Osteoid Osteoma Treatment with Microwave Ablation: A Report of Two Cases
Abstract
1. Introduction
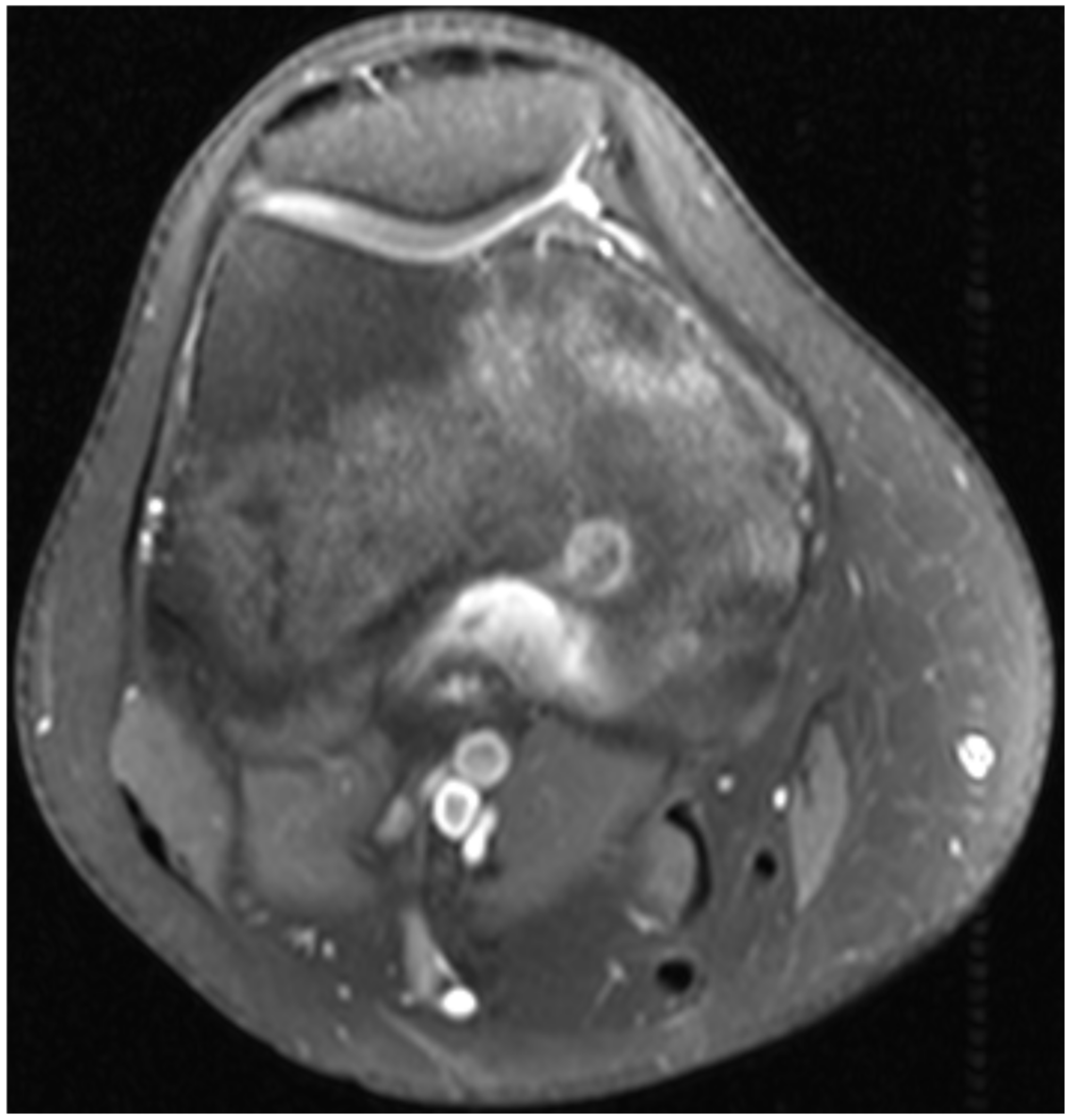
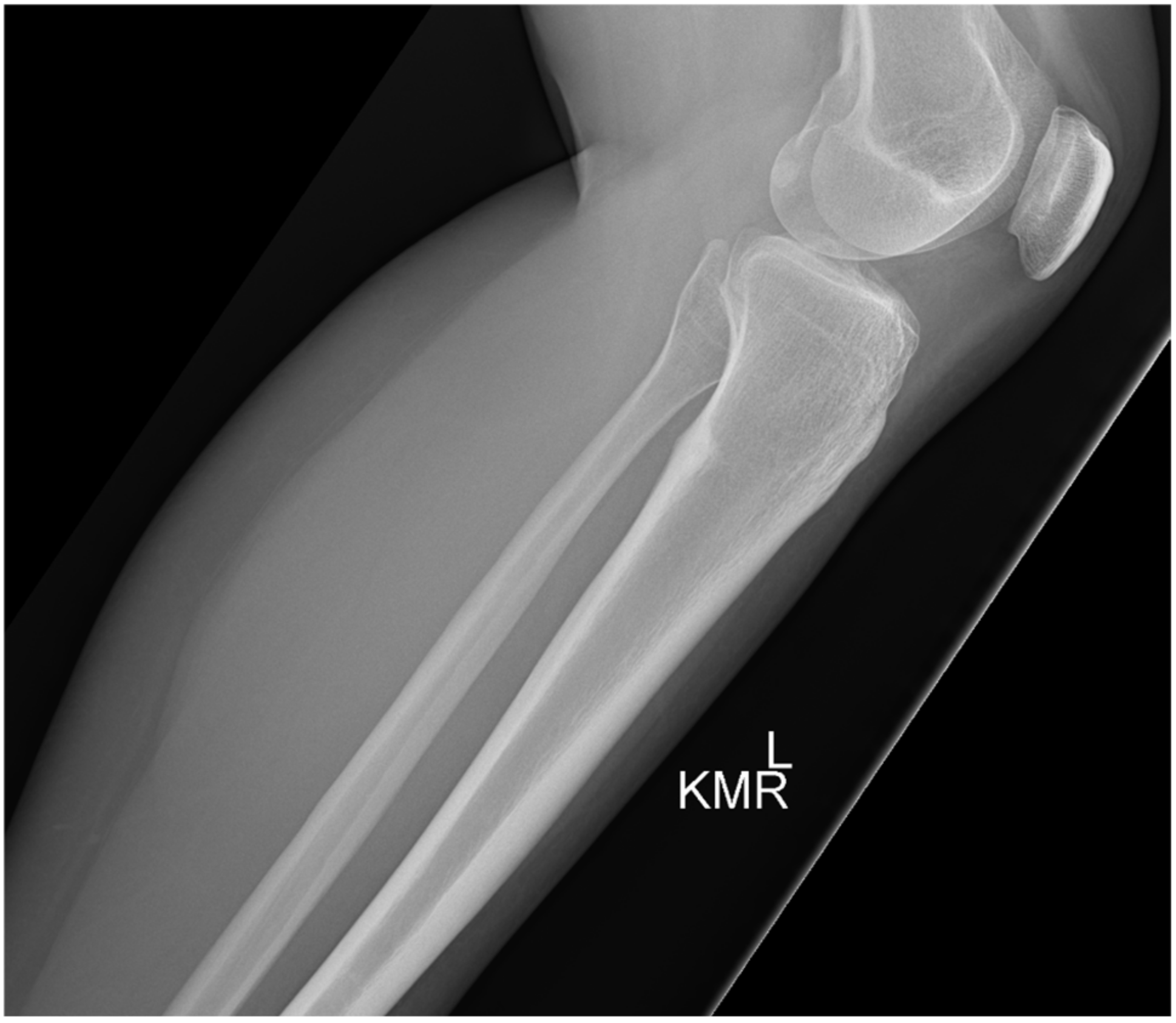
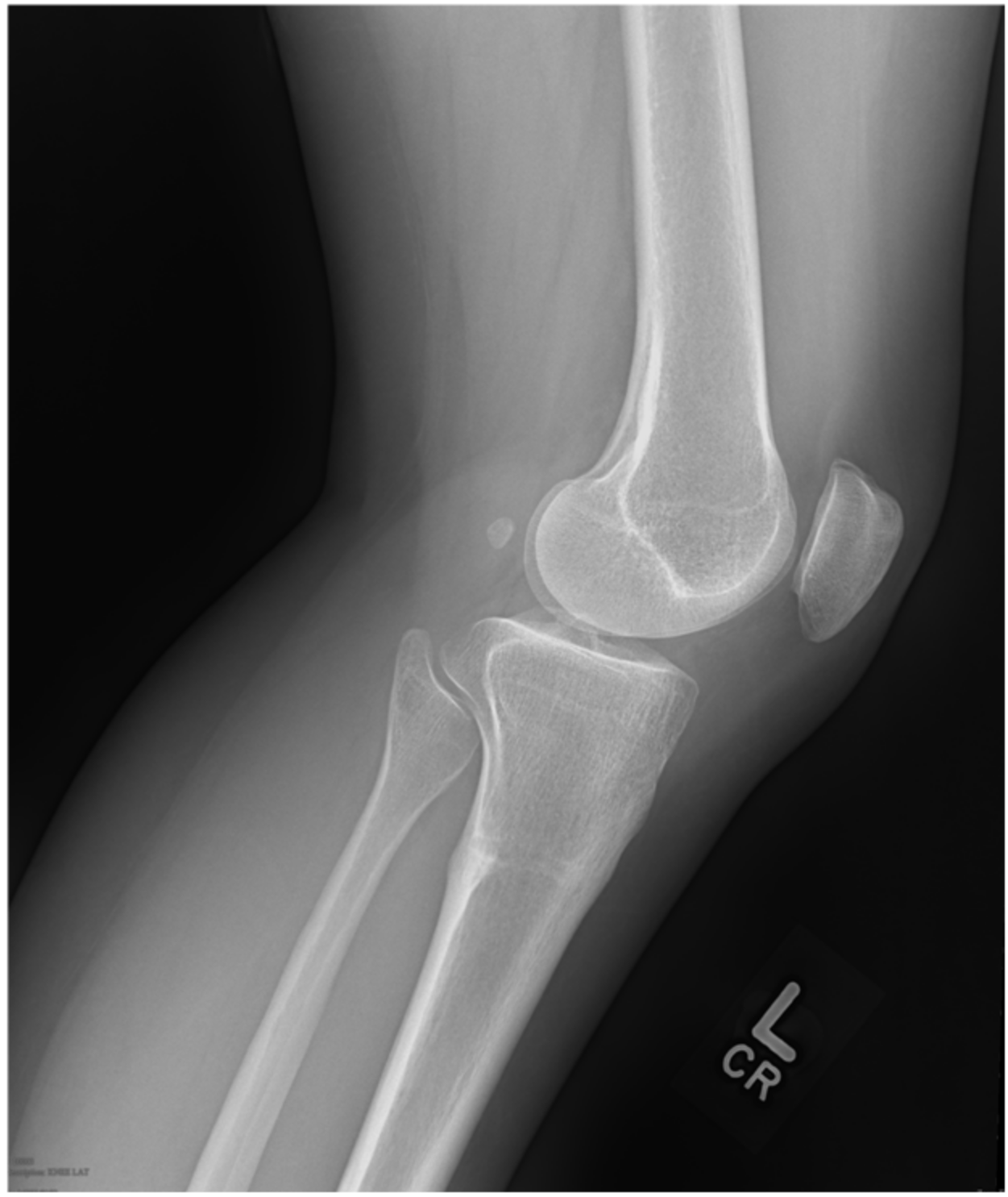
2. Case 1
3. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hakim, D.N.; Pelly, T.; Kulendran, M.; Caris, J.A. Benign tumours of the bone: A review. J. Bone Oncol. 2015, 4, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kransdorf, M.J.; Stull, M.A.; Gilkey, F.W.; Moser, R.P. Osteoid osteoma. Radiographics 1991, 11, 671–696. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Tamburrelli, F.; Ciabattoni, G. Prostaglandins in osteoid osteoma. Int. Orthop. 1991, 15, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Kneisl, J.S.; Simon, M.A. Medical management compared with operative treatment for osteoid-osteoma. J. Bone Joint Surg. Am. 1992, 74, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, D.; Learch, T.J.; Ishimitsu, D.N.; Motamedi, K.; Katz, M.D.; Brien, E.W.; Menendez, L. Thermal ablation of osteoid osteoma: Overview and step-by-step guide. Radiographics 2009, 29, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.I.; Alexander, A.; Rosenberg, A.E.; Springfield, D. Ablation of osteoid osteomas with a percutaneously placed electrode: A new procedure. Radiology 1992, 183, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Decadt, B.; Siriwardena, A.K. Radiofrequency ablation of liver tumours: Systematic review. Lancet Oncol. 2004, 5, 550–560. [Google Scholar] [CrossRef]
- Brace, C.L. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr. Probl. Diagn. Radiol. 2009, 38, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Brace, C.L. Microwave ablation technology: What every user should know. Curr. Probl. Diagn. Radiol. 2009, 38, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Di Maso, M.; Muscatiello, N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Balioğlu, M.B.; Albayrak, A.; Atıcı, Y.; Sökücü, S.; Tacal, M.T.; Kaygusuz, M.A. The effect of simple local resection on pain and scoliotic curve in patients with scoliosis secondary to osteoid osteoma and osteoblastoma in the spine. Acta Orthop. Traumatol. Turc. 2016, 50, 330–338. [Google Scholar] [PubMed]
- Rovella, M.S.; Martins, G.L.; Cavalcanti, C.F.; Bor-Seng-Shu, E.; Camargo, O.P.; Cerri, G.G.; Menezes, M.R. Magnetic Resonance-Guided High-Intensity Focused Ultrasound Ablation of Osteoid Osteoma: A Case Series Report. Ultrasound Med. Biol. 2016, 42, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Napoli, A.; Mastantuono, M.; Cavallo Marincola, B.; Anzidei, M.; Zaccagna, F.; Moreschini, O.; Passariello, R.; Catalano, C. Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology 2013, 267, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Arai, Y.; Myoui, A.; Gobara, H.; Sone, M.; Rosenthal, D.I.; Tsushima, Y.; Kanazawa, S.; Ehara, S.; Endo, K. Phase I/II Multi-Institutional Study of Percutaneous Radiofrequency Ablation for Painful Osteoid Osteoma (JIVROSG-0704). Cardiovasc. Intervent. Radiol. 2016, 39, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Donkol, R.H.; Al-Nammi, A.; Moghazi, K. Efficacy of percutaneous radiofrequency ablation of osteoid osteoma in children. Pediatr. Radiol. 2008, 38, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, D.I.; Hornicek, F.J.; Torriani, M.; Gebhardt, M.C.; Mankin, H.J. Osteoid osteoma: Percutaneous treatment with radiofrequency energy. Radiology 2003, 229, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, C.; Nueffer, J.P.; Runge, M.; Dubut, J.; Kastler, B.; Aubry, S. Prospective pilot study of CT-guided microwave ablation in the treatment of osteoid osteomas. Skeletal. Radiol. 2017, 46, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Rinzler, E.S.; Shivaram, G.M.; Shaw, D.W.; Monroe, E.J.; Koo, K.S.H. Microwave ablation of osteoid osteoma: Initial experience and efficacy. Pediatr. Radiol. 2019, 49, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Chang, Y.; Sharma, A.K. Radiofrequency ablation vs microwave ablation for osteoid osteomas: Long-term results. Skeletal. Radiol. 2020, 49, 1995–2000. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westlake, B.; Mazzi, J.; Tedesco, N. Osteoid Osteoma Treatment with Microwave Ablation: A Report of Two Cases. Medicina 2021, 57, 470. https://doi.org/10.3390/medicina57050470
Westlake B, Mazzi J, Tedesco N. Osteoid Osteoma Treatment with Microwave Ablation: A Report of Two Cases. Medicina. 2021; 57(5):470. https://doi.org/10.3390/medicina57050470
Chicago/Turabian StyleWestlake, Babe, Jessica Mazzi, and Nicholas Tedesco. 2021. "Osteoid Osteoma Treatment with Microwave Ablation: A Report of Two Cases" Medicina 57, no. 5: 470. https://doi.org/10.3390/medicina57050470
APA StyleWestlake, B., Mazzi, J., & Tedesco, N. (2021). Osteoid Osteoma Treatment with Microwave Ablation: A Report of Two Cases. Medicina, 57(5), 470. https://doi.org/10.3390/medicina57050470





