Lung Ultrasound Eight-Point Method in Diagnosing Acute Heart Failure in Emergency Patients with Acute Dyspnea: Diagnostic Accuracy and 72 h Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Statistics
3. Results
3.1. Diagnostic Accuracy
3.2. LUS for Therapeutic Monitoring
3.3. Mortality and Rehospitalization of Patients with Acute Dyspnea
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Christ, M.; Laule-Kilian, K.; Hochholzer, W.; Klima, T.; Breidthardt, T.; Perruchoud, A.P.; Mueller, C. Gender-specific risk stratification with B-type natriuretic peptide levels in patients with acute dyspnea: Insights from the B-type natriuretic peptide for acute shortness of breath evaluation study. J. Am. Coll. Cardiol. 2006, 48, 1808–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martindale, J.L.; Wakai, A.; Collins, S.P.; Levy, P.D.; Diercks, D.B.; Hiestand, B.; Fermann, G.J.; Desouza, I.S.; Sinert, R. Diagnosing acute heart failure in the emergency department: A systematic review and meta-analysis. Acad. Emerg. Med. 2016, 23, 223–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, S.A.; Booth, R.A.; Santaguida, P.L.; Donwauchope, A.C.; Brown, J.A.; Oremus, M.; Ali, U.; Bustamam, A.; Sohel, N.; Mckelvie, R.S.; et al. Use of BNP and NT-proBNP for the diagnosis of heart failure in the emergency department: A systematic review of the evidence. Heart Fail. Rev. 2014, 19, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloeckner, E.; Christ, M.; Geier, F.; Otte, P.; Thiem, U.; Neubauer, S.; Kohfeldt, V.; Singler, K. Accuracy of point-of-care B-line lung ultrasound in comparison to NT-ProBNP for screening acute heart failure. Ultrasound Int. Open 2016, 2, E90–E92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, J.J.V.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; et al. Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2012, 14, 803–869. [Google Scholar]
- Nieminen, M.S.; Priori, S.G.; Garcia, M.A.A.; Budaj, A.; Burgos, E.F.; Lekakis, J.; Mazzotta, G.; Smiseth, O.A.; Dickstein, K.; Crespoleiro, M.G.; et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: The Task Force on Acute Heart Failure of the European Society of Cardiology. Eur. Heart J. 2005, 26, 384–416. [Google Scholar]
- Lichtenstein, D.A.; Meziere, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure*: The BLUE protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Price, S.; Platz, E.; Cullen, L.; Tavazzi, G.; Christ, M.; Cowie, M.R.; Maisel, A.S.; Masip, J.; Miro, O.; Mcmurray, J.J.V.; et al. Expert consensus document: Echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat. Rev. Cardiol. 2017, 14, 427. [Google Scholar] [CrossRef]
- Neskovic, N.A.N.; Hagendorff, A.; Lancellotti, P.; Guarracino, F.; Varga, A.; Cosyns, B.; Flachskampf, F.A.; Popescu, B.A.; Gargani, L.; Zamorano, J.L.; et al. Emergency echocardiography: The European association of cardiovascular imaging recommendations. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Platz, E.; Jhund, P.S.; Campbell, R.T.; McMurray, J.J. Assessment and prevalence of pulmonary oedema in contemporary acute heart failure trials: A systematic review. Eur. J. Heart Fail. 2015, 17, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, E.; Goffi, A.; Lupia, E.; Tizzani, M.; Porrino, G.; Ferreri, E.; Volpicelli, G.; Balzaretti, P.; Banderali, A.; Iacobucci, A.; et al. Lung ultrasound-implemented diagnosis of acute decompensated heart failure in the ED. Chest 2015, 148, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platz, E.; Pivetta, E.; Merz, A.A.; Peck, J.; Rivero, J.; Cheng, S. Impact of device selection and clip duration on lung ultrasound assessment in patients with heart failure. Am. J. Emerg. Med. 2015, 33, 1552–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.L.; Fields, J.M.; Panebianco, N.L.; Jenq, K.Y.; Marin, J.; Dean, A.J. Inter-rater reliability of quantifying pleural B-lines using multiple counting methods. J. Ultrasound Med. 2013, 32, 115–120. [Google Scholar] [CrossRef]
- Anderson, K.L.; Jenq, K.Y.; Fields, J.M.; Panebianco, N.L.; Dean, A.J. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am. J. Emerg. Med. 2013, 31, 1208–1214. [Google Scholar] [CrossRef]
- Bitar, Z.; Maadarani, O.; Almerri, K. Sonographic chest B-lines anticipate elevated B-type natriuretic peptide level, irrespective of ejection fraction. Ann. Int. Care 2015, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Miglioranza, M.H.; Gargani, L.; Santanna, R.T.; Rover, M.; Martins, V.; Mantovani, A.; Weber, C.K.; Moraes, M.A.; Feldman, C.J.; Kalil, R.A.K.; et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: A comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc. Imaging 2013, 6, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, M.; Gupta, M.; Vijan, V.; Vupputuri, A.; Chintamani, S.; Rajendran, B.; Thachathodiyal, R.; Chandrasekaran, R. Use of lung ultrasound for diagnosing acute heart failure in emergency department of southern India. J. Clin. Diagn. Res. 2016, 10, TC05. [Google Scholar] [CrossRef]
- Sartini, S.; Frizzi, J.; Borselli, M.; Sarcoli, E.; Granai, C.; Gialli, V.; Cevenini, G.; Guazzi, G.; Bruni, F.; Gonnelli, S.; et al. Which method is best for an early accurate diagnosis of acute heart failure? Comparison between lung ultrasound, chest X-ray and NT pro-BNP performance: A prospective study. Intern. Emerg. Med. 2017, 12, 861–869. [Google Scholar] [CrossRef]
- Miglioranza, M.H.; Picano, E.; Badano, L.P.; Santanna, R.T.; Rover, M.; Zaffaroni, F.; Sicari, R.; Kalil, R.A.K.; Leiria, T.L.L.; Gargani, L. Pulmonary congestion evaluated by lung ultrasound predicts decompensation in heart failure outpatients. Int. J. Cardiol. 2017, 240, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli, G.; Caramello, V.; Cardinale, L.; Mussa, A.; Bar, F.; Frascisco, M.F. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am. J. Emerg. Med. 2008, 26, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Spevack, R.; Al Shukairi, M.; Jayaraman, D.; Dankoff, J.; Rudski, L.; Lipes, J. Serial lung and IVC ultrasound in the assessment of congestive heart failure. Crit. Ultrasound J. 2017, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prosen, G.; Klemen, P.; Strnad, M.; Grmec, Š. Combination of lung ultrasound (a comet-tail sign) and N-terminal pro-brain natriuretic peptide in differentiating acute heart failure from chronic obstructive pulmonary disease and asthma as cause of acute dyspnea in prehospital emergency setting. Crit. Care 2011, 15, R114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buessler, A.; Chouihed, T.; Duarte, K.; Bassand, A.; Huotmarchand, M.; Gottwalles, Y.; Penine, A.; Andre, E.; Nace, L.; Jaeger, D.; et al. Accuracy of several lung ultrasound methods for the diagnosis of acute heart failure in the ED: A multicenter prospective study. Chest 2020, 157, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Bahrmann, P.; Christ, M.; Hofner, B.; Bahrmann, A.; Achenbach, S.; Sieber, C.C.; Bertsch, T. Prognostic value of different biomarkers for cardiovascular death in unselected older patients in the emergency department. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 568–578. [Google Scholar] [CrossRef] [Green Version]
- Gargani, L.; Pang, P.S.; Frassi, F.; Miglioranza, M.H.; Dini, F.L.; Landi, P.; Picano, E. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: A lung ultrasound study. Cardiovasc. Ultrasound 2015, 13, 40. [Google Scholar] [CrossRef] [Green Version]
- Mueller, C.; Scholer, A.; Laulekilian, K.; Martina, B.; Schindler, C.; Buser, P.; Pfisterer, M.; Perruchoud, A.P. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N. Engl. J. Med. 2004, 350, 647–654. [Google Scholar] [CrossRef]
- Gargani, L.; Frassi, F.; Soldati, G.; Tesorio, P.; Gheorghiade, M.; Picano, E. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: A comparison with natriuretic peptides. Eur. J. Heart Fail. 2008, 10, 70–77. [Google Scholar] [CrossRef]
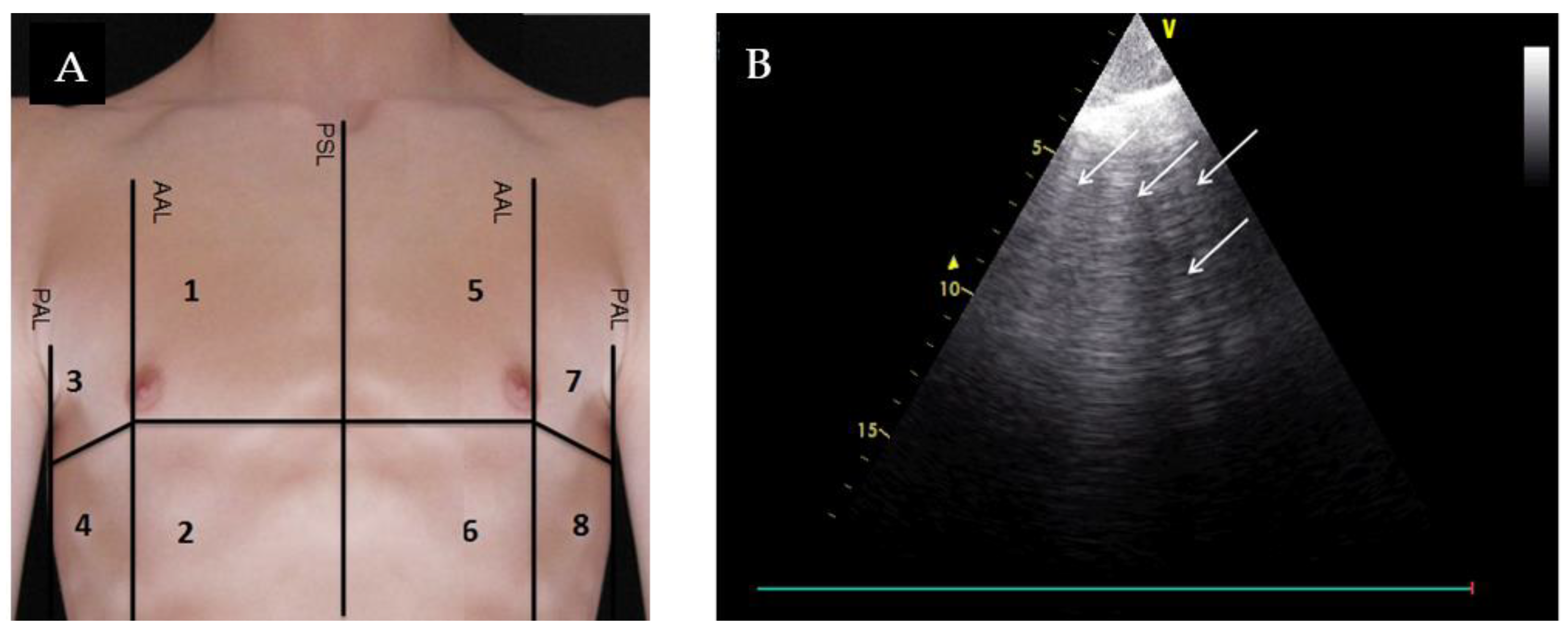




| Overall Cohort (n = 89) | AHF (n = 48) | No AHF (n = 41) | p | |
|---|---|---|---|---|
| Age, Median (IQR), Years | 73 (60–80) | 76 (70–81) | 63(50–75) | <0.001 |
| Males, n (%) | 52 (58.4) | 28 (58.3) | 24 (58.5) | 0.985 |
| BMI, Median (IQR), kg/m2 | 28 (25–32) | 28 (26–33) | 28 (25–31) | 0.545 |
| Care Home Resident, n (%) | 7 (7.9) | 7 (14.6) | 0 (0) | 0.011 |
| NYHA ≥ III, n (%), n = 72 | 59 (81.9) | 38 (95.0) | 21 (65.6) | 0.001 |
| Relevant Comorbidities, n (%) | ||||
| Myocardial Infarction | 9 (10.1) | 7 (14.6) | 2 (4.9) | 0.130 |
| Chronic Heart Failure | 62 (69.7) | 47 (97.9) | 15 (36.6) | <0.001 |
| Peripheral Vascular Disease | 7 (7.9) | 2 (4.2) | 5 (12.2) | 0.161 |
| Chronic Lung Disease | 25 (28.1) | 12 (25) | 13 (31.7) | 0.483 |
| Cerebrovascular Disease | 9 (10.1) | 5 (10.4) | 4 (9.8) | 0.918 |
| Diabetes Mellitus | 29 (32.6) | 22 (45.8) | 7 (17.1) | 0.005 |
| Moderate and Severe Kidney Disease | 33 (37.1) | 25 (52.1) | 8 (19.5) | 0.002 |
| Solid Tumor | 14 (15.7) | 7 (14.6) | 7 (17.1) | 0.748 |
| CCI Overall Score, Median (IQR) | 3 (1–5) | 4 (2–6) | 2 (0–4) | 0.002 |
| Cardiovascular Risk Factors, n (%) | ||||
| Arterial Hypertension | 71 (79.8) | 46 (95.8) | 25 (61) | <0.001 |
| (Ex-)smoker | 55 (64) | 28 (59.6) | 27 (69.2) | 0.353 |
| Hyperlipidemia | 10 (11.2) | 8 (16.7) | 2 (4.9) | 0.079 |
| Hypercholesterolemia | 15 (16.9) | 9 (18.8) | 6 (14.6) | 0.605 |
| Obesity | 49 (55.1) | 25 (52.1) | 24 (58.5) | 0.542 |
| Positive family history | 10 (11.2) | 4 (8.3) | 6 (14.6) | 0.348 |
| Overall Cohort (n = 89) | AHF (n = 48) | No AHF (n = 41) | p | |
|---|---|---|---|---|
| Admission, n (%) | ||||
| Emergency Service with Doctor | 8 (9.0) | 5 (10.4) | 3 (7.3) | 0.610 |
| Emergency Service | 6 (6.7) | 2 (4.2) | 4 (9.8) | 0.295 |
| Self Admission | 16 (18.0) | 5 (10.4) | 11 (26.8) | 0.044 |
| Family Physician | 55 (61.8) | 33 (68.8) | 22 (53.7) | 0.144 |
| Rehabilitation | 4 (4.5) | 3 (6.3) | 1 (2.4) | 0.387 |
| Vital signs in ED, Median (IQR) | ||||
| Respiratory Rate, breaths per minute | 17 (14–20) | 18 (15–20) | 16 (14–20) | 0.373 |
| Systolic Blood Pressure, mmHg | 136 (121–150) | 138 (122–151) | 133 (120–150) | 0.529 |
| Heart Rate, beats per minute | 86 (74–100) | 80 (71–94) | 89 (80–108) | 0.022 |
| Oxygen Saturation, % | 96 (93–98) | 95 (92–98) | 97 (94–99) | 0.037 |
| Lab, Median (IQR) | ||||
| Potassium, mmol/L | 4.2 (3.9–4.5) | 4.3 (3.9–4.7) | 4.2 (3.9–4.4) | 0.496 |
| Sodium, mmol/L | 140 (137–142) | 141 (137–143) | 140 (138–142) | 0.791 |
| Creatinine, mg/dL | 1.06 (0.87–1.51) | 1.24 (0.98–1.77) | 0.93 (0.77–1.18) | <0.001 |
| Urea, mg/dL | 36 (27–57) | 52 (35–74) | 29 (23–36) | <0.001 |
| Hemoglobin, g/dL | 13.2 (11.6–14.6) | 12.4 (11.0–14.1) | 14.1 (12.7–15.1) | 0.003 |
| Leukocytes/nL | 8.7 (7.2–11.0) | 8.5 (7.3–10.5) | 9.2 (6.8–12.0) | 0.301 |
| Glucose, mg/dL | 123 (105–147) | 126 (103–170) | 122 (107–138) | 0.385 |
| NT-proBNP, pg/mL | 2648 (763–5798) | 3912 (2594–8855) | 423 (63–1325) | <0.001 |
| hs cTnT, ng/L | 21 (14–42) | 31 (14–48) | 14 (14–26) | 0.010 |
| Severity of dyspnea on a numeric rating scale (1–10), Median (IQR) | ||||
| At Arrival of Emergency Service (n = 19) | 8 (4–9) | 8 (6–10) | 4 (3–7) | 0.170 |
| At Admission (n = 79) | 5 (2–6) | 5 (3–6) | 3 (1–6) | 0.071 |
| At 24 h (n = 70) | 3 (1–5) | 4 (2–5) | 2 (0–5) | 0.042 |
| At 72 h (n = 57) | 3 (1–4) | 3 (1–4) | 2 (0–5) | 0.542 |
| (n = 89) | AHF (n = 48) | No AHF (n = 41) | p | |
|---|---|---|---|---|
| Positive LUS for acute heart failure, n (%) | ||||
| in ED | 27 (30.3) | 26 (54.2) | 1 (2.4) | <0.001 |
| After 24 h | 11 (15.3) (n = 72) 1 | 8 (18.2) (n = 44) 1 | 3 (10.7) (n = 28) 1 | 0.391 |
| After 72 h | 11 (17.7) (n = 62) 1 | 8 (19.5) (n = 41) 1 | 3 (14.3) (n = 21) 1 | 0.610 |
| Pretreatment with diuretics, n (%) | 16 (18) | 16 (33.3) | 0 (0) | <0.001 |
| SE (95% CI) | SP (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR- (95% CI) |
|---|---|---|---|---|---|
| 54.2 (39.2–68.6) | 97.6 (87.1–99.9) | 96.3 (78.7–99.5) | 64.5 (57.1–71.3) | 22.2 (3.2–156.6) | 0.47 (0.34–0.64) |
| SE (95% CI) | SP (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR- (95% CI) |
|---|---|---|---|---|---|
| 75 (56.6–88.5) | 97.6 (87.1–99.9) | 96 (77.4–99.4) | 83.3 (73.3–90.1) | 30.8 (4.4–215.3) | 0.26 (0.14–0.47) |
| Overall Cohort (n = 89) | AHF (n = 48) | No AHF (n = 41) | p | |
|---|---|---|---|---|
| Length of Stay, Median (IQR) | 7 (4–11) | 8 (6–12) | 4 (1–9) | 0.004 |
| Hospital Mortality, n (%) | 1 (1.1) | 0 (0) | 1 (2.4) | 0.277 |
| Survival After 6 Months, n (%) | 74 (83.1) | 40 (83.3) | 34 (82.9) | 0.934 |
| Dead After 6 Months, n (%) | 5 (5.6) | 3 (6.3) | 2 (4.9) | 0.779 |
| Unknown Survival Status After 6 Months, n (%) | 10 (11.2) | 5 (10.4) | 5 (12.2) | 0.779 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glöckner, E.; Wening, F.; Christ, M.; Dechêne, A.; Singler, K. Lung Ultrasound Eight-Point Method in Diagnosing Acute Heart Failure in Emergency Patients with Acute Dyspnea: Diagnostic Accuracy and 72 h Monitoring. Medicina 2020, 56, 379. https://doi.org/10.3390/medicina56080379
Glöckner E, Wening F, Christ M, Dechêne A, Singler K. Lung Ultrasound Eight-Point Method in Diagnosing Acute Heart Failure in Emergency Patients with Acute Dyspnea: Diagnostic Accuracy and 72 h Monitoring. Medicina. 2020; 56(8):379. https://doi.org/10.3390/medicina56080379
Chicago/Turabian StyleGlöckner, Erika, Felicitas Wening, Michael Christ, Alexander Dechêne, and Katrin Singler. 2020. "Lung Ultrasound Eight-Point Method in Diagnosing Acute Heart Failure in Emergency Patients with Acute Dyspnea: Diagnostic Accuracy and 72 h Monitoring" Medicina 56, no. 8: 379. https://doi.org/10.3390/medicina56080379






