Are Three Weeks of Oral Anticoagulation Sufficient for Safe Cardioversion in Atrial Fibrillation?
Abstract
1. Introduction
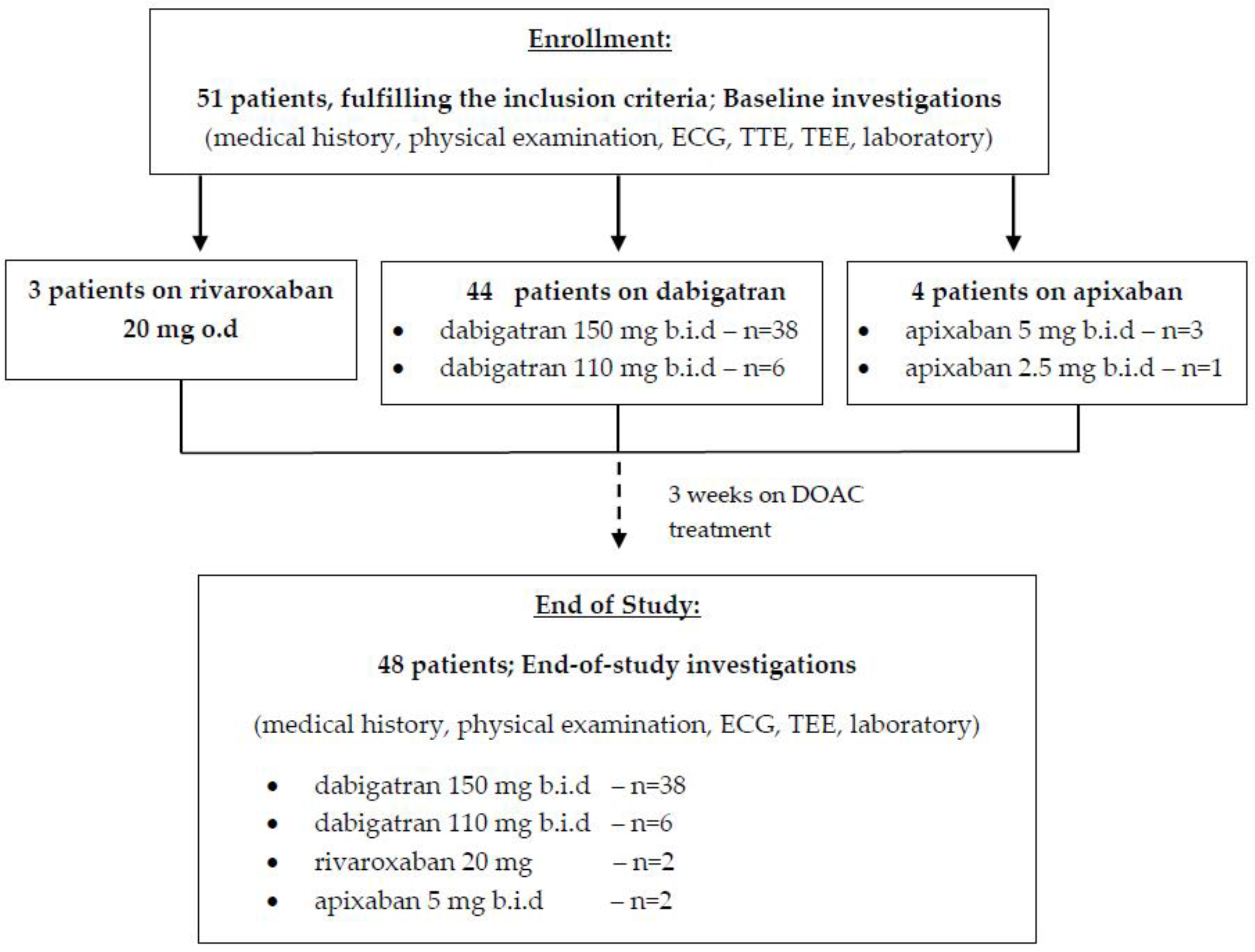
2. Materials and Methods
2.1. Patients’ Selection
2.2. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of nonvitamin K antagonist oral anticoagulants in patients with atrial fibrillation: Executive summary. Europace 2018, 20, 1231–1242. [Google Scholar] [CrossRef]
- Warden, B.A.; MacKay, J.; Jafari, M.; Willman, A.; Stecker, E.C. Use of Direct Oral Anticoagulants among Patients Undergoing Cardioversion: The Importance of Timing before Cardioversion. J. Am. Heart Assoc. 2018, 7, e010854. [Google Scholar] [CrossRef]
- Di Minno, M.N.; Ambrosino, P.; Dello Russo, A.; Casella, M.; Tremoli, E.; Tondo, C. Prevalence of left atrial thrombus in patients with non-valvular atrial fibrillation. A systematic review and meta-analysis of the literature. Thromb. Haemost. 2016, 115, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Farkowski, M.M.; Jubele, K.; Marín, F.; Gandjbakhch, E.; Ptaszynski, P.; Merino, J.L.; Lenarczyk, R.; Potpara, T.S. Diagnosis and management of left atrial appendage thrombus in patients with atrial fibrillation undergoing cardioversion or percutaneous left atrial procedures: Results of the European Heart Rhythm Association survey. Europace 2020, 22, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Reers, S.; Karanatsios, G.; Borowski, M.; Kellner, M.; Reppel, M.; Waltenberger, J. Frequency of atrial thrombus formation in patients with atrial fibrillation under treatment with non-vitamin K oral anticoagulants in comparison to vitamin K antagonists: A systematic review and meta-analysis. Eur. J. Med. Res. 2018, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Palomäki, A.; Mustonen, P.; Hartikainen, J.E.; Nuotio, I.; Kiviniemi, T.; Ylitalo, A.; Hartikainen, P.; Lehtola, H.; Luite, R.; Airaksinen, K.E. Strokes after cardioversion of atrial fibrillation—The FibStroke study. Int. J. Cardiol. 2016, 203, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.K.; Juhlin, T.; Engdahl, J.; Lind, S.; Hagwall, K.; Rorsman, C.; Fodor, E.; Alenholt, A.; Paul Nordin, A.; Rosenqvist, M.; et al. Is one month treatment with dabigatran before cardioversion of atrial fibrillation sufficient to prevent thromboembolism? Europace 2015, 17, 1514–1517. [Google Scholar] [CrossRef]
- Fukuda, S.; Watanabe, H.; Shimada, K.; Aikawa, M.; Kono, Y.; Jissho, S.; Taguchi, H.; Umemura, J.; Yoshiyama, M.; Shiota, T.; et al. Left atrial thrombus and prognosis after anticoagulation therapy in patients with atrial fibrillation. J. Cardiol. 2011, 58, 266–277. [Google Scholar] [CrossRef][Green Version]
- Black, I.W.; Fatkin, D.; Sagar, K.B.; Khandheria, B.K.; Leung, D.Y.; Galloway, J.M.; Feneley, M.P.; Walsh, W.F.; Grimm, R.A.; Stollberger, C.; et al. Exclusion of atrial thrombus by transesophageal echocardiography does not preclude embolism after cardioversion of atrial fibrillation. A multicenter study. Circulation 1994, 89, 2509–2513. [Google Scholar] [CrossRef]
- Mitamura, H.; Nagai, T.; Watanabe, A.; Takatsuki, S.; Okumura, K. Left atrial thrombus formation and resolution during dabigatran therapy: A Japanese Heart Rhythm Society report. J. Arrhythm. 2015, 31, 226–231. [Google Scholar] [CrossRef]
- Shen, X.; Li, H.; Rovang, K.; Hee, T.; Holmberg, M.J.; Mooss, A.N.; Mohiuddin, S.M. Prevalence of intra-atrial thrombi in atrial fibrillation patients with subtherapeutic international normalized ratios while taking conventional anticoagulation. Am. J. Cardiol. 2002, 90, 660–666. [Google Scholar] [CrossRef]
- Miwa, Y.; Minamishima, T.; Sato, T.; Sakata, K.; Yoshino, H.; Soejima, K. Resolution of a warfarin and dabigatran-resistant left atrial appendage thrombus with apixaban. J. Arrhythm. 2016, 32, 233–235. [Google Scholar] [CrossRef]
- Piotrowski, R.; Zaborska, B.; Baran, J.; Sikora-Frąc, M.; Kułakowski, P. Rivaroxaban twice daily for lysis of left atrial appendage thrombus: A potential new therapeutic option. Pol. Arch. Med. Wewn. 2016, 126, 430–431. [Google Scholar] [CrossRef][Green Version]
- Okada, T.; Takaekou, Y.; Idei, N.; Ohashi, N.; Kaseda, S. Resolution of left atrial appendage thrombus with apixaban in a patient with heart failure. Intern. Med. 2017, 56, 2891–2894. [Google Scholar] [CrossRef]
- Watanabe, T.; Shinoda, Y.; Ikeoka, K.; Minamisaka, T.; Fukuoka, H.; Inui, H.; Hoshida, S. Dabigatran therapy resulting in the resolution of rivaroxaban-resistant left atrial appendage thrombi in patients with atrial fibrillation. Intern. Med. 2017, 56, 1977–1980. [Google Scholar] [CrossRef]
- Coleman, C.M.; Khalaf, S.; Mould, S.; Wazni, O.; Kanj, M.; Saliba, W.; Cantillon, D. Novel oral anticoagulants for DC cardioversion procedures: Utilization and clinical outcomes compared with warfarin. Pacing Clin. Electrophysiol. 2015, 38, 731. [Google Scholar] [CrossRef]
- Benamer, S.; Lusty, D.; Everington, T. Dabigatran versus warfarin for direct current cardioversion in atrial fibrillation. Cardiol. Ther. 2016, 5, 215–221. [Google Scholar] [CrossRef][Green Version]
- Yadlapati, A.; Groh, C.; Passman, R. Safety of short-term use of dabigatran or rivaroxaban for direct-current cardioversion in patients with atrial fibrillation and atrial flutter. Am. J. Cardiol. 2014, 113, 1362–1363. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Lip, G. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef]
- Fukuda, S.; Shimada, K.; Kawasaki, T.; Taguchi, H.; Maeda, K.; Fujimoto, H.; Inanami, H.; Yoshida, K.; Jissho, S.; Yoshiyama, M.; et al. Transnasal transesophageal echocardiography in the detection of left atrial thrombus. J. Cardiol. 2009, 54, 425–431. [Google Scholar] [CrossRef]
- Pollick, C.; Taylor, D. Assessment of left atrial appendage function by transesophageal echocardiography. Implications for the development of thrombus. Circulation 1991, 84, 223–231. [Google Scholar] [CrossRef]
- Bansal, M.; Kasliwal, R.R. Echocardiography for left atrial appendage structure and function. Indian Heart J. 2012, 64, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Hammerstingl, C.; Marin, F.; Cappato, R.; Meng, I.L.; Kirsch, B.; van Eickels, M.; Cohen, A.; X-TRA Study and CLOT-AF Registry Investigators. Left atrial thrombus resolution in atrial fibrillation or flutter: Results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am. Heart J. 2016, 178, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, M.D.; Pollack, C.V.J.; Halperin, J.L.; England, R.D.; VanPelt Nguyen, S.; Spahr, J.; Sudworth, M.; Cater, N.B.; Breazna, A.; Oldgren, J.; et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: The EMANATE trial. Eur. Heart J. 2018, 39, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.J.; Silverman, D.I.; Douglas, P.S.; Manning, W.J. Cardioversion of nonrheumatic atrial fibrillation. Reduced thromboembolic complications with 4 weeks of precardioversion anticoagulation are related to atrial thrombus resolution. Circulation 1995, 92, 160–163. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Total | Males | Females | p |
|---|---|---|---|---|
| n = 51 | n = 26 | n = 25 | ||
| Age (years) | 67.3 ± 7.4 | 66.4 ± 5.7 | 68.6 ± 6.1 | 0.126 |
| Body mass index | 28.8 ± 3.4 | 28.7 ± 5.2 | 29.5 ± 4.9 | 0.472 |
| HTN, n (%) | 46 (90.2%) | 22 (84.6) | 24 (96.0) | 0.160 |
| Type 2 DM, n (%) | 10 (19.6) | 4 (15.4) | 6 (24.0) | 0.048 |
| Vascular disease *,#, n (%) | 6 (11.8) | 3 (11.5) | 3 (12.0) | 0.742 |
| VHD &, n (%) | 2 (3.9%) | 1 (3.9) | 1 (4.0) | 0.778 |
| CHA2DS2-VASc (points) | 3.92 ± 1.12 | 3.86 ± 1.04 | 4.17 ± 0.96 | 0.010 |
| HAS-BLED (points) | 2.33 ± 0.88 | 2.47 ± 1.07 | 2.26 ± 0.66 | 0.622 |
| CrCl (ml/min.) | 57.4 ± 7.02 | 54.1 ± 6.23 | 59.7 ± 8.16 | 0.276 |
| Indexed LA volume (mL/m2) | 31.8 ± 6.44 | 30.2 ± 5.04 | 32.6 ± 7.38 | 0.213 |
| LVEF (%) | 64.8 ± 6.46 | 66.2 ± 7.22 | 62.9 ± 8.17 | 0.381 |
| dabigatran 150 b.i.d | 38 (74.5) | 18 (69.2) | 20 (80.0) | 0.340 |
| dabigatran 110 b.i.d | 6 (11.8) | 4 (15.4) | 2 (8.0) | 0.220 |
| apixaban 5 mg b.i.d | 3 (5.9) | 1 (3.8) | 2 (8.0) | 0.338 |
| apixaban 2.5 mg b.i.d | 1 (2.0) | 0 (0) | 1 (4) | 0.191 |
| rivaroxaban 20 o.d | 3 (5.9) | 3 (11.5) | 0 (0) | 0.083 |
| Parameters | SEC/LAT (−) n = 15 | SEC/LAT (+) n = 36 | p |
|---|---|---|---|
| Indexed LA volume (mL/m2) | 27.8 ± 10.2 | 35.4 ± 8.6 | <0.001 |
| LAA filling velocity (cm/s) | 43.4 ± 7.2 | 39.5 ± 10.6 | 0.218 |
| LAA emptying velocity (cm/s) | 29.4 ± 11.3 | 18.7 ± 14.4 | 0.033 |
| Number of LAA lobes, n (%) | |||
| 1 | 10 (66.7) | 5 (13.9) | <0.001 |
| 2 | 3 (20.0) | 13 (36.1) | 0.045 |
| 3 | 2 (13.3) | 12 (33.3) | 0.012 |
| >3 | 0 (0) | 6 (16.7) | <0.001 |
| Parameters | SEC/LAA Thrombus * (−) n = 29 | SEC/LAA Thrombus (+) n = 19 | p |
|---|---|---|---|
| Indexed LA volume (mL/m2) | 32.3 ± 6.2 | 37.4 ± 9.5 | 0.020 |
| LAA filling velocity (cm/s) | 40.4 ± 8.1 | 33.6 ± 9.5 | 0.014 |
| LAA emptying velocity (cm/s) | 24.8 ± 7.1 | 15.6 ± 9.2 | <0.001 |
| Number of LAA lobes, n (%) | |||
| 1 | 13 (44.8) | 0 (0) | <0.001 |
| 2 | 12 (41.4) | 4 (21.1) | <0.001 |
| 3 | 4 (13.8) | 9 (47.4) | 0.040 |
| >3 | 0 (0) | 6 (31.6) | <0.001 |
| Variable | OR | 95% Confidence Interval for OR | p | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Indexed LA volume ≥34 mL/m2 vs. 34 mL/m2 | 1.37 | 1.28 | 1.88 | 0.036 |
| LAA emptying velocity <20 cm/s vs. ≥20 cm/s | 2.82 | 1.94 | 4.38 | <0.001 |
| Number of LAA lobes >2 vs. ≤2 | 1.84 | 1.42 | 2.93 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naydenov, S.; Runev, N.; Manov, E. Are Three Weeks of Oral Anticoagulation Sufficient for Safe Cardioversion in Atrial Fibrillation? Medicina 2021, 57, 554. https://doi.org/10.3390/medicina57060554
Naydenov S, Runev N, Manov E. Are Three Weeks of Oral Anticoagulation Sufficient for Safe Cardioversion in Atrial Fibrillation? Medicina. 2021; 57(6):554. https://doi.org/10.3390/medicina57060554
Chicago/Turabian StyleNaydenov, Stefan, Nikolay Runev, and Emil Manov. 2021. "Are Three Weeks of Oral Anticoagulation Sufficient for Safe Cardioversion in Atrial Fibrillation?" Medicina 57, no. 6: 554. https://doi.org/10.3390/medicina57060554
APA StyleNaydenov, S., Runev, N., & Manov, E. (2021). Are Three Weeks of Oral Anticoagulation Sufficient for Safe Cardioversion in Atrial Fibrillation? Medicina, 57(6), 554. https://doi.org/10.3390/medicina57060554






