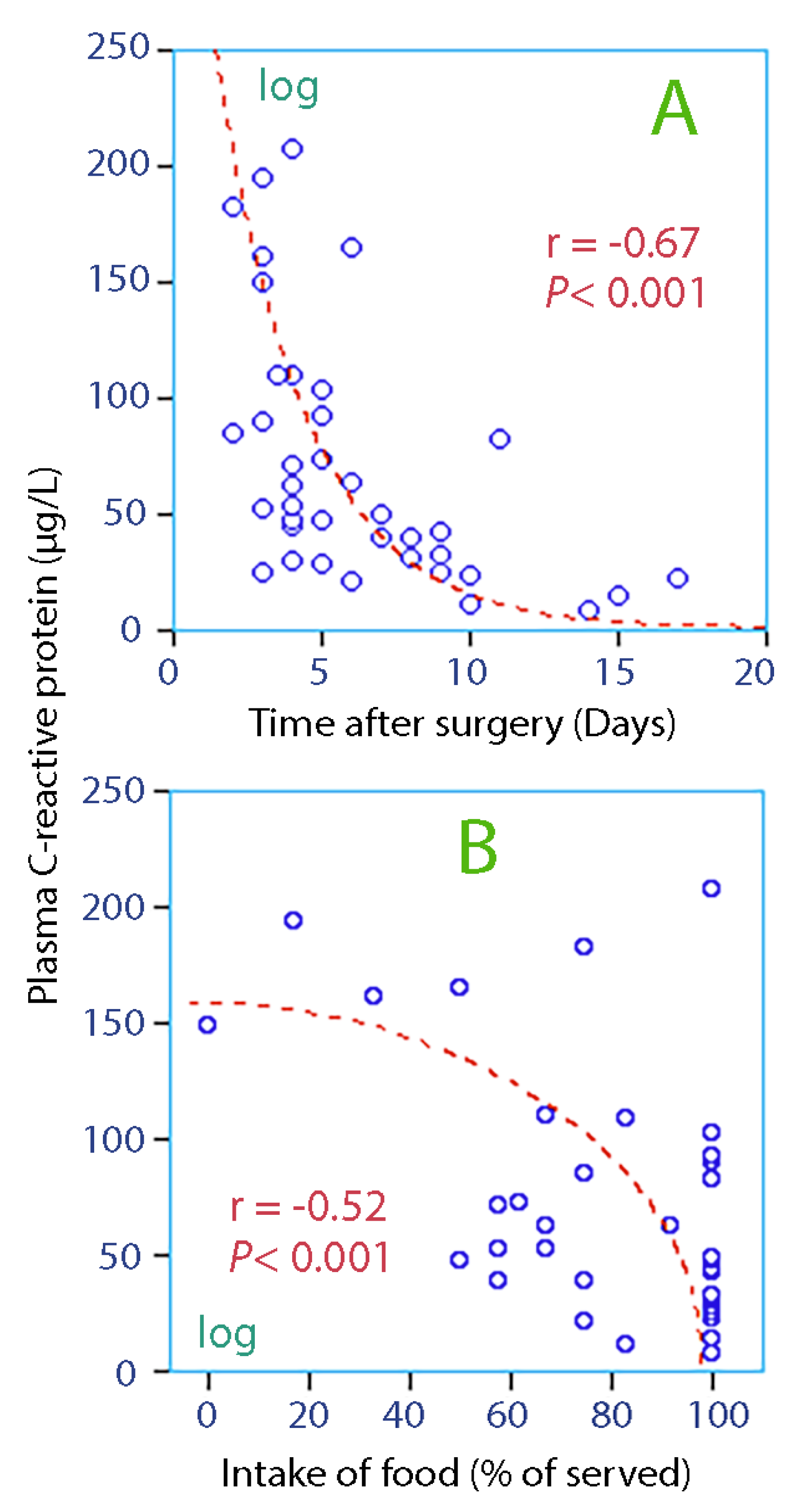
3.3. Linear Correlations
3.4. Indices of Dehydration
3.4.1. Hyperosmolality
3.4.2. Low Fluid Intake
3.4.3. Concentrated Urine
3.4.4. Agreement between Methods
3.4.5. Fluid Retention Index
4. Discussion
4.1. Setting
4.2. Hyperosmolality
4.3. Low Fluid Intake
4.4. Concentrated Urine
4.5. Physical Examination
4.6. Thirst
4.7. Laboratory Analyses
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abrahamsen, B.; van Staa, T.; Ariely, R.; Olson, M.; Cooper, C. Excess mortality following hip fracture: A systematic epidemiological review. Osteoporos. Int. 2009, 20, 1633–1650. [Google Scholar] [CrossRef] [PubMed]
- Zaki, H.E.; Mousa, S.M.; El Said, S.M.S.; Mortagy, A.K. Morbidity and mortality following surgery for hip fractures in elderly patients. J. Aging. Res. 2019, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, B. Hydration and physical performance. J. Am. Coll. Nutr. 2007, 26, 542S–548S. [Google Scholar] [CrossRef]
- Masento, N.A.; Golightly, M.; Field, D.T.; Butler, L.T.; van Reekum, C.M. Effects of hydration status on cognitive performance and mood. Br. J. Nutr. 2014, 111, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Fortes, M.B.; Owen, J.A.; Raymond-Barker, P.; Bishop, C.; Elghenzai, S.; Oliver, S.J.; Walsh, N.P. Is this elderly patient dehydrated? Diagnostic accuracy of hydration using physical signs, urine, and saliva markers. JAMDA 2015, 16, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, R.G.; Waldréus, N. An aggregate urine analysis tool to detect acute dehydration. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 303–311. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Soto, J.A.; Hacker, F.T.; Casa, D.J.; Kavouras, S.A.; Maresh, C.M. Urinary indices during dehydration, exercise and rehydration. Int. J. Sport Nutr. Exerc. Metab., 1998, 8, 345–355. [Google Scholar] [CrossRef]
- Hahn, R.G.; Grankvist, N.; Krizhanovskii, C. Urinary analysis of fluid retention in the general population: A cross-sectional study. PLoS ONE 2016, 11, e0164152. [Google Scholar] [CrossRef]
- Johnson, P.; Hahn, R.G. Signs of dehydration in nursing home residents. JAMDA 2018, 19, 1124–1128. [Google Scholar] [CrossRef]
- Popowski, L.A.; Oppliger, R.A.; Lambert, P.G.; Johnson, A.K.; Gisolfi, C.V. Blood and urinary measures of hydration status during progressive acute dehydration. Med. Sci. Sports Exerc. 2001, 33, 747–753. [Google Scholar] [CrossRef]
- Oppliger, R.A.; Magnes, S.A.; Popowski, L.A.; Gisolfi, C.V. Accuracy of urine specific gravity and osmolality as indicators of hydration status. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Schoeller, D.A.; Subar, A.F.; Troiano, R.P.; Schatzkin, A.; Harris, T.; Bauer, D.; Bingham, S.A.; Everhart, J.E.; Newman, A.B.; et al. Water turnover in 458 American adults 40–79 yr of age. Am. J. Physiol. Ren. Physiol. 2004, 286, F394–F401. [Google Scholar] [CrossRef]
- Vivanti, A.; Harvey, K.; Ash, S.; Battistuta, D. Clinical assessment of dehydration in older people admitted to hospital. What are the strongest indicators? Arch. Gerontol. Geriatr. 2008, 47, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.; Harvey, K.; Ash, S. Developing a quick and practical screen to improve the identification of poor hydration in geriatric and rehabilitative care. Arch. Gerontol. Geriatr. 2010, 50, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, A.D.; Minaker, K.L. Dehydration. Evaluation and management in older adults. Council on Scientific Affairs, American Medical Association. JAMDA 1995, 274, 1552–1556. [Google Scholar] [CrossRef]
- Waldréus, N.; Sjöstrand, F.; Hahn, R.G. Thirst in the elderly with and without heart failure. Arch. Gerontol. Geriatr. 2011, 53, 174–178. [Google Scholar] [CrossRef]
- Waldréus, N.; Hahn, R.G.; Engvall, J.; Skoog, J.; Ewerman, L.; Lindenberger, M. Thirst response to acute hypovolemia in healthy women and women prone to vasovagal syncope. Physiol. Behav. 2013, 120, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Perrier, E.; Vergne, S.; Klein, A.; Poupin, M.; Rondeau, P.; Le Bellegro, L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br. J. Nutr. 2013, 109, 1678–1687. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.; Huffman, A.E.; Yoder, H.; Dolci, A.; Perrier, E.T.; Larson-Meyer, D.E.; Armstrong, L.E. Urinary markers of hydration during 3-day water restriction and graded rehydration. Eur. J. Nutr. 2020, 59, 2171–2181. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.; Waldreus, N.; Hahn, R.G.; Stenström, H.; Sjöstrand, F. Fluid retention index predicts the 30-day mortality in geriatric care. Scand. J. Clin. Lab. Investig. 2015, 75, 444–451. [Google Scholar] [CrossRef]
- Ylinenvaara, S.I.; Elisson, O.; Berg, K.; Zdolsek, J.H.; Krook, H.; Hahn, R.G. Preoperative urine-specific weight and the incidence of complications after hip fracture surgery. A prospective, observational study. Eur. J. Anaesthesiol. 2014, 31, 85–90. [Google Scholar] [CrossRef] [PubMed]



| Mean or Median | SD or 25–75 th Percentiles | Extreme Values | |
|---|---|---|---|
| Blood analyses | |||
| Plasma albumin (g/L) | 26 | 4 | ≤25 (n = 18) |
| Plasma sodium (mmol/L) | 136.5 | 3.8 | ≤130 (n = 4) |
| Plasma potassium (mmol/L) | 3.9 | 0.5 | 2.8 and 5.3 |
| Plasma creatinine (µmol/L) | 68 | 25 | >100 (n = 6) |
| Plasma C-reactive protein (µg/L) | 61 | 21–92 | >100 (n = 9) |
| Plasma urea (mmol/L) | 6 | 5–11 | >10 (n = 10) |
| Serum osmolality (mosmol/kg) | 291 | 286–298 | 299 (n = 7) and 265 (n = 1) |
| Fractional sodium excretion (%) | 0.47 | 0.28–0.72 | <0.3 (n = 10) |
| Urine analyses | |||
| Urine osmolality (mosmol/kg) | 548 | 149 | 132 to 916 |
| Urine creatinine (mmol/L) | 7.8 | 4.2 | >10 (n = 8) |
| Urine potassium (mmol/L) | 41 | 15 | 12 to 74 |
| Urine-specific gravity (no unit) | 1.020 | 1.015–1.020 | ≥1.025 (n = 8) |
| Urine sodium (mmol/L) | 69 | 53–100 | <30 (n = 5) |
| Urine albumin (mg/L) | 14 | 4–52 | >100 (n = 6) |
| Urine albumin/creatinine (mg/mmol) | 3.4 | 1.2–9.6 | >10 (n = 7) |
| Urine color * | 5 | 3–6 | Darkest color 7 (n = 6) |
| Clinical indices | |||
| Age (years) | 82 | 9 | 65 to 97 |
| Body weight (kg) | 67 | 14 | 44 to 98 |
| Breathing frequency (breaths/min) | 16 | 3 | ≥20 (n = 6) |
| Thirst severity (VAS scale 0–100) | 41 | 26 | <10 (n = 5) and > 80 (n = 3) |
| Daily medications (N) | 10 | 7–12 | 3 to 18 |
| Tongue furrows (graded 1/2) | 1.5 | - | Present = 1, Absent = 2 |
| Poor skin turgor (graded 1/2) | 1.8 | - | Present = 1, Absent = 2 |
| Hemodynamics | |||
| Heart rate (bpm) | 83 | 13 | ≥100 (n = 6) |
| Systolic arterial pressure (mmHg) | 123 | 18 | ≤100 (n = 5) |
| Diastolic arterial pressure (mmHg) | 65 | 11 | ≤50 (n = 11) |
| Mean arterial pressure (mmHg) | 84 | 12 | <70 (n = 5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekman, L.; Johnson, P.; Hahn, R.G. Signs of Dehydration after Hip Fracture Surgery: An Observational Descriptive Study. Medicina 2020, 56, 361. https://doi.org/10.3390/medicina56070361
Ekman L, Johnson P, Hahn RG. Signs of Dehydration after Hip Fracture Surgery: An Observational Descriptive Study. Medicina. 2020; 56(7):361. https://doi.org/10.3390/medicina56070361
Chicago/Turabian StyleEkman, Louise, Peter Johnson, and Robert G. Hahn. 2020. "Signs of Dehydration after Hip Fracture Surgery: An Observational Descriptive Study" Medicina 56, no. 7: 361. https://doi.org/10.3390/medicina56070361




