Follow-Up Analysis of Pulmonary Function, Exercise Capacity, Radiological Changes, and Quality of Life Two Months after Recovery from SARS-CoV-2 Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- radiologically chest X-ray- and/or CT-confirmed lung injury
- they were willing and capable to complete the pulmonary functions test, 6-min walking test, and the health status -elated quality of life short form questionnaire (SF-36) at follow-up
- no chronic lung disease prior the infection (to avoid radiological and functional overdiagnosis)
- they were able to understand and sign the informed consent to participate in the study.
- moderate disease (clinically and radiologically confirmed pneumonia, with no requirement for supplemental oxygen; oxygen saturation >93%)
- severe disease (having radiological evidence of bilateral pneumonia with lung injury >v50% and any of the following: respiratory rate ≥30 breaths/min, oxygen saturation ≤93% at rest; oxygen therapy ≤10 L/min; no need for treatment in the Intensive Care Unit (ICU));
- critical disease (respiratory failure with the need of high-flow oxygen therapy or intubation, shock, or other organ failure that requires ICU care).
2.2. Data Collection
2.2.1. Pulmonary Function Testing and 6 MWT
2.2.2. Chest CT Protocols and Image Analysis
2.2.3. SF-36 Questionnaire
2.2.4. Laboratory Parameters
2.3. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Lung Function Testing and 6 MWT
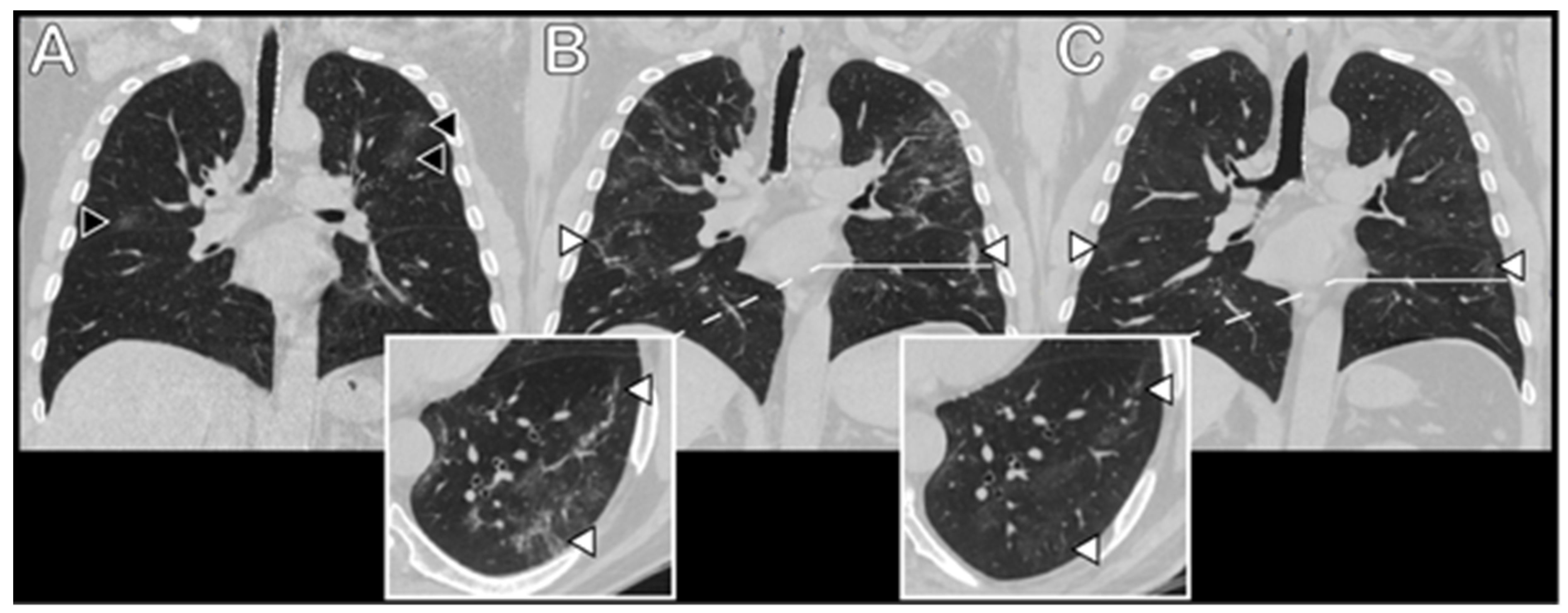
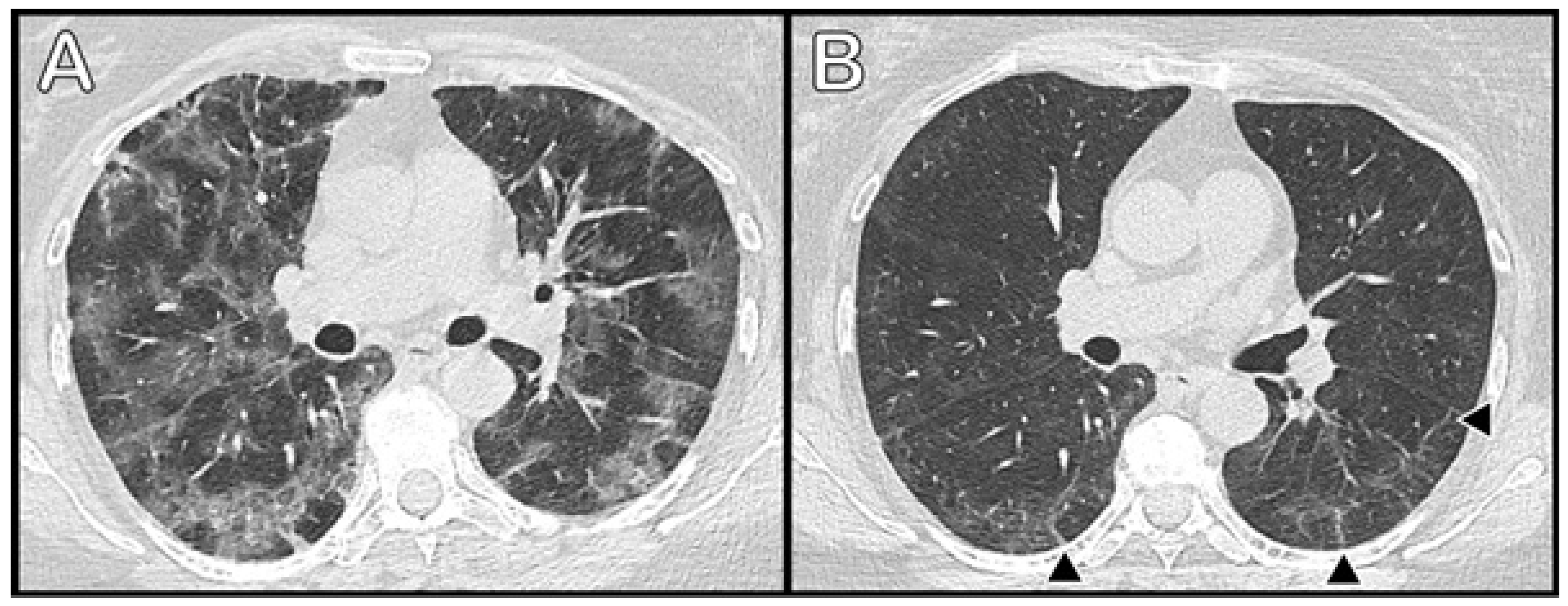
3.3. Radiological Data
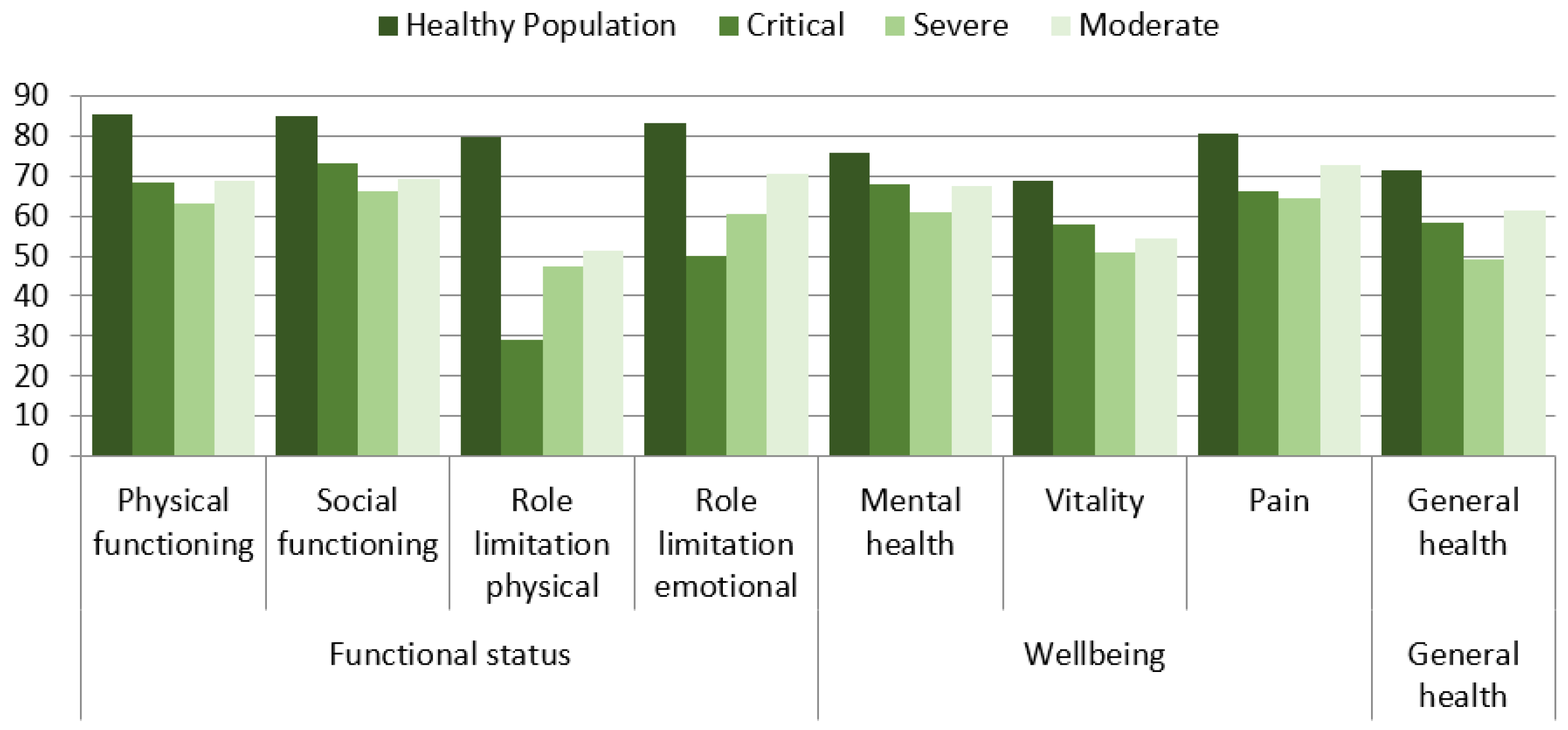
3.4. Health-Related Quality of Life
3.5. Laboratory Data
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://covid19.who.int (accessed on 15 February 2021).
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Sahanic, S.; Pizzini, A.; Luger, A.; Schwabl, C.; Sonnweber, B.; Kurz, K.; Koppelstätter, S.; Haschka, D.; Petzer, V.; et al. Cardiopulmonary recovery after COVID-19: An observational prospective multicenter trial. Eur. Respir. J. 2021, 57, 2003481. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Zheng, J.P.; Mok, Y.W.; Li, Y.M.; Liu, Y.N.; Chu, C.M.; Ip, M.S. SARS: Prognosis, outcome and sequelae. Respirology 2003, 8, S36–S40. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Y.; Fan, B.; Xiao, Y.; Tian, Q.; Chen, L.; Zhao, H.; Chen, W. Dynamic changes of serum SARS-coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge. Respir. Res. 2005, 6, 5. [Google Scholar] [CrossRef]
- Das, K.M.; Lee, E.Y.; Singh, R.; Enani, M.A.; Al Dossari, K.; Van Gorkom, K.; Larsson, S.G.; Langer, R.D. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J. Radiol. Imaging 2017, 27, 342–349. [Google Scholar] [CrossRef]
- WHO. Clinical Management of Severe Acute Respiratory Infection when COVID-19 Disease Is Suspected: Interim Guidance, 13 March 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- National Health Commission of the People’s Republic of China. Chinese Management Guideline for COVID-19 (Chinese Version, Version 7.0). The General Office of National Health Commissinos, Office of State TCM Administrations. Available online: https://www.chinadaily.com.cn/pdf/2020/1.Clinical.Protocols.for.the.Diagnosis.and.Treatment.of.COVID-19.V7.pdf (accessed on 3 March 2020).
- Brusasco, V.; Crapo, R.; Viegi, G. Coming together: The ATS/ERS consensus on clinical pulmonary function testing. Eur. Respir. J. 2005, 26, 1–2. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Antonio, G.E.; Wong, K.T.; Hui, D.S.; Wu, A.; Lee, N.; Yuen, E.H.; Leung, C.B.; Rainer, T.H.; Cameron, P.; Chung, S.S.; et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: Preliminary experience. Radiology 2003, 228, 810–815. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S. SF-36 Physical and Mental Summary Scales: A User’s Manual; New England Medical Center, The Health Institute: Boston, MA, USA, 1994. [Google Scholar]
- Aaronson, N.K.; Muller, M.; Cohen, P.D.; Essink-Bot, M.L.; Fekkes, M.; Sanderman, R.; Sprangers, M.A.; Te Velde, A.; Verrips, E. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J. Clin. Epidemiol. 1998, 51, 1055–1068. [Google Scholar] [CrossRef]
- Garcia, P.D.W.; Fumeaux, T.; Guerci, P.; Heuberger, D.M.; Montomoli, J.; Roche-Campo, F.; Schuepbach, R.A.; Hilty, M.P.; RISC-19-ICU Investigators. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine 2020, 25, 100449. [Google Scholar] [CrossRef]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Lavena Marzio, M.A.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef]
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197. [Google Scholar] [CrossRef]
- Liang, L.; Yang, B.; Jiang, N.; Fu, W.; He, X.; Zhou, Y.; Ma, W.L.; Wang, X. Three-month Follow-up Study of Survivors of Coronavirus Disease 2019 after Discharge. J. Korean Med. Sci. 2020, 35, e418. [Google Scholar] [CrossRef] [PubMed]
- Rovere Querini, P.; De Lorenzo, R.; Conte, C.; Brioni, E.; Lanzani, C.; Yacoub, M.R.; Chionna, R.; Martinenghi, S.; Vitali, G.; Tresoldi, M.; et al. Post-COVID-19 follow-up clinic: Depicting chronicity of a new disease. Acta Biomed. 2020, 91 (Suppl. 9), 22–28. [Google Scholar] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2020, 75, e13746. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Shang, Y.M.; Song, W.B.; Li, Q.Q.; Xie, H.; Xu, Q.F.; Jia, J.L.; Li, L.M.; Mao, H.L.; Zhou, X.M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xiong, Y.; Sun, D.; Liu, Y.; Fan, Y.; Zhao, L.; Li, X.; Zhu, W. Clinical and High-Resolution CT Features of the COVID-19 Infection: Comparison of the Initial and Follow-up Changes. Investig. Radiol. 2020, 55, 332–339. [Google Scholar] [CrossRef]
- Talman, S.; Boonman-de Winter, L.J.M.; de Mol, M.; Hoefman, E.; van Etten, R.W.; De Backer, I.C. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir. Med. 2021, 176, 106272. [Google Scholar]
- Lerum, T.V.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.; Ikdahl, E.; Lund, K.M.A.; Durheim, M.T.; Rodriguez, J.R.; Meltzer, C.; Tonby, K.; et al. Dyspnoea, lung function and CT findings three months after hospital admission for COVID-19. Eur. Respir. J. 2020, 57, 2003448. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Patel, K.; Greenwood, D.C.; Halpin, S.; Lewthwaite, P.; Salawu, A.; Eyre, L.; Breen, A.; O’Connor, R.; Jones, A.; et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, jrm00063. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tan, C.; Wu, J.; Chen, M.; Wang, Z.; Luo, L.; Zhou, X.; Liu, X.; Huang, X.; Yuan, S.; et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cao, Y.; Jiang, N.; Chen, Y.; Alwalid, O.; Zhang, X.; Gu, J.; Dai, M.; Liu, J.; Zhu, W.; et al. Novel Coronavirus Disease 2019 (COVID-19) Pneumonia Progression Course in 17 Discharged Patients: Comparison of Clinical and Thin-Section Computed Tomography Features During Recovery. Clin. Infect. Dis. 2020, 71, 723–731. [Google Scholar] [CrossRef]
- Ng, C.K.; Chan, J.W.; Kwan, T.L.; To, T.S.; Chan, Y.H.; Ng, F.Y.; Mok, T.Y. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax 2004, 59, 889–891. [Google Scholar] [CrossRef]
- Marvisi, M.; Ferrozzi, F.; Balzarini, L.; Mancini, C.; Ramponi, S.; Uccelli, M. First report on clinical and radiological features of COVID-19 pneumonitis in a Caucasian population: Factors predicting fibrotic evolution. Int. J. Infect. Dis. 2020, 99, 485–488. [Google Scholar] [CrossRef]
- Chen, K.Y.; Li, T.; Gong, F.H.; Zhang, J.S.; Li, X.K. Predictors of Health-Related Quality of Life and Influencing Factors for COVID-19 Patients, a Follow-Up at One Month. Front. Psychiatry. 2020, 11, 668. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020, 39, 101166. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Gomersall, C.D.; Joynt, G.M.; Chan, D.P.; Leung, P.; Hui, D.S. Long-term outcome of acute respiratory distress syndrome caused by severe acute respiratory syndrome (SARS): An observational study. Crit. Care Resusc. 2006, 8, 302–308. [Google Scholar] [PubMed]
- Ngai, J.C.; Ko, F.W.; Ng, S.S.; To, K.W.; Tong, M.; Hui, D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010, 15, 543–550. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 51) | COVID-19 Severity | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Critical (n = 12) | Severe (n = 21) | Moderate (n = 18) | |||||
| Age years | Mean (SD) Min-Max | 56.0 (11.7) 29–79 | 54.8(9.7) | 60.0 (11.9) | 52.1 (11.8) | 0.104 | |
| Age group | 18–30 | n (%) | 1 (2.0) | - | - | 1 (5.6) | 0.290 |
| 31–40 | n (%) | 3 (5.9) | - | 1 (4.8) | 2 (11.1) | ||
| 41–50 | n (%) | 13 (25.5) | 5 (41.7) | 3 (14.3) | 5 (27.8) | ||
| 51–60 | n (%) | 18 (35.3) | 3 (25.0) | 9 (42.9) | 6 (33.3) | ||
| 61–70 | n (%) | 10 (19.6) | 4 (33.3) | 3 (14.3) | 3 (16.7) | ||
| 71–80 | n (%) | 6 (11.8) | - | 5 (23.8) | 1 (5.6) | ||
| Gender | Female | n (%) | 26 (51.0) | 1 (8.3) | 11 (52.4) | 14 (77.8) | <0.001 |
| Male | n (%) | 25 (49.0) | 11 (91.7) | 10 (47.6) | 4 (22.2) | ||
| Medical history | Hypertension | n (%) | 26 (51.0) | 6 (50.0) | 13 (61.9) | 7 (38.9) | 0.377 |
| Cardiac failure | n (%) | 13 (25.5) | 4 (33.3) | 7 (33.3) | 2 (11.1) | 0.195 | |
| Atrial fibrillation | n (%) | 9 (17.6) | 3 (25.0) | 4 (19.0) | 2 (11.1) | 0.588 | |
| Diabetes | n (%) | 8 (15.7) | 3 (25.0) | 3 (14.3) | 2 (11.1) | 0.624 | |
| Other diseases | n (%) | 23 (45.1) | 6 (50.0) | 11 (52.4) | 6 (33.3) | 0.452 | |
| BMI groups | Normal weight | n (%) | 9 (17.6) | 1 (8.3) | 2 (9.5) | 6 (33.3) | 0.018 |
| Overweight | n (%) | 14 (27.5) | 2 (16.7) | 8 (38.1) | 4 (22.2) | ||
| I° obesity | n (%) | 15 (29.4) | 6 (50.0) | 7 (33.3) | 2 (11.1) | ||
| II° obesity | n (%) | 9 (17.6) | - | 4 (19.0) | 5 (27.8) | ||
| III° obesity | n (%) | 4 (7.8) | 3 (25.0) | - | 1 (5.6) | ||
| Total (n = 51) | COVID-19 Severity | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Critical (n = 12) | Severe (n = 21) | Moderate (n = 18) | |||||
| FVC | Normal | n (%) | 47 (92.2) | 8 (66.7) | 21 (100.0) | 18 (100.0) | 0.002 |
| <LLN | n (%) | 4 (7.8) | 4 (33.3) | - | - | ||
| FEV1 | Normal | n (%) | 46 (90.2) | 8 (66.7) | 20 (95.2) | 18 (100.0) | 0.012 |
| <LLN | n (%) | 5 (9.8) | 4 (33.3) | 1 (4.8) | - | ||
| FEV1/FVC | Normal | n (%) | 50 (98.0) | 12 (100.0) | 21 (100.0) | 17 (94.4) | 0.588 |
| <LLN | n (%) | 1 (2.0) | - | - | 1 (5.6) | ||
| TLC | Normal | n (%) | 40 (78.4) | 8 (66.7) | 17 (81.0) | 15 (83.3) | 0.567 |
| <LLN | n (%) | 11 (21.6) | 4 (33.3) | 4 (19.0) | 3 (16.7) | ||
| VC | Normal | n (%) | 42 (82.4) | 7 (58.3) | 21 (100.0) | 14 (77.8) | 0.003 |
| <LLN | n (%) | 9 (17.6) | 5 (41.7) | - | 4 (22.2) | ||
| DLCO | Normal | n (%) | 36 (70.6) | 6 (50.0) | 14 (66.7) | 16 (88.9) | 0.063 |
| <LLN | n (%) | 15 (29.4) | 6 (50.0) | 7 (33.3) | 2 (11.1) | ||
| 6MWD, m | Mean (SD) | 553.5 (86.96) | 518.8 (45.98) | 550.7 (97.60) | 575.4 (91.54) | 0.258 | |
| 6MWD, % | Mean (SD) | 100.9 (14.97) | 88.7 (14.30) *,** | 102.6 (13.39) ** | 106.1 (13.50) * | 0.008 | |
| Radiological score, acute disease, total | Mean (SD) | 13.6 (5.77) | 19.3 (4.05) *,** | 13.48 (3.89) * | 9.8 (5.58) ** | <0.001 | |
| radiological score, follow-up, GGO | Mean (SD) | 7.1 (5.62) | 11.4 (6.64) * | 7.3 (4.94) | 3.8 (3.31) * | <0.001 | |
| radiological score, follow-up, fibrotic/reticular | Mean (SD) | 3.9 (4.60) | 7.1 (5.87) * | 4.1 (4.49) | 1.4 (1.58) * | 0.002 | |
| radiological score, follow-up, total | Mean (SD) | 10.9 (8.87) | 18.5 (9.34) *,** | 11.5 (8.21) *,*** | 5.22 (4.49) **,*** | <0.001 | |
| Total (n = 51) | COVID-19 Severity | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Critical (n = 12) | Severe (n = 21) | Moderate (n = 18) | |||||
| Max WBC, × 109/L | Mean (SD) | 8.63 (3.725) | 9.60 (4.746) | 9.49 (3.932) | 6.98 (1.895) | 0.063 | |
| Min LYM count, × 109/L | Mean (SD) | 1.00 (0.413) | 0.74 (0.292) * | 0.97 (0.347) | 1.22 (0.455) * | 0.006 | |
| CRP, mg/L | Mean (SD) | 100.1 (97.97) | 212.1 (122.08) *,** | 84.3 (56.78) * | 43.9 (47.72) ** | <0.001 | |
| D-dimer, mcg/L | Mean (SD) | 658.6 (689.47) | 1302.5 (863.36) *,** | 416.0 (225.05) * | 512.3 (684.26) ** | <0.001 | |
| IL-6, ng/L | Mean (SD) | 71.9 (171.99) | 189.0 (320.12) *,** | 43.2 (42.86) * | 18.4 (10.86) ** | 0.019 | |
| Ferritin, mcg/L | Mean (SD) | 902.5 (992.28) | 1823.0 (1113.63) *,** | 895.7 (914.27) * | 261.0 (212.31) ** | <0.001 | |
| LDH, U/L | Mean (SD) | 324.3 (106.09) | 412.1 (91.88) *,** | 331.6 (102.58) *,*** | 253.2 (65.52) **,*** | <0.001 | |
| Hepatic injury | Yes | n (%) | 38 (74.5) | 12 (100.0) | 17 (81.0) | 9 (50.0) | 0.005 |
| No | n (%) | 13 (25.5) | - | 4 (19.0) | 9 (50.0) | ||
| Renal injury | Yes | n (%) | 18 (35.3) | 11 (91.7) | 6 (28.6) | 1 (5.6) | <0.001 |
| No | n (%) | 33 (64.7) | 1 (8.3) | 15 (71.4) | 17 (94.4) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strumiliene, E.; Zeleckiene, I.; Bliudzius, R.; Samuilis, A.; Zvirblis, T.; Zablockiene, B.; Strumila, A.; Gruslys, V.; Malinauskiene, L.; Kasiulevicius, V.; et al. Follow-Up Analysis of Pulmonary Function, Exercise Capacity, Radiological Changes, and Quality of Life Two Months after Recovery from SARS-CoV-2 Pneumonia. Medicina 2021, 57, 568. https://doi.org/10.3390/medicina57060568
Strumiliene E, Zeleckiene I, Bliudzius R, Samuilis A, Zvirblis T, Zablockiene B, Strumila A, Gruslys V, Malinauskiene L, Kasiulevicius V, et al. Follow-Up Analysis of Pulmonary Function, Exercise Capacity, Radiological Changes, and Quality of Life Two Months after Recovery from SARS-CoV-2 Pneumonia. Medicina. 2021; 57(6):568. https://doi.org/10.3390/medicina57060568
Chicago/Turabian StyleStrumiliene, Edita, Ingrida Zeleckiene, Rytis Bliudzius, Arturas Samuilis, Tadas Zvirblis, Birute Zablockiene, Arunas Strumila, Vygantas Gruslys, Laura Malinauskiene, Vytautas Kasiulevicius, and et al. 2021. "Follow-Up Analysis of Pulmonary Function, Exercise Capacity, Radiological Changes, and Quality of Life Two Months after Recovery from SARS-CoV-2 Pneumonia" Medicina 57, no. 6: 568. https://doi.org/10.3390/medicina57060568
APA StyleStrumiliene, E., Zeleckiene, I., Bliudzius, R., Samuilis, A., Zvirblis, T., Zablockiene, B., Strumila, A., Gruslys, V., Malinauskiene, L., Kasiulevicius, V., & Jancoriene, L. (2021). Follow-Up Analysis of Pulmonary Function, Exercise Capacity, Radiological Changes, and Quality of Life Two Months after Recovery from SARS-CoV-2 Pneumonia. Medicina, 57(6), 568. https://doi.org/10.3390/medicina57060568






