Atrial and Ventricular Structural and Functional Alterations in Obese Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics, Consent and Permissions
2.3. Conventional Echocardiography
2.4. Doppler Imaging
2.5. 2D Speckle Tracking Imaging
2.6. Reproducibility
2.7. Statistical Analysis
3. Results
3.1. Population
3.2. Conventional and Doppler Echocardiographic Characteristics
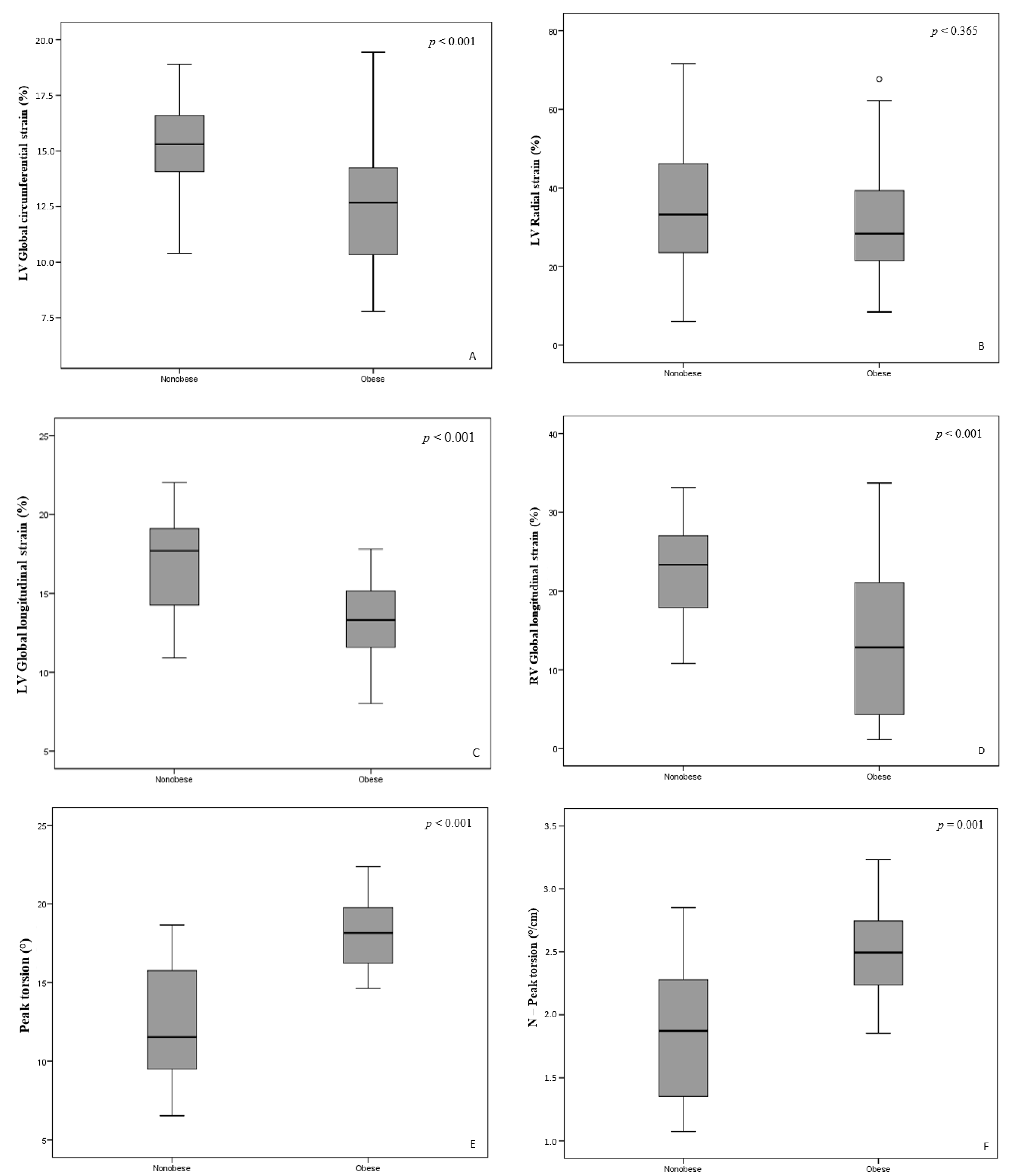
3.3. Ventricular 2D Speckle Tracking Analysis
3.4. Atrial Volumetric Data and 2D Strain Analysis
3.5. Multivariate Stepwise Linear Regression Analysis
3.6. Reproducibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nittari, G.; Scuri, S.; Petrelli, F.; Pirillo, I.; di Luca, N.M.; Grappasonni, I. Fighting obesity in children from European World Health Organisation member states. Epidemiological data, medical-social aspects, and prevention programs. Clin. Ter. 2019, 170, e223–e230. [Google Scholar]
- Barlow, S.E. Expert committee. Recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120, 164–192. [Google Scholar] [CrossRef] [Green Version]
- Mangner, N.; Scheuermann, K.; Winzer, E.; Wagner, I.; Hoellriegel, R.; Sandri, M.; Zimmer, M.; Mende, M.; Linke, A.; Kiess, W.; et al. Childhood obesity. Impact on cardiac geometry and function. JACC Cardiovasc. Imaging 2014, 7, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, M.K.; Chiuve, S.E.; Rimm, E.B.; Dethlefsen, C.; Tjonneland, A.; Joensen, A.M.; Overvad, K. Obesity, Behavioral Lifestyle Factors, and Risk of Acute Coronary Events. Circulation 2008, 117, 3062–3069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltijeral, A.; Isla, L.P.; Pérez-Rodríguez, O.; Rueda, S.; Fernandez-Golfin, C.; Almeria, C. Early myocardial deformation changes associated to isolated obesity: A study based on 3D-wall motion tracking analysis. Obesity 2011, 9, 2268–2273. [Google Scholar] [CrossRef]
- Abel, A.D.; Litwin, S.E.; Sweeney, G. Cardiac remodelling in obesity. Physiol. Rev. 2008, 88, 1399–1404. [Google Scholar] [CrossRef]
- Cole, T.; Bellizzi, M.; Flegal, K.; Dietz, W. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachkamf, F.A.; Foster, E.; Golstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quintification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoit, B.D. Left atrial size and function. JACC 2014, 63, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.J.; Mackle, A.S.; Mitsnefes, M.; Ali, H.; Mamber, S.; Colan, S.D. A novel method of expressing left ventricular mass relative to body size in children. Circulation 2008, 117, 2769–2775. [Google Scholar] [CrossRef] [Green Version]
- Kampmann, C.; Wiethoff, C.M.; Wenzel, A.; Stolz, G.; Betacor, M.; Wippermann, C.F.; Huth, R.G.; Habermehl, P.; Knuf, M.; Emschermann, T.; et al. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and childen in Central Europe. Heart 2000, 83, 667–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, R.M.; Demirkol, S.; Buakhamsri, A.; Greenberg, N.; Popovic, Z.B.; Thomas, J.D.; Klein, A.L. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J. Am. Soc. Echocardiogr. 2010, 23, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Otsuji, Y.; Lang, R.M. Evaluation of left ventricular twist and torsion parameters. Curr. Cardiol. Rep. 2009, 11, 225–230. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickhank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef] [Green Version]
- Alpert, M.A. Obesity cardiomyopathy: Pathophysiology and evolution of the clinical syndrome. Am. J. Med. Sci. 2001, 321, 225–236. [Google Scholar] [CrossRef]
- Van Putte-Katier, N.; Rooman, R.P.; Haas, L.; Verhulst, S.L.; Desager, K.N.; Ramet, J.; Suys, B.E. Early cardiac abnormalities in obese children: Importance of obesity per se versus associated cardiovascular risk factors. Pediatr. Res. 2008, 64, 205–209. [Google Scholar] [CrossRef]
- Peterson, L.R.; Herrero, P.; Schechtman, K.B.; Racette, S.B.; Waggoner, A.D.; Kisrieva-Ware, Z.; Dence, C.; Klein, S.; Marsala, J.; Meyer, T.; et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004, 109, 2191–2196. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.Y.; Byrne, N.M.; O’ Moore-Sullivan, T.; Hills, A.P.; Prins, J.B.; Marwick, T.H. Effect of weight loss due to lifestyle intervention on subclinical cardiovascular dysfunction in obesity (body mass index > 30kg/m2). Am. J. Cardiol. 2006, 98, 1593–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cote, A.T.; Harris, K.C.; Panagiotopoulos, C.; Sandor, G.G.S.; Devlin, A.M. Childhood obesity and cardiovascular dysfunction. J. Am. Coll. Cardiol. 2013, 62, 1309–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labombarda, F.; Zangl, E.; Dugue, A.E.; Bougle, D.; Pellissier, A.; Ribault, V.; Maragnes, P.; Milliez, P.; Saloux, E. Alterations of left ventricular myocardial strain in obese children. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Cantinotti, M.; Kutty, S.; Giordano, R.; Assanta, N.; Murzi, B.; Crocetti, M.; Marotta, M.; Iervasi, G. Review and status report of pediatric left systolic strain and strane rate nomograms. Heart Fail Rev. 2015, 20, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Marcus, K.A.; Mavinkurve-Groothuis, A.M.; Barends, M.; van Dijk, A.; Feuth, T.; de Korte, C.; Kapusta, L. References values for myocardial two-dimensional strain echocardiographyin a healthy pediatric and young adult cohort. J. Am. Soc. Echocardiogr. 2011, 24, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H.; Leano, R.L.; Brown, J.; Sun, J.P.; Hoffmann, R.; Lysyansky, P.; Becker, M.; Thomas, J.D. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: Definition of normal range. JACC Cardiovasc. Imaging 2009, 2, 80–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Salvo, G.; Del Giudice, E.M.; Natale, F.; Verrengia, M.; Rea, A.; Fratta, F.; Castaldi, B.; D’Andrea, A.; Miele, T.; Coppola, F.; et al. Abnormal myocardial deformation properties in obese, non-hypertensive children: An ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur. Heart J. 2006, 27, 2689–2695. [Google Scholar] [CrossRef] [Green Version]
- Biswas, M.; Sudhakar, S.; Nanda, N.C.; Buckberg, G.; Pradhan, M.; Roomi, A.U.; Gorissen, W.; Houle, H. Two- and three-dimensional speckle tracking echocardiography: Clinical applications and future directions. Echocardiography 2013, 30, 88–105. [Google Scholar] [CrossRef]
- Di Bello, V.; Santini, F.; Di Cori, A.; Pucci, A.; Palagi, C.; Donne, M.G.D.; Fierabracci, P.; Marsili, A.; Talini, E.; Giannetti, M.; et al. Obesity Cardiomyopathy: Is It a Reality? An Ultrasonic Tissue Characterization Study. J. Am. Soc. Echocardiogr. 2006, 19, 1063–1071. [Google Scholar] [CrossRef]
- Wong, C.Y.; O’Moore-Sullivan, T.; Leano, R.; Byrne, N.; Beller, E.; Marwick, T.H. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 2004, 110, 3081–3087. [Google Scholar] [CrossRef] [Green Version]
- Lorch, S.M.; Sharkey, A. Myocardial velocity, strain, and strain rate abnormalities in healthy obese children. J. Cardiometab. Syndr. 2007, 2, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Dervan, J.P.; Ilercil, A.; Kane, P.B.; Anagnostopoulos, C. Fatty infiltration: Another restrictive cardiomyopathic pattern. Cathet. Cardiovasc. Diagn. 1991, 22, 184–189. [Google Scholar] [CrossRef]
- Green, D.; Kalra, P.R.; Kalra, P.A. Echocardiographic abnormalities in dialysis pateints with normal ejection fraction. Nephrol. Dial. Transplant. 2012, 27, 4256–4259. [Google Scholar] [CrossRef] [Green Version]
- Wakami, K.; Ohte, N.; Asada, K.; Fukuta, H.; Goto, T.; Mukai, S.; Narita, H.; Kimura, G. Correlation between Left Ventricular End-diastolic Pressure and Peak Left Atrial Wall Strain during Left Ventricular Systole. J. Am. Soc. Echocardiogr. 2009, 22, 847–851. [Google Scholar] [CrossRef] [PubMed]
| Variable | Obese n = 35 | Nonobese n = 37 | p Value |
|---|---|---|---|
| Gender, boys/girls, n | 20/15 | 18/19 | 0.490 |
| Age, years | 13.51 (2.1) | 13.43 (1.7) | 0.945 |
| Heart rate, bpm | 86.43 (10.73) | 78.12 (12.28) | 0.003 |
| Systolic blood pressure, mmHg | 126.14 (13.76) | 113.22 (7.22) | <0.001 |
| Diastolic blood pressure, mmHg | 78.74 (8.59) | 70.70 (6.71) | <0.001 |
| Height, median (IQR), cm | 168 (138–178) | 172.61 (140–191) | 0.015 |
| Weight, kg | 91.06 (18.65) | 56.11 (9.91) | <0.001 |
| Body mass index, kg/m2 | 33.12 (5.15) | 19.08 (3.08) | <0.001 |
| Body mass index, z-score | 0.88 (0.63) | −0.83 (0.38) | <0.001 |
| Body surface area, m2 | 2.01 (0.26) | 1.64 (0.17) | <0.001 |
| Total cholesterol, mmol/L | 4.46 (1.03) | 3.34 (0.71) | <0.001 |
| LDLP, mmol/L | 2.74 (1.1) | 2.14 (0.58) | 0.060 |
| HDLP, median (IQR), mmol/L | 1.09 (0.72–3.21) | 1.14 (1–1.65) | 0.045 |
| Triglycerides, median (IQR), mmol/L | 1.26 (0.50–3.16) | 1.24 (0.85–1.84) | 0.502 |
| Parameters | Obese n = 35 | Nonobese n = 37 | p Value |
|---|---|---|---|
| LV end-diastolic volume, mL | 100.54 (21.42) | 85.05 (22.30) | 0.04 |
| LV end-systolic volume, mL | 24.94 (8.23) | 24.68 (12.45) | 0.915 |
| Interventricular septum, mm | 8.71 (1.34) | 8.24 (1.21) | 0.158 |
| LV posterior wall, mm | 8.83 (1.29) | 7.70 (1.31) | <0.001 |
| LV ejection fraction, % | 69.29 (6.98) | 67.32 (8.97) | 0.243 |
| LV fractional shortening, % | 43.71 (4.71) | 45.35 (5.66) | 0.188 |
| LV mass, g | 115.81 (32.22) | 80.12 (18.31) | <0.001 |
| LV mass/height 2.7, g/m 2.7 | 30.35 (6.12) | 19.0 (3.93) | <0.001 |
| LV end-diastolic volume z-score | 0.13 (0.98) | −0.12 (1.02) | 0.291 |
| LV end-systolic volume z-score | 0.01 (0.78) | −0.01 (1.18) | 0.915 |
| Interventricular septum z-score | 0.18 (1.04) | −0.18 (0.94) | 0.122 |
| LV posterior wall z-score | 0.41 (0.92) | −0.39 (0.93) | <0.001 |
| LV ejection fraction z-score | 0.12 (0.86) | −0.12 (1.11) | 0.306 |
| LV mass z-score | 0.15 (0.94) | −0.13 (0.97) | 0.012 |
| LV early filling velocity, m/s | 0.88 (0.18) | 0.86 (0.09) | 0.609 |
| LV late filling velocity, m/s | 0.59 (0.15) | 0.55 (0.09) | 0.284 |
| E/Am | 1.52 (0.40) | 1.59 (0.38) | 0.459 |
| LV early filling deceleration time, median (IQR), m/s ms | 147 (24–217) | 152 (120–230) | 0.238 |
| Sm, median (IQR), cm/s | 0.092 (0.04–0.4) | 0.11 (0.06–0.19) | 0.024 |
| Em, median (IQR), cm/s | 0.16 (0.07–0.24) | 0.19 (0.08–0.24) | 0.013 |
| Am, median (IQR), cm/s | 0.06 (0.04–0.11) | 0.16 (0.06–0.16) | <0.001 |
| E/Em, median (IQR) | 7 (4.1–16.4) | 7.5 (3–14.6) | 0.451 |
| RV basal diameter, mm | 29.1 (3.20) | 28.3 (2.32) | 0.843 |
| RV early filling velocity, m/s | 1.08 (14.65) | 1.06 (20.24) | 0.172 |
| RV late filling velocity, m/s | 0.61 (0.12) | 0.58 (0.11) | 0.295 |
| Tricuspid annulus motion amplitude, mm | 20.44 (0.42) | 18.53 (0.81) | 0.752 |
| St, median (IQR), cm/s | 0.76 (0.041–0.9) | 0.09 (0.05–0.15) | 0.004 |
| Et, median (IQR), cm/s | 0.13 (0.09–0.22) | 0.13 (0.06–0.19) | 0.544 |
| At, median (IQR), cm/s | 0.06 (0.04–0.11) | 0.08 (0.06–0.16) | 0.004 |
| Parameter | Obese n = 35 | Nonobese n = 37 | p Value |
|---|---|---|---|
| LA peak negative strain, % | −2.05 (2.33) | −4.87 (2.97) | <0.001 |
| LA peak positive strain, % | 29.98 (10.36) | 27.88 (12.62) | 0.297 |
| Total LA strain, % | 31.88 (12.49) | 28.97 (15.18) | 0.126 |
| LAVi max, mL/m2 | 29.55 (17.01) | 24.66 (10.75) | <0.001 |
| LAVi min, mL/m2 | 9.94 (3.76) | 6.66 (1.94) | <0.001 |
| Total LA stroke volume, mL | 18.93 (10.38) | 15.03 (5.86) | 0.02 |
| LARVi, mL/m2 | 28.72 (8.50) | 32.81 (7.65) | 0.348 |
| LARVi/ height 2.7, mL/m 2.7 | 9.79 (3.52) | 6.36 (1.82) | <0.001 |
| Total LA emptying fraction, % | 47.16 (19.42) | 54.14 (12.65) | 0.286 |
| RA peak negative strain, % | −4.79 (6.60) | −4.88 (2.45) | 0.818 |
| RA peak positive strain, % | 36.05 (26.22) | 34.77 (20.29) | 0.927 |
| Total RA strain, % | 32.99 (26.83) | 31.88 (22.21) | 0.219 |
| RAVi max, mL/m2 | 19.54 (6.50) | 15.83 (5.77) | 0.011 |
| RAVi min, mL/m2 | 11.06 (3.85) | 7.50 (2.56) | 0.001 |
| Total RA stroke volume, mL | 17.78 (10.19) | 11.94 (6.43) | 0.01 |
| RARVi, mL/m2 | 29.66 (8.50) | 24.44 (7.65) | 0.01 |
| RARVi/ height 2.7 | 10.15 (3.16) | 6.07 (2.14) | <0.001 |
| Total RA emptying fraction, % | 43.96 (15.53) | 46.10 (12.26) | 0.374 |
| Parameters | LV Circumferential Strain | LV Radial Strain | LV Longitudinal Strain | RV Longitudinal Strain | ||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| BMI z-score | −1.548 (−2.75; −0.35) | 0.012 | −6.817 (−11.43; −2.21) | 0.004 | −1.353 (−2.44; −0.27) | 0.016 | −4.576 (−7.02; −2.13) | 0.001 |
| HR z-score | 0.240 (−0.78; 1.26) | 0.640 | 1.205 (−2.80; 5.21) | 0.550 | 0.219 (−0.75; 1.19) | 0.653 | 0.536 (−1.64; 2.71) | 0.624 |
| SBP z-score | 0.214 (−1.00; 1.43) | 0.727 | 0.609 (−4.02; 5.24) | 0.793 | −0.139 (−1.27; 0.99) | 0.807 | −0.197 (−2.69; 2.29) | 0.875 |
| DBP z-score | −0.968 (−2.25; 0.32) | 0.137 | 2.464 (−2.40; 7.33) | 0.315 | −0.661 (−1.83; 0.51) | 0.264 | 0.678 (−2.01; 3.36) | 0.615 |
| LDLP z-score | −0.044 (−1.04; 0.95) | 0.930 | 3.905 (−0.314; 8.13) | 0.069 | −0.978 (−1.97; 0.02) | 0.055 | −1.585 (−3.79; 0.63) | 0.157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šileikienė, R.; Adamonytė, K.; Ziutelienė, A.; Ramanauskienė, E.; Vaškelytė, J.J. Atrial and Ventricular Structural and Functional Alterations in Obese Children. Medicina 2021, 57, 562. https://doi.org/10.3390/medicina57060562
Šileikienė R, Adamonytė K, Ziutelienė A, Ramanauskienė E, Vaškelytė JJ. Atrial and Ventricular Structural and Functional Alterations in Obese Children. Medicina. 2021; 57(6):562. https://doi.org/10.3390/medicina57060562
Chicago/Turabian StyleŠileikienė, Rima, Karolina Adamonytė, Aristida Ziutelienė, Eglė Ramanauskienė, and Jolanta Justina Vaškelytė. 2021. "Atrial and Ventricular Structural and Functional Alterations in Obese Children" Medicina 57, no. 6: 562. https://doi.org/10.3390/medicina57060562





