Chronic Obstructive Pulmonary Disease as a Phenotype of Bronchiectasis for Long-Term Clinical Presentation and Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Design
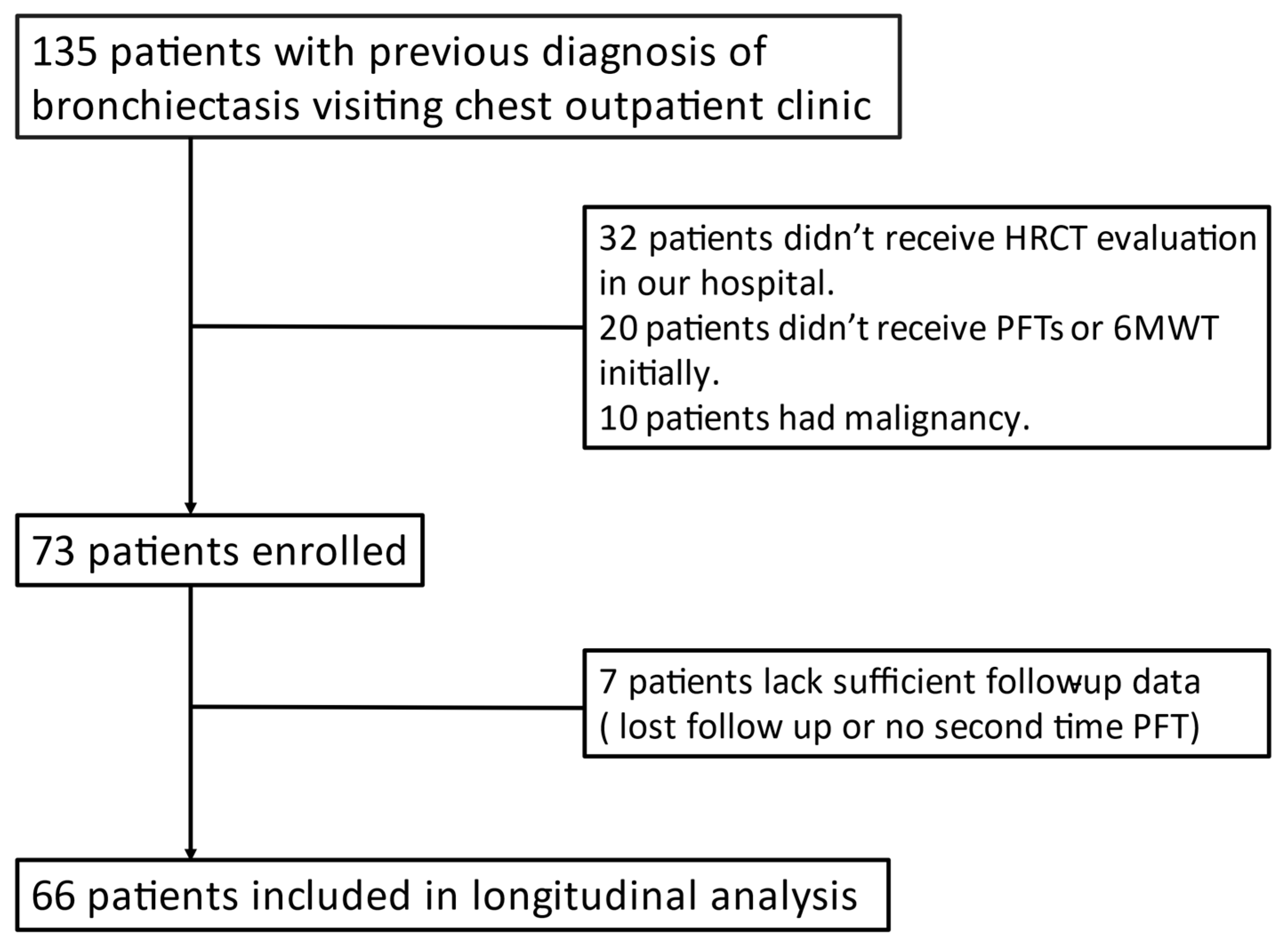
2.3. Inclusion and Exclusion Criteria
2.4. 6MWT, PFT, and Sputum Culture
2.5. Data collection: Medical Record, Lung Function Test, 6MWT, and Culture
2.6. Bronchiectasis Extension Score
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Q.; Jin, J.; Liu, X.; Sun, Y. Bronchiectasis as a comorbidity of chronic obstructive pulmonary disease: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0150532. [Google Scholar]
- Martínez-García, M.Á.; Soler-Cataluña, J.J.; Sanz, Y.D.; Serra, P.C.; Lerma, M.A.; Vicente, J.B.; Perpiñá-Tordera, M.J.C. Factors associated with bronchiectasis in patients with COPD. Chest 2011, 140, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.S.; Vlahos, I.; Wilkinson, T.M.; Lloyd-Owen, S.J.; Donaldson, G.C.; Wilks, M.; Reznek, R.H.; Wedzicha, J.A. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004, 170, 400–407. [Google Scholar] [CrossRef]
- Hurst, J.R.; Elborn, J.S.; De Soyza, A. COPD–bronchiectasis overlap syndrome. Chest 2015, 45, 310–313. [Google Scholar] [CrossRef]
- Blasi, F.; Chalmers, J.D.; Aliberti, S. COPD and bronchiectasis: Phenotype, endotype or co-morbidity? J. Chronic Obstr. Pulm. Dis. 2014, 11, 603–604. [Google Scholar] [CrossRef]
- Radovanovic, D.; Santus, P.; Blasi, F.; Sotgiu, G.; D’Arcangelo, F.; Simonetta, E.; Contarini, M.; Franceschi, E.; Goeminne, P.C.; Chalmers, J.D.; et al. A comprehensive approach to lung function in bronchiectasis. Respir. Med. 2018, 145, 120–129. [Google Scholar] [CrossRef]
- Chung, W.-S.; Lin, C.-L. Acute respiratory events in patients with bronchiectasis–COPD overlap syndrome: A population-based cohort study. Respir. Med. 2018, 140, 6–10. [Google Scholar] [CrossRef] [PubMed]
- King, P.T.; Holdsworth, S.R.; Freezer, N.J.; Villanueva, E.; Holmes, P.W. Microbiologic follow-up study in adult bronchiectasis. Respir. Med. 2007, 101, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.J.; Aliberti, S.; Goeminne, P.C.; Restrepo, M.I.; Finch, S.; Pesci, A.; Dupont, L.J.; Fardon, T.C.; Wilson, R.; Loebinger, M.R.; et al. Comorbidities and the risk of mortality in patients with bronchiectasis: An international multicentre cohort study. Lancet Respir. Med. 2016, 4, 969–979. [Google Scholar] [CrossRef]
- Chalmers, J.D.; Goeminne, P.; Aliberti, S.; McDonnell, M.J.; Lonni, S.; Davidson, J.; Poppelwell, L.; Salih, W.; Pesci, A.; Dupont, L.J.; et al. The bronchiectasis severity index. An international derivation and validation study. Am. J. Respir. Crit. Care Med. 2014, 189, 576–585. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.; Athanazio, R.; Girón, R.; Máiz-Carro, L.; De la Rosa, D.; Olveira, C.; de Gracia, J.; Vendrell, M.; Prados-Sánchez, C.; Gramblicka, G.; et al. Predicting high risk of exacerbations in bronchiectasis: The E-FACED score. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 275. [Google Scholar] [CrossRef]
- Brody, A.S.; Klein, J.S.; Molina, P.L.; Quan, J.; Bean, J.A.; Wilmott, R.W. High-resolution computed tomography in young patients with cystic fibrosis: Distribution of abnormalities and correlation with pulmonary function tests. J. Pediatr. 2004, 145, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Reiff, D.B.; Wells, A.U.; Carr, D.H.; Cole, P.J.; Hansell, D.M. CT findings in bronchiectasis: Limited value in distinguishing between idiopathic and specific types. Am. J. Roentgenol. 1995, 165, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, M.; Turcios, N.; Aponte, V.; Jenkins, M.; Leitman, B.; McCauley, D.; Naidich, D.J.R. Cystic fibrosis: Scoring system with thin-section CT. Radiology 1991, 179, 783–788. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- De la Rosa, D.; Martínez-Garcia, M.-A.; Giron, R.M.; Vendrell, M.; Olveira, C.; Borderias, L.; Maiz, L.; Torres, A.; Martinez-Moragon, E.; Rajas, O.J.P.O. Clinical impact of chronic obstructive pulmonary disease on non-cystic fibrosis bronchiectasis. A study on 1,790 patients from the Spanish Bronchiectasis Historical Registry. PLoS ONE 2017, 12, e0177931. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Miravitlles, M. Bronchiectasis in COPD patients: More than a comorbidity? Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1401. [Google Scholar] [CrossRef]
- Nicotra, M.B.; Rivera, M.; Dale, A.M.; Shepherd, R.; Carter, R.J.C. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest 1995, 108, 955–961. [Google Scholar] [CrossRef] [PubMed]
- King, P.T.; Holdsworth, S.R.; Freezer, N.J.; Villanueva, E.; Gallagher, M.; Holmes, P.W. Outcome in adult bronchiectasis. J. Chronic Obstr. Pulm. Dis. 2005, 2, 27–34. [Google Scholar] [CrossRef]
- Ikan, A.; Adir, Y.; Stein, N.; Schneer, S.; Shteinberg, M. Lung function decline in patients with bronchiectasis. Eur. Respir. J. 2018, 52, PA2668. [Google Scholar] [CrossRef]
- King, P.T.; Holdsworth, S.R.; Freezer, N.J.; Villanueva, E.; Holmes, P.W. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir. Med. 2006, 100, 2183–2189. [Google Scholar] [CrossRef]
- Monso, E.; Garcia-Aymerich, J.; Soler, N.; Farrero, E.; Felez, M.; Anto, J.; Torres, A. Bacterial infection in exacerbated COPD with changes in sputum characteristics. Epidemiol. Infect. 2003, 131, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.; Seemungal, T.; Wilks, M.; Lloyd-Owen, S.; Donaldson, G.; Wedzicha, J.J.T. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002, 57, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.A.; Newell, J.; Hale, V.; Dyer, D.; Corkery, K.; Fox, N.L.; Gerend, P.; Fick, R. Correlation of CT findings with clinical evaluations in 261 patients with symptomatic bronchiectasis. Am. J. Roentgenol. 1999, 173, 53–58. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef]
- King, P.J.D. Is there a role for inhaled corticosteroids and macrolide therapy in bronchiectasis? Drugs 2007, 67, 965–974. [Google Scholar] [CrossRef]
- McShane, P.J.; Naureckas, E.T.; Tino, G.; Strek, M.E. Non–cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 2013, 188, 647–656. [Google Scholar] [CrossRef]
- Latorre, M.; Novelli, F.; Vagaggini, B.; Braido, F.; Papi, A.; Sanduzzi, A.; Santus, P.; Scichilone, N.; Paggiaro, P. Differences in the efficacy and safety among inhaled corticosteroids (ICS)/long-acting beta2-agonists (LABA) combinations in the treatment of chronic obstructive pulmonary disease (COPD): Role of ICS. Pulm. Pharmacol. Ther. 2015, 30, 44–50. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, H.; Carriere, K.C.; Kim, J.H.; Han, J.-H.; Shin, B.; Jeong, B.-H.; Koh, W.-J.; Kwon, O.J.; Park, H.Y. Effects of long-term bronchodilators in bronchiectasis patients with airflow limitation based on bronchodilator response at baseline. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2757. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Soler-Cataluña, J.J.; Catalán-Serra, P.; Román-Sánchez, P.; Tordera, M.P.J.C. Clinical efficacy and safety of budesonide-formoterol in non-cystic fibrosis bronchiectasis. Chest 2012, 141, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Elborn, J.S.; Floto, R.A.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.J.T. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019, 74, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, R.; Wells, A.; Copley, S.; Desai, S.; Howling, S.; Cole, P.; Wilson, R.; Hansell, D.M. A comparison of serial computed tomography and functional change in bronchiectasis. Eur. Respir. J. 2002, 20, 581–587. [Google Scholar] [CrossRef]
- Martínez-García, M.A.; Soler-Cataluña, J.-J.; Perpiñá-Tordera, M.; Román-Sánchez, P.; Soriano, J.J.C. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007, 132, 1565–1572. [Google Scholar] [CrossRef]
| Bronchiectasis Patients with COPD (n = 21) | Bronchiectasis Patients without COPD (n = 45) | p-Value | |
|---|---|---|---|
| Age | 56.95 ± 15.47 | 58.2 ± 12.81 | 0.732 |
| Body weight | 53.48 ± 9.10 | 58.29 ± 11.26 | 0.087 |
| BMI | 20.90 ± 2.91 | 22.62 ± 3.56 | 0.057 |
| Smoking history | 7 (15.6%) | 5 (23.8%) | 0.499 |
| Past history | |||
| Cardiovascular | 4 (8.9%) | 0 (0.0%) | 0.298 |
| Diabetes mellitus | 5 (11.1%) | 2 (9.5%) | 1.000 |
| TB history (%) | 2 (4.4%) | 1 (4.8%) | 1.000 |
| Lung function test | |||
| FVC | 1.63 ± 0.66 | 2.16 ± 0.79 | 0.010 |
| FVC% | 53.33 ± 17.85 | 70.58 ± 17.17 | 0.000 |
| FEV1 | 0.86 (0.64–1.28) | 1.41 (1.17–2.07) | 0.000 |
| FEV1% | 40.90 ± 18.15 | 69.04 ± 19.98 | 0.000 |
| FEV1/FVC | 62.70 (51.70–65.65) | 78.00 (72.00–83.00) | 0.000 |
| 6-min walking test | |||
| Walking distance | 421.71 ± 102.47 | 463.95 ± 80.20 | 0.073 |
| Pre-test saturation | 95.00 (92.00–96.00) | 96.00 (95.00–97.00) | 0.01 |
| Post-test saturation | 84.00 (68.75–89.75) | 91.00 (85.50–93.00) | 0.02 |
| Saturation loss | 9.00 (5.50–18.25) | 4.50 (3.00–8.00) | 0.29 |
| Bronchiectasis Patients with COPD (n = 21) | Bronchiectasis Patients without COPD (n = 45) | p-Value | |
|---|---|---|---|
| Image study HRCT extension score Clinical performance variation per year | 32.21 ± 13.09 | 21.89 ± 10.08 | 0.001 |
| Δ FVC/year | −0.62 (−1.80–−0.27) | −0.03 (−0.11–0.00) | 0.406 |
| Δ FVC predicted/year | −2.17 (−5.75–−0.86) | −1.00 (−3.98–0.43) | 0.444 |
| Δ FEV1/year | −0.03 (−0.08–0.03) | −0.04 (−0.10–0.00) | 0.374 |
| Δ FEV1 predicted/year | −1.20 (−5.00–0.86) | −0.83 (−5.12–0.72) | 0.940 |
| Δ FEV1/FVC/year | 0.41 (−2.70–2.27) | −0.53 (−4.92–−0.08) | 0.064 |
| Δ 6MWT-distance/year | −9.92 (−40.04–−5.33) | −1.85 (−20.91–6.82) | 0.164 |
| Δ saturation-loss/year | 0.33 (−0.54–1.49) | −0.09 (−1.00–1.00) | 0.205 |
| Bronchiectasis Patients with COPD (n = 21) | Bronchiectasis Patients without COPD (n = 45) | p-Value | |
|---|---|---|---|
| Sputum production | |||
| Sputum production at recruitment | 95.2% | 66.7% | 0.012 |
| Sputum production after 3 years | 82.4% | 81.6% | 0.945 |
| Sputum culture | |||
| Pseudomonas positive during follow-up | 23.8% | 24.4% | 0.560 |
| Other bacteria positive during follow-up | 33.3% | 8.9% | 0.001 |
| Clinical treatment | |||
| Long term antibiotics use 1 | 80.9% | 64.4% | 0.174 |
| ICS and/or LABA use after 3 years | 47.6% | 22.2% | 0.020 |
| ICS and/or LABA use after 5 years | 64.7% | 24.2% | 0.012 |
| ICS and/or LABA use after 10 years | 57.1% | 40.0% | 0.195 |
| LAMA use | 23.8% | 11.1% | 0.287 |
| OR | 95% CI of OR | p-Value | |
|---|---|---|---|
| With COPD | 1.54 | 0.44–5.32 | 0.500 |
| Bronchiectasis extent score | 1.06 | 1.00–1.12 | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-Y.; Poon, Y.-Y.; Chen, Y.-W.; Hsieh, M.-H.; Lin, H.-C.; Fang, W.-F. Chronic Obstructive Pulmonary Disease as a Phenotype of Bronchiectasis for Long-Term Clinical Presentation and Treatment. Medicina 2021, 57, 579. https://doi.org/10.3390/medicina57060579
Hsu C-Y, Poon Y-Y, Chen Y-W, Hsieh M-H, Lin H-C, Fang W-F. Chronic Obstructive Pulmonary Disease as a Phenotype of Bronchiectasis for Long-Term Clinical Presentation and Treatment. Medicina. 2021; 57(6):579. https://doi.org/10.3390/medicina57060579
Chicago/Turabian StyleHsu, Chih-Yi, Yan-Yuen Poon, Yu-Wei Chen, Meng-Heng Hsieh, Horng-Chyuan Lin, and Wen-Feng Fang. 2021. "Chronic Obstructive Pulmonary Disease as a Phenotype of Bronchiectasis for Long-Term Clinical Presentation and Treatment" Medicina 57, no. 6: 579. https://doi.org/10.3390/medicina57060579
APA StyleHsu, C.-Y., Poon, Y.-Y., Chen, Y.-W., Hsieh, M.-H., Lin, H.-C., & Fang, W.-F. (2021). Chronic Obstructive Pulmonary Disease as a Phenotype of Bronchiectasis for Long-Term Clinical Presentation and Treatment. Medicina, 57(6), 579. https://doi.org/10.3390/medicina57060579







