Uncommon Metastasis of Ovarian Dysgerminoma: A Case Report and Review of the Literature
Abstract
:1. Introduction
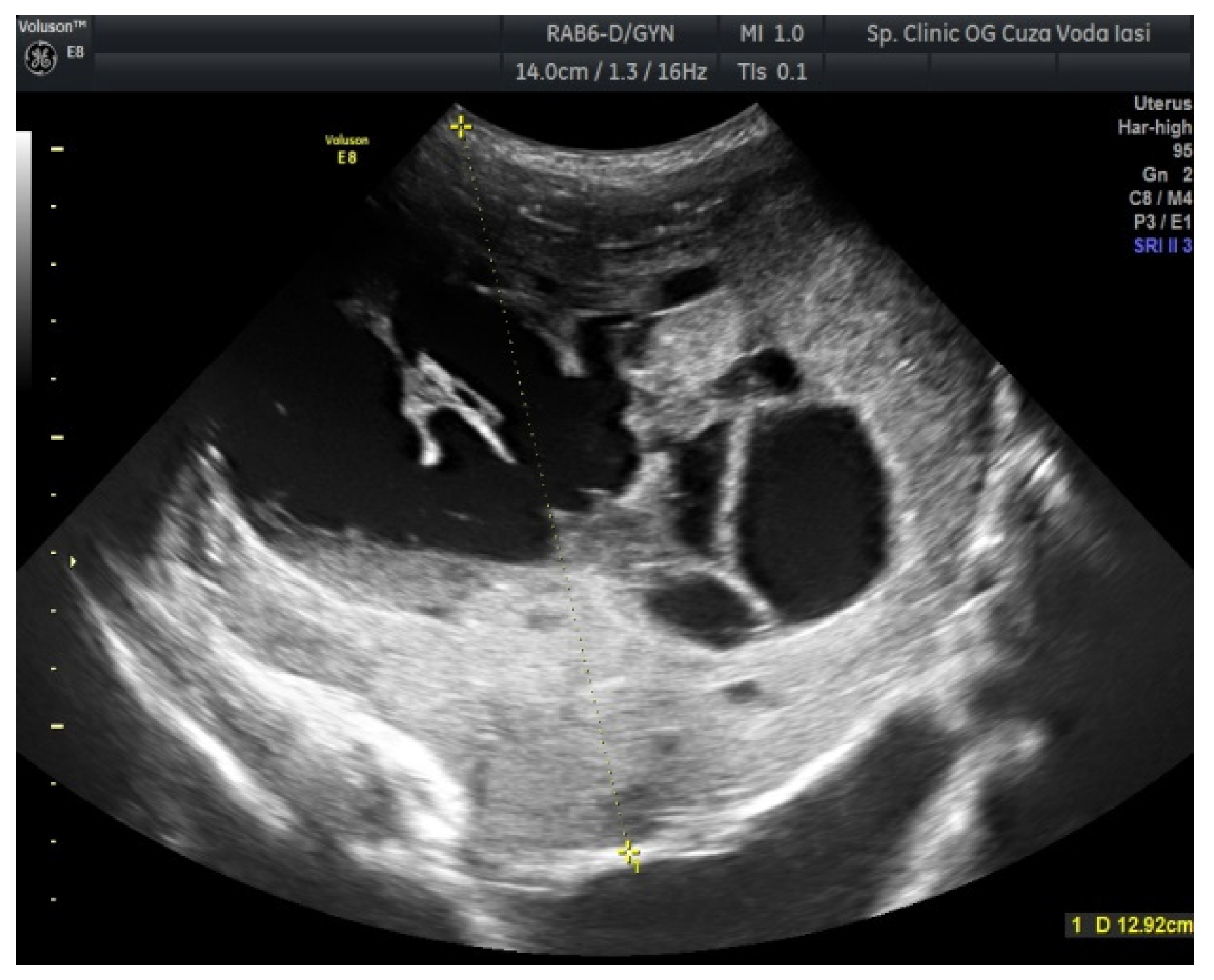
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, A.N.; Ellenson, L.H.; Gilks, C.B.; Kim, K.R.; Kong, C.S.; Lax, S.F.; Longacre, T.A.; Malpica, A.; McCluggage, W.G.; Oliva, E.; et al. Tumors of the Ovary. In Female Genital Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 4. [Google Scholar]
- Kaur, B. Pathology of malignant ovarian germ cell tumours. Diagn. Histopathol. 2020, 26, 289–297. [Google Scholar] [CrossRef]
- Zogbi, L.; Gonçalves, C.V.; Tejada, V.F.; Martins, D.; Karam, F.; Dos Santos, S.M.; Caldeira, R.R.; Senhorin, G.Z.; Lauz, S. Treatment of Bilateral Ovarian Dysgerminoma with 11-Year Follow-up: A Case Report. Ann. Med. Surg. 2018, 33, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.O.; Berwick, M.; Verschraegen, C.F.; Wiggins, C.; Lansing, L.; Muller, C.Y.; Qualls, C.R. Incidence and Survival Rates for Female Malignant Germ Cell Tumors. Obstet. Gynecol. 2006, 107, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Bidus, M.A.; Elkas, J.C.; Rose, G.S. Germ cell, stromal, and other ovarian tumors. In Clinical Gynecologic Oncology, 8th ed.; Di Saia, P.J., Creasman, W.T., Eds.; Elsevier/Saunders: Philadelphia, PA, USA, 2012; pp. 329–356. [Google Scholar]
- Gordon, A.; Lipton, D.; Woodruff, J.D. Dysgerminoma: A Review of 158 Cases from the Emil Novak Ovarian Tumor Registry. Obstet. Gynecol. 1981, 58, 497–504. [Google Scholar] [PubMed]
- Shaaban, A.M.; Rezvani, M.; Elsayes, K.M.; Baskin, H.; Mourad, A.; Foster, B.R.; Jarboe, E.A.; Menias, C.O. Ovarian Malignant Germ Cell Tumors: Cellular Classification and Clinical and Imaging Features. Radiographics 2014, 34, 777–801. [Google Scholar] [CrossRef] [PubMed]
- Susnerwala, S.S.; Pande, S.C.; Shrivastava, S.K.; Dinshaw, K.A. Dysgerminoma of the Ovary: Review of 27 Cases. J. Surg. Oncol. 1991, 46, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Valentin, L.; Bourne, T.H.; Collins, W.P.; Verrelst, H.; Vergote, I. International Ovarian Tumor Analysis (IOTA) Group Terms, Definitions and Measurements to Describe the Sonographic Features of Adnexal Tumors: A Consensus Opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef]
- Abramowicz, J.S.; Timmerman, D. Ovarian Mass-Differentiating Benign from Malignant: The Value of the International Ovarian Tumor Analysis Ultrasound Rules. Am. J. Obstet. Gynecol. 2017, 217, 652–660. [Google Scholar] [CrossRef]
- Husaini, H.A.L.; Soudy, H.; Darwish, A.E.D.; Ahmed, M.; Eltigani, A.; Mubarak, M.A.L.; Abu Sabaa, A.; Edesa, W.; AL-Tweigeri, T.; Al-Badawi, I.A. Pure Dysgerminoma of the Ovary: A Single Institutional Experience of 65 Patients. Med. Oncol. 2012, 29, 2944–2948. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Zhai, L.-R.; Huang, D.-W.; Jiang, Z.-D.; Yu, T.; Liu, S.-Y.; Cui, M.-H. Pregnancy with Giant Ovarian Dysgerminoma: A Case Report and Literature Review. Medicine 2020, 99, e21214. [Google Scholar] [CrossRef]
- Pectasides, D.; Pectasides, E.; Kassanos, D. Germ Cell Tumors of the Ovary. Cancer Treat. Rev. 2008, 34, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, K.; Ahmad, S.S.; Kumar, A.; Afshan, N. Dysgerminoma with Pregnancy and Viable Baby: A Case Report. Oman Med. J. 2011, 26, 198–200. [Google Scholar] [CrossRef]
- Buller, R.E.; Darrow, V.; Manetta, A.; Porto, M.; DiSaia, P.J. Conservative Surgical Management of Dysgerminoma Concomitant with Pregnancy. Obstet. Gynecol. 1992, 79, 887–890. [Google Scholar]
- Lazebnik, N.; Balog, A.; Bennett, S.; Redline, R.; Liu, J. Ovarian Dysgerminoma: A Challenging Clinical and Sonographic Diagnosis. J. Ultrasound Med. 2009, 28, 1409–1415. [Google Scholar] [CrossRef]
- Guerriero, S.; Testa, A.C.; Timmerman, D.; Van Holsbeke, C.; Ajossa, S.; Fischerova, D.; Franchi, D.; Leone, F.P.G.; Domali, E.; Alcazar, J.L.; et al. Imaging of Gynecological Disease (6): Clinical and Ultrasound Characteristics of Ovarian Dysgerminoma. Ultrasound Obstet. Gynecol. 2011, 37, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Tewari, K.S.; Waller, S.; Cheung, M.K.; Shin, J.Y.; Osann, K.; Kapp, D.S. The Influence of Conservative Surgical Practices for Malignant Ovarian Germ Cell Tumors. J. Surg. Oncol. 2008, 98, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Milewicz, T.; Mrozińska, S.; Szczepański, W.; Białas, M.; Kiałka, M.; Doroszewska, K.; Kabzińska-Turek, M.; Wojtyś, A.; Ludwin, A.; Chmura, Ł. Dysgerminoma and Gonadoblastoma in the Course of Swyer Syndrome. Pol. J. Pathol. 2016, 67, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Patterson, D.M.; Murugaesu, N.; Holden, L.; Seckl, M.J.; Rustin, G.J.S. A Review of the Close Surveillance Policy for Stage I Female Germ Cell Tumors of the Ovary and Other Sites. Int. J. Gynecol. Cancer 2008, 18, 43–50. [Google Scholar] [CrossRef]
- Nourani, M.; Manera, R.B. Pediatric Ovarian Dysgerminoma Presenting with Hypercalcemia and Chronic Constipation: A Case Report. J. Pediatr. Hematol. Oncol. 2013, 35, e272–e273. [Google Scholar] [CrossRef] [Green Version]
- Thomakos, N.; Diakosavvas, M.; Machairiotis, N.; Fasoulakis, Z.; Zarogoulidis, P.; Rodolakis, A. Rare Distant Metastatic Disease of Ovarian and Peritoneal Carcinomatosis: A Review of the Literature. Cancers 2019, 11, 1044. [Google Scholar] [CrossRef] [Green Version]
- Mangili, G.; Sigismondi, C.; Lorusso, D.; Cormio, G.; Scollo, P.; Viganò, R.; Gamucci, T.; Candiani, M.; Pignata, S. Is Surgical Restaging Indicated in Apparent Stage IA Pure Ovarian Dysgerminoma? The MITO Group Retrospective Experience. Gynecol. Oncol. 2011, 121, 280–284. [Google Scholar] [CrossRef]
- Beck, T.L.; Momose, H.; Dym, J.M.; Rao, V.Y.; Bohart, R.; Goldstein, B.H. Cranial and Intra-Axial Metastasis Originating from a Primary Ovarian Dysgerminoma. Gynecol. Oncol. Rep. 2019, 29, 55–57. [Google Scholar] [CrossRef]
- Takemori, M.; Ichimura, T.; Nishimura, R.; Hasegawa, K. Ovarian Dysgerminoma with Massive Metastases to Para-Aortic Lymph Nodes. Gynecol. Obstet. Investig. 2000, 49, 211–213. [Google Scholar] [CrossRef]
- Kasenda, B.; Harter, P.; Hirsch, T.; Ast, A.; Buhrmann, C.; Glaser, F.; Du Bois, A. Para-Aortic Lymph Node Metastasis in Malignant Dysgerminoma of the Ovary. Acta Obstet. Gynecol. Scand. 2009, 88, 1288–1290. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, M.; Bhuyan, G.; Mandal, A.K. Extragonadal dysgerminoma presenting as neck metastasis and masquerading as a thyroid swelling. Clin. Cancer Investig. J. 2016, 5, 43–45. [Google Scholar] [CrossRef]
- Maekawa, K.; Tokumitsu, T.; Minematsu, E.; Noguchi, H.; Nakamura, E.; Asada, Y.; Nakayama, T.; Sameshima, H.; Sato, Y. Cervical Lymph Node Metastasis of Ovarian Dysgerminoma: A Case Report with Fine Needle Aspiration Cytology. Diagn. Cytopathol. 2020, 48, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Mandeville, F.B.; Sahyoun, P.F.; Sutton, L.E. Dysgerminoma of the Ovary in a 4-Year-Old Girl with Metastases Clinically Simulating Wilms’ Tumor and Adrenal Neuroblastoma. J. Pediatr. 1949, 34, 70–75. [Google Scholar] [CrossRef]
- Kumar, L.; Bhargava, V.L.; Rao, R.C.; Rath, G.K.; Kataria, S.P. Bone Metastasis in Ovarian Cancer. Asia Ocean. J. Obstet. Gynaecol. 1992, 18, 309–313. [Google Scholar] [CrossRef]
- Rabban, J.T.; Zaloudek, C.J. A Practical Approach to Immunohistochemical Diagnosis of Ovarian Germ Cell Tumours and Sex Cord-Stromal Tumours. Histopathology 2013, 62, 71–88. [Google Scholar] [CrossRef]
- Adekunle, O.O.; Zayyan, M.; Kolawole, A.O. Case report: A rare case of dysgerminoma presenting with skin and breast metastasis. Case Rep. Clin. Med. 2013, 2, 170–172. [Google Scholar] [CrossRef] [Green Version]
- Afridi, M.A.; Vongtama, V.; Tsukada, Y.; Piver, M.S. Dysgerminoma of the Ovary: Radiation Therapy for Recurrence and Metastases. Am. J. Obstet. Gynecol. 1976, 126, 190–194. [Google Scholar] [CrossRef]
- Jolles, C.J.; Karayianis, S.; Smotkin, D.; DeLia, J.E. Advanced Ovarian Dysgerminoma with Cure of Tumor Persistent in Meninges. Gynecol. Oncol. 1989, 33, 389–391. [Google Scholar] [CrossRef]
- Kerr, V.E.; Cadman, E. Pulmonary Metastases in Ovarian Cancer. Analysis of 357 Patients. Cancer 1985, 56, 1209–1213. [Google Scholar] [CrossRef]
- Cantisani, V.; Mortele, K.J.; Kalantari, B.N.; Glickman, J.N.; Tempany, C.; Silverman, S.G. Vaginal Metastasis from Uterine Leiomyosarcoma. Magnetic Resonance Imaging Features with Pathological Correlation. J. Comput. Assist. Tomogr. 2003, 27, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Binda, M.C.; Cejas, C.; Eli, A.; Bozo, C.; Pineda, D. Leiomiosarcoma uterino con metástasis vaginal A propósito de un caso. Rev. Argent. Radiol. 2006, 7, 207–212. [Google Scholar]
- Sandberg, A.A. Updates on the Cytogenetics and Molecular Genetics of Bone and Soft Tissue Tumors: Leiomyosarcoma. Cancer Genet. Cytogenet. 2005, 161, 1–19. [Google Scholar] [CrossRef]
- Benbrahim, Z.; Chouaib, A.; Mazeron, R.; Leger-Ravet, M.B.; Lefort, C.; Lhommé, C.; El Mesbahi, O.; Escudier, B. Gynecologic Bleeding Revealing Vaginal Metastasis of Renal Cell Carcinoma. Pan Afr. Med. J. 2013, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.K.; Yu, C.S.; Lee, S.-W.; Kim, J.; Song, I.; Lee, J.L.; Kim, C.W.; Yoon, Y.S.; Park, I.J.; Lim, S.-B.; et al. Isolated Vaginal Metastasis from Stage I Colon Cancer: A Case Report. World J. Clin. Cases 2020, 8, 527–534. [Google Scholar] [CrossRef]
- Weitzner, S.; Dressner, S.A. Vaginal Metastasis from Adenocarcinoma of Pancreas. Am. Surg. 1974, 40, 256–258. [Google Scholar]
- Kumar, S.; Shah, J.P.; Bryant, C.S.; Imudia, A.N.; Cote, M.L.; Ali-Fehmi, R.; Malone, J.M.; Morris, R.T. The Prevalence and Prognostic Impact of Lymph Node Metastasis in Malignant Germ Cell Tumors of the Ovary. Gynecol. Oncol. 2008, 110, 125–132. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, X.; Jin, H.; Lu, X. Swyer Syndrome, 46,XY Gonadal Dysgenesis, a Sex Reversal Disorder with Dysgerminoma: A Case Report and Literature Review. Clin. Exp. Obstet. Gynecol. 2011, 38, 414–418. [Google Scholar] [PubMed]
- Behtash, N.; Zarchi, M.K. Dysgerminoma in Three Patients with Swyer Syndrome. World J. Surg. Oncol. 2007, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Howitt, B.E.; Lee, K.R.; Muto, M.G.; Nucci, M.R.; Crum, C.P. The Pathology of Pelvic-Ovarian Epithelial (Epithelial-Stromal) Tumors. In Diagnostic Gynecologic and Obstetric Pathology, 3rd ed.; Crum, C.P., Nucci, M.R., Howitt, B.E., Granter, S.R., Parast, M.M., Boyd, T.K., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 865–948. ISBN 978-0-323-44732-4. [Google Scholar]
- Piura, B. Hypercalcemia in malignancies of the female genital tract. Harefuah 2008, 147, 229–234, 277. [Google Scholar] [PubMed]
- Hosseini, B.; Leibl, M.; Stoffman, J.; Morris, A. Two Cases of Hypercalcemia in Pediatric Ovarian Dysgerminoma. J. Obstet. Gynaecol. Can. 2019, 41, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Zaloudek, C.J.; Tavassoli, F.A.; Norris, H.J. Dysgerminoma with Syncytiotrophoblastic Giant Cells. A Histologically and Clinically Distinctive Subtype of Dysgerminoma. Am. J. Surg. Pathol. 1981, 5, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.M.; Oliva, E. Germ cell tumors of the ovary. In Gynecologic Pathology; Nucci, M.R., Oliva, E., Eds.; Elsevier Churchill Livingstone: London, UK, 2009; pp. 501–507. [Google Scholar]
- Song, E.-S.; Lee, J.-P.; Han, J.-H.; Kim, H.-Y.; Mun, S.-H.; Ryu, H.-S.; Chang, K.-H. Dysgerminoma of the Ovary with Precocious Puberty: A Case Report. Gynecol. Endocrinol. 2007, 23, 34–37. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tîrnovanu, M.C.; Florea, I.D.; Tănase, A.; Toma, B.F.; Cojocaru, E.; Ungureanu, C.; Lozneanu, L. Uncommon Metastasis of Ovarian Dysgerminoma: A Case Report and Review of the Literature. Medicina 2021, 57, 534. https://doi.org/10.3390/medicina57060534
Tîrnovanu MC, Florea ID, Tănase A, Toma BF, Cojocaru E, Ungureanu C, Lozneanu L. Uncommon Metastasis of Ovarian Dysgerminoma: A Case Report and Review of the Literature. Medicina. 2021; 57(6):534. https://doi.org/10.3390/medicina57060534
Chicago/Turabian StyleTîrnovanu, Mihaela Camelia, Irina Daniela Florea, Adina Tănase, Bogdan Florin Toma, Elena Cojocaru, Carmen Ungureanu, and Ludmila Lozneanu. 2021. "Uncommon Metastasis of Ovarian Dysgerminoma: A Case Report and Review of the Literature" Medicina 57, no. 6: 534. https://doi.org/10.3390/medicina57060534
APA StyleTîrnovanu, M. C., Florea, I. D., Tănase, A., Toma, B. F., Cojocaru, E., Ungureanu, C., & Lozneanu, L. (2021). Uncommon Metastasis of Ovarian Dysgerminoma: A Case Report and Review of the Literature. Medicina, 57(6), 534. https://doi.org/10.3390/medicina57060534





