TBX2, a Novel Regulator of Labour
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. TBX2 Downregulates TNFα and Interferon Signaling in Myometrial Cells
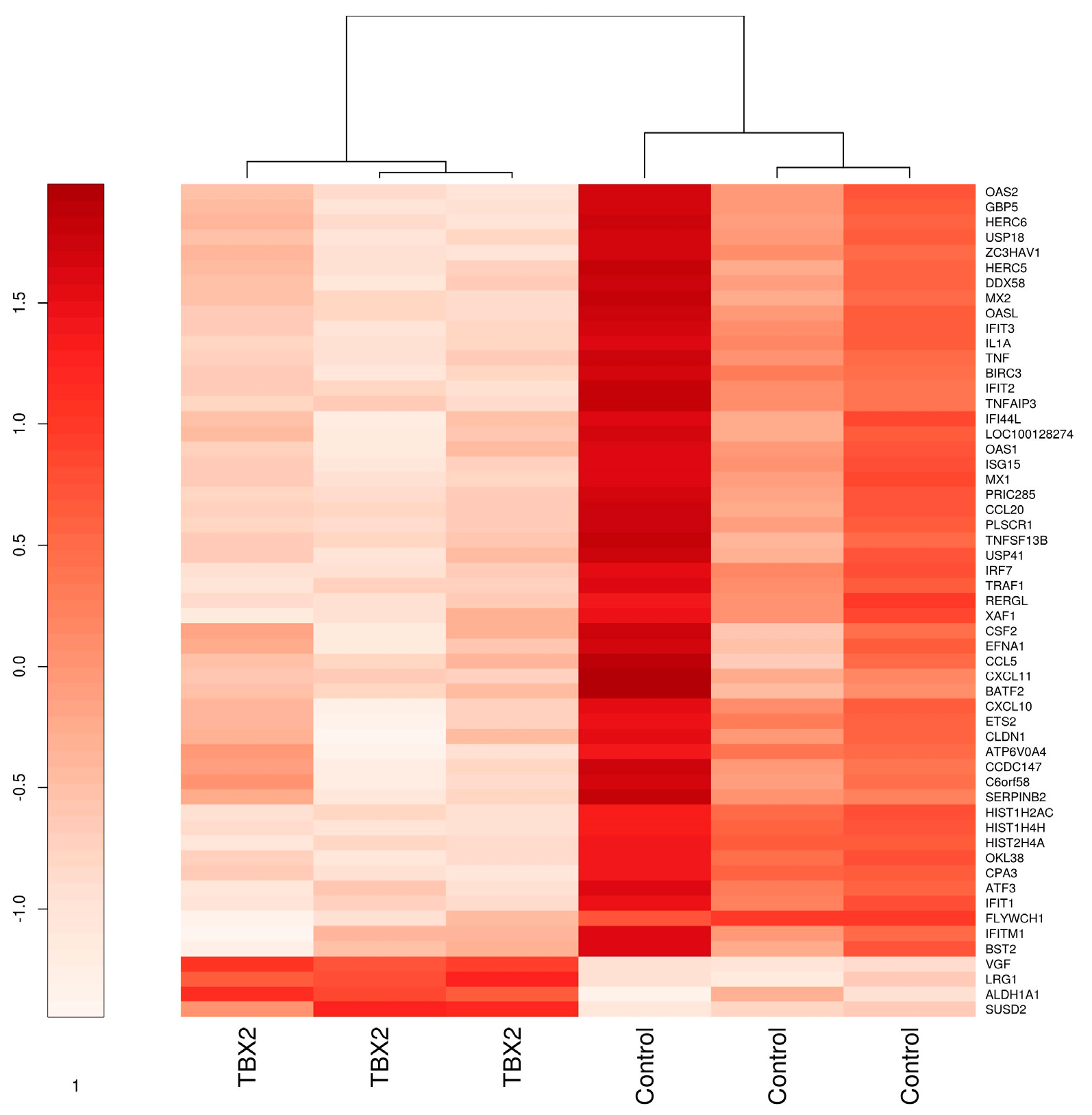
3.1.1. Microarray Analysis of hTERT-HM Cells with TBX2 Overexpression Compared to Control
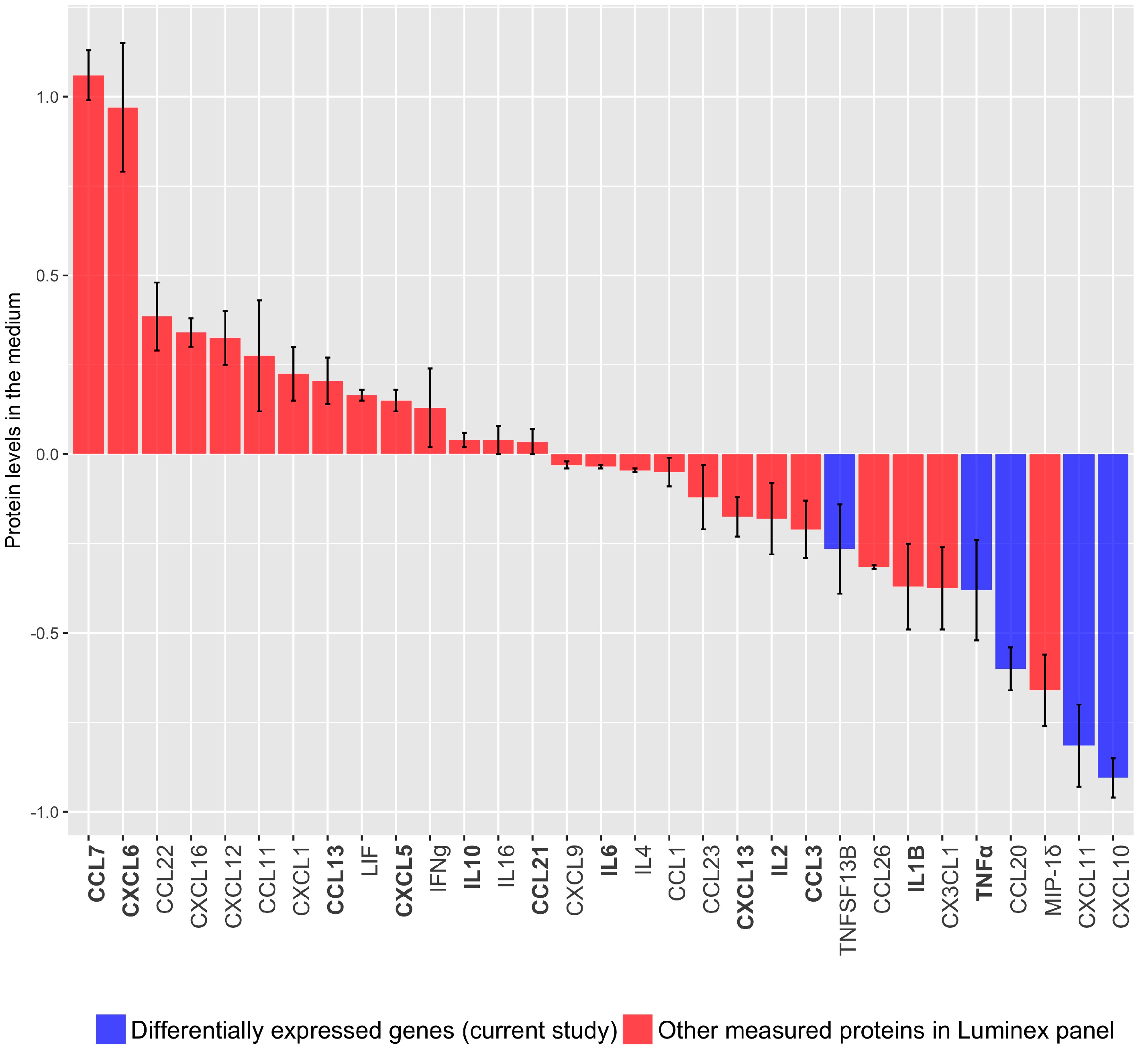
3.1.2. Cytokine/Chemokine Release after TBX2 Overexpression in hTERT-HM Cells
3.2. TBX2 Inhibits Crucial Upstream Regulators in Preterm and Term Labour
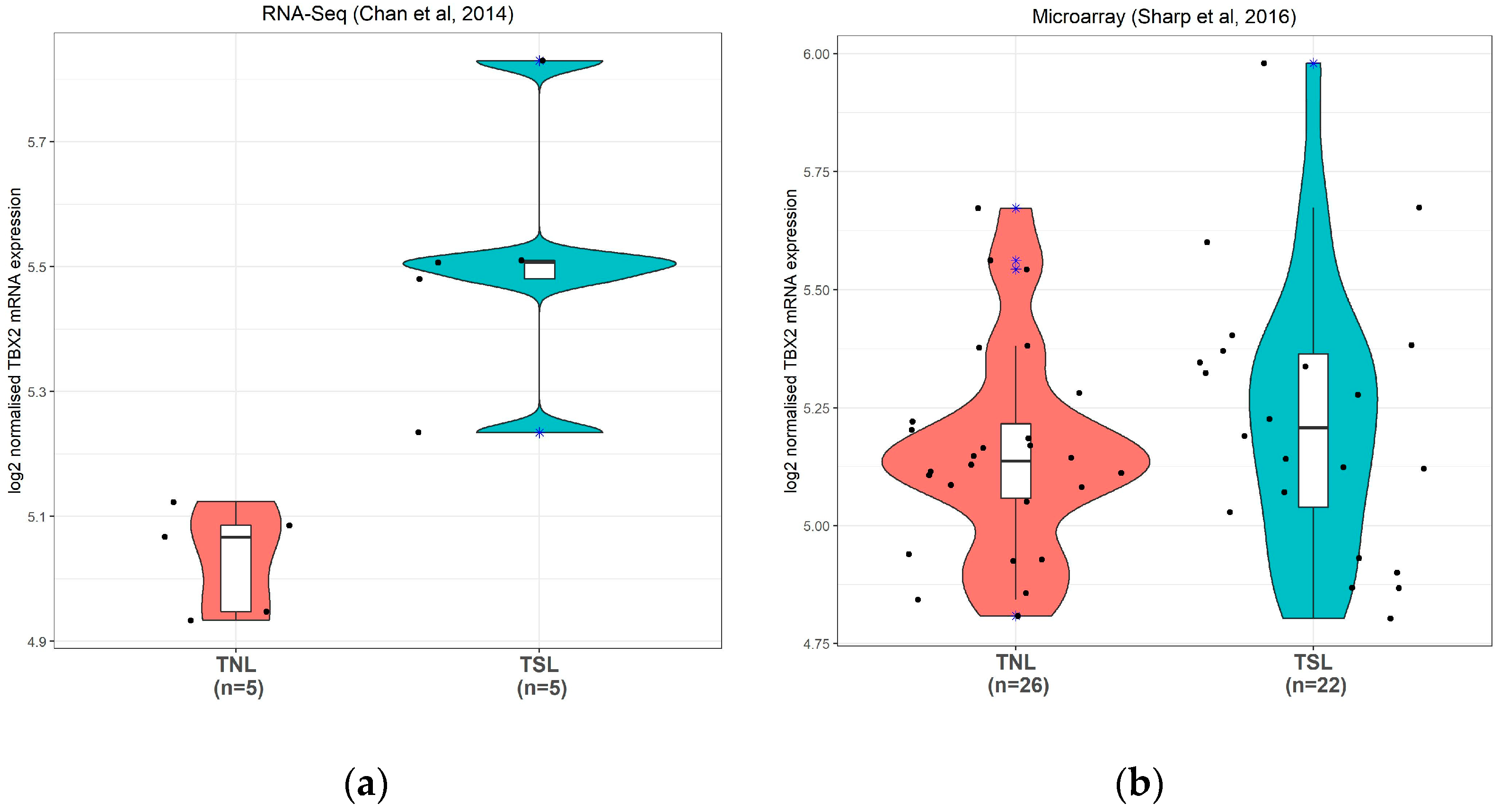
3.2.1. TBX2 mRNA Expression in Human Preterm Myometrium Samples
3.2.2. TBX2 mRNA Expression in Human Term Myometrium Samples
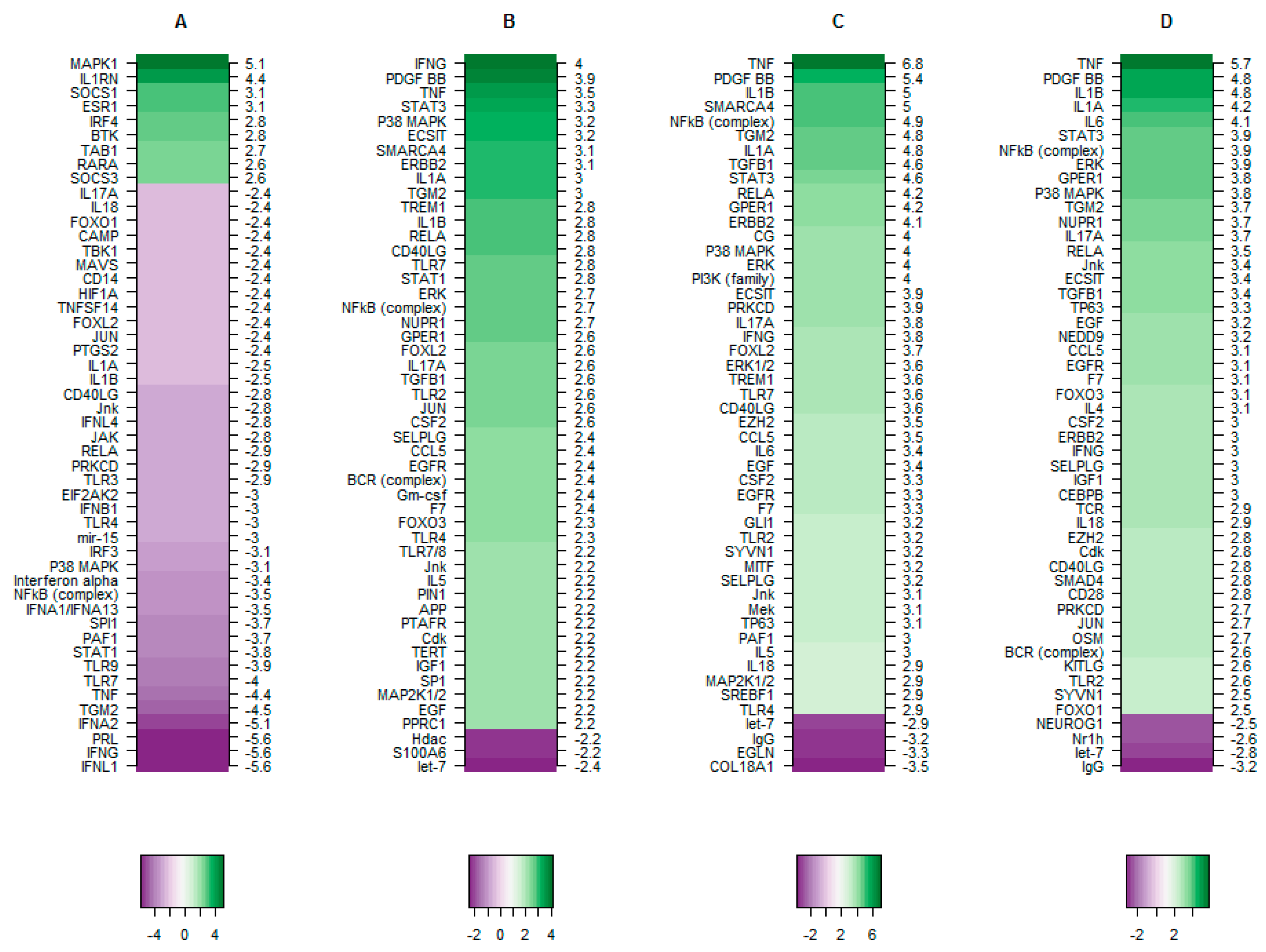
3.2.3. The Putative Role of TBX2 in Myometrium during Labour
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef]
- Linsell, L.; Malouf, R.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or With Very Low Birth Weight: A Systematic Review. JAMA Pediatr. 2015, 169, 1162–1172. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.H.; Larson, J.; Blencowe, H.; Spong, C.Y.; Howson, C.P.; Cairns-Smith, S.; Lackritz, E.M.; Lee, S.K.; Mason, E.; Serazin, A.C.; et al. Preventing preterm births: Analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 2013, 381, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113 (Suppl. 3), 17–42. [Google Scholar] [CrossRef] [PubMed]
- Condon, J.C.; Jeyasuria, P.; Faust, J.M.; Wilson, J.W.; Mendelson, C.R. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc. Natl. Acad. Sci. USA 2003, 100, 9518–9523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, L.; Shynlova, O.; Matysiak-Zablocki, E.; Mesiano, S.; Dong, X.; Lye, S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. 2016, 7, 11565. [Google Scholar] [CrossRef] [Green Version]
- Renthal, N.E.; Williams, K.C.; Montalbano, A.P.; Chen, C.C.; Gao, L.; Mendelson, C.R. Molecular Regulation of Parturition: A Myometrial Perspective. Cold Spring Harb. Perspect. Med. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Talati, A.N.; Hackney, D.N.; Mesiano, S. Pathophysiology of preterm labour with intact membranes. Semin. Perinatol. 2017, 41, 420–426. [Google Scholar] [CrossRef]
- Allport, V.C.; Pieber, D.; Slater, D.M.; Newton, R.; White, J.O.; Bennett, P.R. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol. Hum. Reprod. 2001, 7, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Bennett, P.R.; Terzidou, V. Advances in the role of oxytocin receptors in human parturition. Mol. Cell. Endocrinol. 2017, 449, 56–63. [Google Scholar] [CrossRef]
- Smith, R. Parturition. N. Engl. J. Med. 2007, 356, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoven, E.; Wissink, S.; van der Saag, P.T.; van der Burg, B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J. Biol. Chem. 1996, 271, 6217–6224. [Google Scholar] [CrossRef] [Green Version]
- Hardy, D.B.; Janowski, B.A.; Corey, D.R.; Mendelson, C.R. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol. Endocrinol. 2006, 20, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yi, L.; Rote, N.S.; Hurd, W.W.; Mesiano, S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: Implications for progesterone actions in human pregnancy and parturition. J. Clin. Endocrinol. Metab. 2012, 97, E719–E730. [Google Scholar] [CrossRef]
- Amini, P.; Michniuk, D.; Kuo, K.; Yi, L.; Skomorovska-Prokvolit, Y.; Peters, G.A.; Tan, H.; Wang, J.; Malemud, C.J.; Mesiano, S. Human Parturition Involves Phosphorylation of Progesterone Receptor-A at Serine-345 in Myometrial Cells. Endocrinology 2016, 157, 4434–4445. [Google Scholar] [CrossRef]
- Peters, G.A.; Yi, L.; Skomorovska-Prokvolit, Y.; Patel, B.; Amini, P.; Tan, H.; Mesiano, S. Inflammatory Stimuli Increase Progesterone Receptor-A Stability and Transrepressive Activity in Myometrial Cells. Endocrinology 2017, 158, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Hadley, E.E.; Richardson, L.S.; Torloni, M.R.; Menon, R. Gestational tissue inflammatory biomarkers at term labour: A systematic review of literature. Am. J. Reprod. Immunol. 2018, 79. [Google Scholar] [CrossRef]
- Kirby, M.A.; Heuerman, A.C.; Custer, M.; Dobyns, A.E.; Strilaeff, R.; Stutz, K.N.; Cooperrider, J.; Elsissy, J.G.; Yellon, S.M. Progesterone Receptor-Mediated Actions Regulate Remodeling of the Cervix in Preparation for Preterm Parturition. Reprod. Sci. 2016, 23, 1473–1483. [Google Scholar] [CrossRef] [Green Version]
- Romero, R.; Conde-Agudelo, A.; Da Fonseca, E.; O’Brien, J.M.; Cetingoz, E.; Creasy, G.W.; Hassan, S.S.; Nicolaides, K.H. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: A meta-analysis of individual patient data. Am. J. Obstet. Gynecol. 2018, 218, 161–180. [Google Scholar] [CrossRef] [Green Version]
- Jarde, A.; Lutsiv, O.; Beyene, J.; McDonald, S.D. Vaginal progesterone, oral progesterone, 17-OHPC, cerclage, and pessary for preventing preterm birth in at-risk singleton pregnancies: An updated systematic review and network meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 556–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanprakob, T.; Laopaiboon, M.; Lumbiganon, P.; Sangkomkamhang, U.S. Cyclo-oxygenase (COX) inhibitors for preventing preterm labour. Cochrane Database Syst. Rev. 2012, 10, CD007748. [Google Scholar] [CrossRef] [PubMed]
- Reinebrant, H.E.; Pileggi-Castro, C.; Romero, C.L.; Santos, R.A.N.d.; Kumar, S.; Souza, J.P.; Flenady, V. Cyclo-oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst. Rev. 2015, CD001992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papatsonis, D.N.; Van Geijn, H.P.; Ader, H.J.; Lange, F.M.; Bleker, O.P.; Dekker, G.A. Nifedipine and ritodrine in the management of preterm labour: A randomized multicenter trial. Obstet. Gynecol. 1997, 90, 230–234. [Google Scholar] [CrossRef] [Green Version]
- Flenady, V.; Reinebrant, H.E.; Liley, H.G.; Tambimuttu, E.G.; Papatsonis, D.N. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst. Rev. 2014, CD004452. [Google Scholar] [CrossRef] [Green Version]
- Fernando, F.; Boussata, S.; Jongejan, A.; van der Post, J.A.; Afink, G.; Ris-Stalpers, C. In silico analysis of the Mus musculus uterine gene expression landscape during pregnancy identifies putative upstream regulators for labour. PLoS ONE 2018, 13, e0204236. [Google Scholar] [CrossRef]
- Papaioannou, V.E.; Silver, L.M. The T-box gene family. Bioessays 1998, 20, 9–19. [Google Scholar] [CrossRef]
- Campbell, C.; Goodrich, K.; Casey, G.; Beatty, B. Cloning and mapping of a human gene (TBX2) sharing a highly conserved protein motif with the Drosophila omb gene. Genomics 1995, 28, 255–260. [Google Scholar] [CrossRef]
- Papaioannou, V.E. The T-box gene family: Emerging roles in development, stem cells and cancer. Development 2014, 141, 3819–3833. [Google Scholar] [CrossRef] [Green Version]
- Douglas, N.C.; Papaioannou, V.E. The T-box transcription factors TBX2 and TBX3 in mammary gland development and breast cancer. J. Mammary Gland Biol. Neoplasia 2013, 18, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Rana, M.S.; Christoffels, V.M.; Moorman, A.F. A molecular and genetic outline of cardiac morphogenesis. Acta Physiol. 2013, 207, 588–615. [Google Scholar] [CrossRef]
- Stanfield, Z.; Amini, P.; Wang, J.; Yi, L.; Tan, H.; Cgance, M.R.; Koyutürk, M.; Mesiano, S. Interplay of transcriptional signaling by progesterone, cyclic AMP, and inflamation in myomtrial cells; implications for the control of human parturition. Mol. Hum. Reprod. 2019, 25, 408–422. [Google Scholar] [CrossRef]
- Chan, Y.W.; van den Berg, H.A.; Moore, J.D.; Quenby, S.; Blanks, A.M. Assessment of myometrial transcriptome changes associated with spontaneous human labour by high-throughput RNA-seq. Exp. Physiol. 2014, 99, 510–524. [Google Scholar] [CrossRef]
- Sharp, G.C.; Hutchinson, J.L.; Hibbert, N.; Freeman, T.C.; Saunders, P.T.; Norman, J.E. Transcription Analysis of the Myometrium of Labouring and Non-Labouring Women. PLoS ONE 2016, 11, e0155413. [Google Scholar] [CrossRef] [Green Version]
- Condon, J.; Yin, S.; Mayhew, B.; Word, R.A.; Wright, W.E.; Shay, J.W.; Rainey, W.E. Telomerase immortalization of human myometrial cells. Biol. Reprod. 2002, 67, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Murphy, P.G.; Henderson, D.T.; Adams, M.D.; Horlick, E.A.; Dixon, E.P.; King, L.M.; Avissar, P.L.; Brown, C.A.; Fischer, T.J.; Malinowski, D.P. Isolation of RNA from cell lines and cervical cytology specimens stored in BD SurePath preservative fluid and downstream detection of housekeeping gene and HPV E6 expression using real time RT-PCR. J. Virol. Methods 2009, 156, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.A.; Quispe-Ricalde, A.; Montes de Oca, F.; Foronda, P.; Hernandez, M.M. A high-throughput open-array qPCR gene panel to identify housekeeping genes suitable for myometrium and leiomyoma expression analysis. Gynecol. Oncol. 2014, 134, 138–143. [Google Scholar] [CrossRef]
- Marullo, M.; Zuccato, C.; Mariotti, C.; Lahiri, N.; Tabrizi, S.J.; di Donato, S.; Cattaneo, E. Expressed Alu repeats as a novel, reliable tool for normalization of real-time quantitative RT-PCR data. Genome Biol. 2010, 11, R9. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.J.; Fortier, M.A.; Lopez Bernal, A. Prostaglandin pathway gene expression in human placenta, amnion and choriodecidua is differentially affected by preterm and term labour and by uterine inflammation. BMC Pregnancy Childbirth 2014, 14, 241. [Google Scholar] [CrossRef] [Green Version]
- Christoffels, V.M.; Hoogaars, W.M.; Tessari, A.; Clout, D.E.; Moorman, A.F.; Campione, M. T-box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers. Dev. Dyn. 2004, 229, 763–770. [Google Scholar] [CrossRef]
- Harrelson, Z.; Kelly, R.G.; Goldin, S.N.; Gibson-Brown, J.J.; Bollag, R.J.; Silver, L.M.; Papaioannou, V.E. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development 2004, 131, 5041–5052. [Google Scholar] [CrossRef] [Green Version]
- Abrahams, A.; Parker, M.I.; Prince, S. The T-box transcription factor Tbx2: Its role in development and possible implication in cancer. IUBMB Life 2010, 62, 92–102. [Google Scholar] [CrossRef]
- Douglas, N.C.; Heng, K.; Sauer, M.V.; Papaioannou, V.E. Dynamic expression of Tbx2 subfamily genes in development of the mouse reproductive system. Dev. Dyn. 2012, 241, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Bethin, K.E.; Nagai, Y.; Sladek, R.; Asada, M.; Sadovsky, Y.; Hudson, T.J.; Muglia, L.J. Microarray analysis of uterine gene expression in mouse and human pregnancy. Mol. Endocrinol. 2003, 17, 1454–1469. [Google Scholar] [CrossRef] [Green Version]
- Carreira, S.; Dexter, T.J.; Yavuzer, U.; Easty, D.J.; Goding, C.R. Brachyury-related transcription factor Tbx2 and repression of the melanocyte-specific TRP-1 promoter. Mol. Cell. Biol. 1998, 18, 5099–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condon, J.C.; Jeyasuria, P.; Faust, J.M.; Mendelson, C.R. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc. Natl. Acad. Sci. USA 2004, 101, 4978–4983. [Google Scholar] [CrossRef] [Green Version]
- Romero, R.; Espinoza, J.; Goncalves, L.F.; Kusanovic, J.P.; Friel, L.; Hassan, S. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 2007, 25, 21–39. [Google Scholar] [CrossRef]
- MacIntyre, D.A.; Lee, Y.S.; Migale, R.; Herbert, B.R.; Waddington, S.N.; Peebles, D.; Hagberg, H.; Johnson, M.R.; Bennett, P.R. Activator protein 1 is a key terminal mediator of inflammation-induced preterm labour in mice. FASEB J. 2014, 28, 2358–2368. [Google Scholar] [CrossRef]
- Pirianov, G.; MacIntyre, D.A.; Lee, Y.; Waddington, S.N.; Terzidou, V.; Mehmet, H.; Bennett, P.R. Specific inhibition of c-Jun N-terminal kinase delays preterm labour and reduces mortality. Reproduction 2015, 150, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Menon, R.; Papaconstantinou, J. p38 Mitogen activated protein kinase (MAPK): A new therapeutic target for reducing the risk of adverse pregnancy outcomes. Expert Opin. Ther. Targets 2016, 20, 1397–1412. [Google Scholar] [CrossRef]
- Toyoshima, K.; Narahara, H.; Furukawa, M.; Frenkel, R.A.; Johnston, J.M. Platelet-activating factor. Role in fetal lung development and relationship to normal and premature labour. Clin. Perinatol. 1995, 22, 263–280. [Google Scholar] [CrossRef]
- Mendelson, C.R.; Montalbano, A.P.; Gao, L. Fetal-to-maternal signaling in the timing of birth. J. Steroid Biochem. Mol. Biol. 2017, 170, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Hoffman, D.R.; Hwang, S.B.; Miyaura, S.; Johnston, J.M. Prolongation of parturition in the pregnant rat following treatment with a platelet activating factor receptor antagonist. Biol. Reprod. 1991, 44, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Capece, A.; Vasieva, O.; Meher, S.; Alfirevic, Z.; Alfirevic, A. Pathway analysis of genetic factors associated with spontaneous preterm birth and pre-labour preterm rupture of membranes. PLoS ONE 2014, 9, e108578. [Google Scholar] [CrossRef] [Green Version]
- Varner, M.W.; Esplin, M.S. Current understanding of genetic factors in preterm birth. BJOG Int. J. Obstet. Gynaecol. 2005, 112 (Suppl. 1), 28–31. [Google Scholar] [CrossRef] [PubMed]
- Prearo Moco, N.; Camargo Batista, R.A.; Fernandes Martin, L.; de Oliveira, L.G.; Garcia de Lima Parada, C.M.; Alarcão Dias-Melicio, L.; de Assis Golim, M.; Guimarães da Silva, M. Toll-Like Receptor-2 and -4 Expression by Maternal Neutrophils in Preterm Labour. Gynecol. Obstet. Invest. 2018, 83, 1–8. [Google Scholar] [CrossRef]
- Lappas, M. Forkhead box O1 (FOXO1) in pregnant human myometrial cells: A role as a pro-inflammatory mediator in human parturition. J. Reprod. Immunol. 2013, 99, 24–32. [Google Scholar] [CrossRef]
- Hagberg, H.; Mallard, C.; Jacobsson, B. Role of cytokines in preterm labour and brain injury. BJOG Int. J. Obstet. Gynaecol. 2005, 112 (Suppl. 1), 16–18. [Google Scholar] [CrossRef]
- Shynlova, O.; Lee, Y.H.; Srikhajon, K.; Lye, S.J. Physiologic uterine inflammation and labour onset: Integration of endocrine and mechanical signals. Reprod. Sci. 2013, 20, 154–167. [Google Scholar] [CrossRef]
- Nehme, E.; Rahal, Z.; Sinjab, A.; Khalil, A.; Chami, H.; Nemer, G.; Kadara, H. Epigenetic suppression of the T-box subfamily 2 (TBX2) in human non-small cell lung cancer. Int. J. Mol. Sci. 2019, 20, 1159. [Google Scholar] [CrossRef] [Green Version]
- Milton, A.D.; Okkema, P.G. Caenorhabditis elegans TBX-2 directly regulates its own expression in a negative autoregulatory loop. G3 Genes Genomes Genet. 2015, 5, 1177–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| KEGG ID | Pathway Description | Gene Count | FDR * | Matching Proteins in Network |
|---|---|---|---|---|
| 4668 | TNF signaling pathway | 8 | 9.74 × 10−8 | BIRC3, CCL20, CCL5, CSF2, CXCL10, TNF, TNFAIP3, TRAF1 |
| 4064 | NF-kappa B signaling pathway | 6 | 8.37 × 10−6 | BIRC3, DDX58, TNF, TNFAIP3, TNFSF13B, TRAF1 |
| 4622 | RIG-I-like receptor signaling pathway | 5 | 3.60 × 10−5 | CXCL10, DDX58, IRF7, ISG15, TNF |
| 4620 | Toll-like receptor signaling pathway | 5 | 0.000199 | CCL5, CXCL10, CXCL11, IRF7, TNF |
| 4621 | NOD-like receptor signaling pathway | 4 | 0.000375 | BIRC3, CCL5, TNF, TNFAIP3 |
| 4623 | Cytosolic DNA-sensing pathway | 4 | 0.000484 | CCL5, CXCL10, DDX58, IRF7 |
| 4062 | Chemokine signaling pathway | 4 | 0.0276 | CCL20, CCL5, CXCL10, CXCL11 |
| GO ID Pathway | Pathway Description | Gene Count | FDR | Matching Proteins in Network |
|---|---|---|---|---|
| GO.0060337 | type I interferon signaling pathway | 14 | 2.45 × 10−20 | BST2, IFIT1, IFIT2, IFIT3, IFITM1, IRF7, ISG15, MX1, MX2, OAS1, OAS2, OASL, USP18, XAF1 |
| GO.0019221 | cytokine-mediated signaling pathway | 19 | 3.98 × 10−17 | BST2, CCL5, CXCL10, CXCL11, HERC5, IFIT1, IFIT2, IFIT3, IFITM1, IL1A, IRF7, ISG15, MX1, MX2, OAS1, OAS2, OASL, USP18, XAF1 |
| GO.0007166 | cell surface receptor signaling pathway | 22 | 2.18 × 10−7 | ATP6V0A4, BIRC3, BST2, CCL5, CXCL10, CXCL11, EFNA1, HERC5, IFIT1, IFIT2, IFIT3, IFITM1, IL1A, IRF7, ISG15, MX1, MX2, OAS1, OAS2, OASL, USP18, XAF1 |
| GO.0060333 | interferon-gamma-mediated signaling pathway | 4 | 4.74 × 10−3 | IRF7, OAS1, OAS2, OASL |
| GO.2001236 | regulation of extrinsic apoptotic signaling pathway | 5 | 8.79 × 10−3 | ATF3, CSF2, IL1A, TNFAIP3, TRAF1 |
| GO.0002753 | cytoplasmic pattern recognition receptor signaling pathway | 3 | 1.37 × 10−2 | DDX58, IRF7, TNFAIP3 |
| GO.0039528 | cytoplasmic pattern recognition receptor signaling pathway in response to virus | 2 | 1.46 × 10−2 | DDX58, IRF7 |
| GO.0070424 | regulation of nucleotide-binding oligomerization domain containing signaling pathway | 2 | 1.91 × 10−2 | BIRC3, TNFAIP3 |
| GO.0034121 | regulation of toll-like receptor signaling pathway | 3 | 1.97 × 10−2 | BIRC3, IRF7, TNFAIP3 |
| GO.0070098 | chemokine-mediated signaling pathway | 3 | 2.16 × 10−2 | CCL5, CXCL10, CXCL11 |
| GO.0039535 | regulation of RIG-I signaling pathway | 2 | 3.99 × 10−2 | BIRC3, ZC3HAV1 |
| GO.0002221 | pattern recognition receptor signaling pathway | 4 | 4.51 × 10−2 | BIRC3, DDX58, IRF7, TNFAIP3 |
| Preterm Spontaneous Labour (PSL) * n = 8 | Preterm No Labour (PNL) * n = 8 | p Value | |
|---|---|---|---|
| Gestational age at delivery in days (mean and range) | 198 (179–233) | 213 (189–236) | 0.10 # |
| Gravidity (mean) | 2.25 | 2.75 | 0.62 # |
| Parity (mean) | 1 | 0.75 | 0.95 # |
| Male neonatal gender | 8 | 5 | 0.2 ## |
| Neonatal weight in grams (mean and range) | 1258 (820–2054) | 1137 (580–1970) | 0.44 # |
| Spontaneous Contractions | 6 | 0 | <0.01 ## |
| Spontaneous Rupture of Membranes | 2 | 0 | 0.47 ## |
| Contractions at any stage during pregnancy | 8 | 0 | <0.01 ## |
| Hypertension during pregnancy | 0 | 6 | <0.01 ## |
| Tocolytics during pregnancy | 6 | 2 ** | 0.13 ## |
| Protein | Level in TBX2 Transduced hTERT-HM Cells Compared to Mock Transduced Cells * | p Value ** |
|---|---|---|
| NFκB | 1.06 ± 0.20 | 0.694 |
| RelB p68 | 0.46 ± 0.17 | 0.047 |
| Progesterone receptor A isoform | - | |
| Progesterone receptor B isoform | 0.69 ± 0.39 | 0.380 |
| Estrogen receptor α | 3.68 ± 0.89 | 0.051 |
| Oxytocin receptor | 0.40 ± 0.13 | 0.022 |
| Connexin43 | 0.67 ± 0.04 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, F.; Veenboer, G.J.M.; Oudijk, M.A.; Kampman, M.A.M.; Heida, K.Y.; Lagendijk, L.J.M.; van der Post, J.A.M.; Jongejan, A.; Afink, G.B.; Ris-Stalpers, C. TBX2, a Novel Regulator of Labour. Medicina 2021, 57, 515. https://doi.org/10.3390/medicina57060515
Fernando F, Veenboer GJM, Oudijk MA, Kampman MAM, Heida KY, Lagendijk LJM, van der Post JAM, Jongejan A, Afink GB, Ris-Stalpers C. TBX2, a Novel Regulator of Labour. Medicina. 2021; 57(6):515. https://doi.org/10.3390/medicina57060515
Chicago/Turabian StyleFernando, Febilla, Geertruda J.M. Veenboer, Martijn A. Oudijk, Marlies A.M. Kampman, Karst Y. Heida, Louise J.M. Lagendijk, Joris A.M. van der Post, Aldo Jongejan, Gijs B. Afink, and Carrie Ris-Stalpers. 2021. "TBX2, a Novel Regulator of Labour" Medicina 57, no. 6: 515. https://doi.org/10.3390/medicina57060515
APA StyleFernando, F., Veenboer, G. J. M., Oudijk, M. A., Kampman, M. A. M., Heida, K. Y., Lagendijk, L. J. M., van der Post, J. A. M., Jongejan, A., Afink, G. B., & Ris-Stalpers, C. (2021). TBX2, a Novel Regulator of Labour. Medicina, 57(6), 515. https://doi.org/10.3390/medicina57060515






