Severe Coronary Artery Disease in a Person Living with HIV
Abstract
:1. Introduction
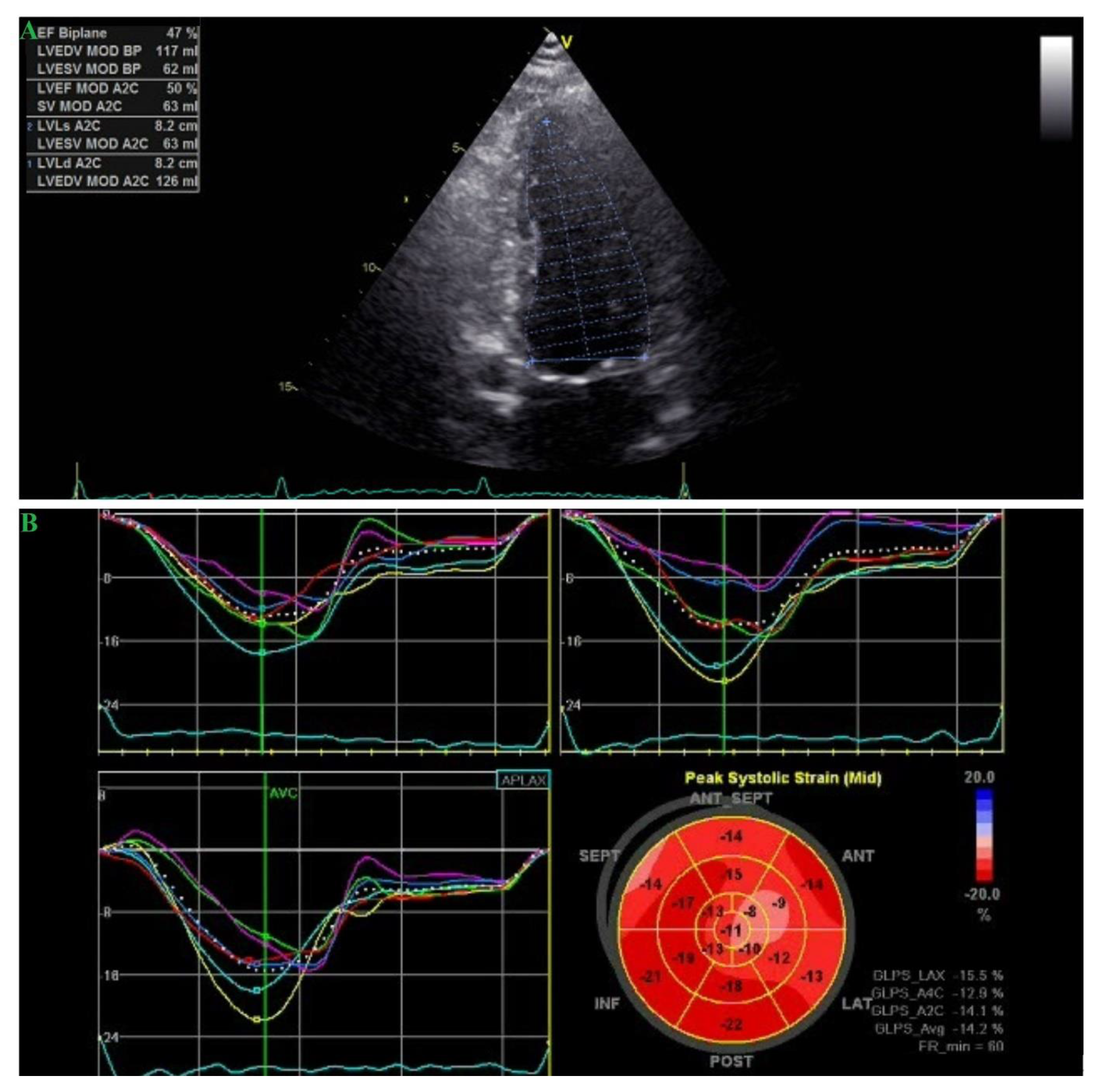
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worm, S.W.; Sabin, C.; Weber, R.; Reiss, P.; El-Sadr, W.; Dabis, F.; De Wit, S.; Law, M.M.; Monforte, A.D.; Friis-Moller, N.; et al. Risk of Myocardial Infarction in Patients with HIV Infection Exposed to Specific Individual Antiretroviral Drugs from the 3 Major Drug Classes: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study. J. Infect. Dis. 2010, 201, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Lewden, C.; May, T.; Rosenthal, E.; Burty, C.; Bonnet, F.; Costagliola, D.; Jougla, E.; Semaille, C.; Morlat, P.; Salmon, D.; et al. Changes in Causes of Death Among Adults Infected by HIV Between 2000 and 2005: The “Mortalité 2000 and 2005” Surveys (ANRS EN19 and Mortavic). J. Acquir. Immune Defic. Syndr. 2008, 48, 590–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Antiretroviral Therapy Cohort Collaboration. Causes of Death in HIV-1–Infected Patients Treated with Antiretroviral Therapy, 1996–2006: Collaborative Analysis of 13 HIV Cohort Studies. Clin. Infect. Dis. 2010, 50, 1387–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palella, F.J.; Baker, R.K.; Moorman, A.C.; Chmiel, J.S.; Wood, K.C.; Brooks, J.T.; Holmberg, S.D. Mortality in the Highly Active Antiretroviral Therapy Era. JAIDS J. Acquir. Immune Defic. Syndr. 2006, 43, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearns, A.; Gordon, J.; Burdo, T.H.; Qin, X. HIV-1—Associated Atherosclerosis: Unraveling the Missing Link. J. Am. Coll. Cardiol. 2017, 69, 3084–3098. [Google Scholar] [CrossRef] [PubMed]
- Seecheran, V.; Giddings, S.L.; Seecheran, N.A. Acute coronary syndromes in patients with HIV. Coron. Artery Dis. 2017, 28, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Boccara, F.; Mary-Krause, M.; Teiger, E.; Lang, S.; Lim, P.; Wahbi, K.; Beygui, F.; Milleron, O.; Steg, P.G.; Funck-Brentano, C.; et al. Acute coronary syndrome in human immunodeficiency virus-infected patients: Characteristics and 1 year prognosis. Eur. Heart J. 2010, 32, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccara, F.; Mary-Krause, M.; Potard, V.; Teiger, E.; Lang, S.; Hammoudi, N.; Chauvet, M.; Ederhy, S.; Dufour-Soulat, L.; Ancedy, Y.; et al. HIV Infection and Long-Term Residual Cardiovascular Risk After Acute Coronary Syndrome. J. Am. Heart Assoc. 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Triant, V.A.; Lee, H.; Hadigan, C.; Grinspoon, S.K. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J. Clin. Endocrinol. Metab. 2007, 92, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S.; Chang, C.-C.H.; Kuller, L.H.; Skanderson, M.; Lowy, E.; Kraemer, K.L.; Butt, A.A.; Goetz, M.B.; Leaf, D.; Oursler, K.A.; et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern. Med. 2013, 173, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Escaut, L.; Monsuez, J.J.; Chironi, G.; Merad, M.; Teicher, E.; Smadja, D.; Simon, A.; Vittecoq, D. Coronary artery disease in HIV infected patients. Intensiv. Care Med. 2003, 29, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.J.; Khan, I.A. HIV-Associated Coronary Artery Disease. Angiology 2003, 54, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Boccara, F.; Cohen, A.; Di Angelantonio, E.; Meuleman, C.; Ederhy, S.; Dufaitre, G.; Odi, G.; Teiger, E.; Barbarini, G.; Barbaro, G.; et al. Coronary artery bypass graft in HIV-infected patients: A multicenter case control study. Curr. HIV Res. 2008, 6, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Khandelwal, A.R.; Rogers, L.K.; Hebert, V.Y.; Kleinedler, J.J.; Zavecz, J.H.; Shi, W.; Orr, A.; Dugas, T.R. Antiretrovirals Induce Endothelial Dysfunction via an Oxidant-Dependent Pathway and Promote Neointimal Hyperplasia. Toxicol. Sci. 2010, 117, 524–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsousi, N.; Daali, Y.; Fontana, P.; Reny, J.; Ancrenaz-Sirot, V.; Calmy, A.; Rudaz, S.; Desmeules, J.A.; Samer, C.F. Impact of Boosted Antiretroviral Therapy on the Pharmacokinetics and Efficacy of Clopidogrel and Prasugrel Active Metabolites. Clin. Pharmacokinet. 2018, 57, 1347–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajdechi, M.; Micu, C.-E.; Rugină, S.; Stoian, G.-E.; Gurghean, A. Metabolic Syndrome and Myocardial Involvement in HIV-Infected Patients. Intern. Med. 2019, 16, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Erdem, H.; Puca, E.; Ruch, Y.; Santos, L.; Ghanem-Zoubi, N.; Argemi, X.; Hansmann, Y.; Guner, R.; Tonziello, G.; Mazzucotelli, J.-P.; et al. Portraying infective endocarditis: Results of multinational ID-IRI study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1753–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, A.; Reinsch, N.; Neuhaus, K.; Esser, S.; Brockmeyer, N.; Potthoff, A.; Pankuweit, S.; Erbel, R.; Maisch, B.; Neumann, T.; et al. Pericardial effusion of HIV-infected patients? Results of a prospective multicenter cohort study in the era of antiretroviral therapy. Eur. J. Med. Res. 2011, 16, 480. [Google Scholar] [PubMed] [Green Version]
- Torres-Macho, J.; Aro, T.; Bruckner, I.; Cogliati, C.; Gilja, O.; Gurghean, A.; Karlafti, E.; Krsek, M.; Monhart, Z.; Müller-Marbach, A.; et al. Point-of-care ultrasound in internal medicine: A position paper by the ultrasound working group of the European federation of internal medicine. Eur. J. Intern. Med. 2020, 73, 67–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbaz, S.; Manicardi, M.; Guaraldi, G.; Raggi, P. Cardiovascular disease in human immunodeficiency virus infected patients: A true or perceived risk? World J. Cardiol. 2015, 7, 633–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajdechi, M.; Mihai, C.; Scafa-Udriste, A.; Cherry, A.; Zamfir, D.; Dumitru, I.; Cernat, R.; Rugina, S. Severe Coronary Artery Disease in a Person Living with HIV. Medicina 2021, 57, 595. https://doi.org/10.3390/medicina57060595
Bajdechi M, Mihai C, Scafa-Udriste A, Cherry A, Zamfir D, Dumitru I, Cernat R, Rugina S. Severe Coronary Artery Disease in a Person Living with HIV. Medicina. 2021; 57(6):595. https://doi.org/10.3390/medicina57060595
Chicago/Turabian StyleBajdechi, Mircea, Cosmin Mihai, Alexandru Scafa-Udriste, Ali Cherry, Diana Zamfir, Irina Dumitru, Roxana Cernat, and Sorin Rugina. 2021. "Severe Coronary Artery Disease in a Person Living with HIV" Medicina 57, no. 6: 595. https://doi.org/10.3390/medicina57060595
APA StyleBajdechi, M., Mihai, C., Scafa-Udriste, A., Cherry, A., Zamfir, D., Dumitru, I., Cernat, R., & Rugina, S. (2021). Severe Coronary Artery Disease in a Person Living with HIV. Medicina, 57(6), 595. https://doi.org/10.3390/medicina57060595






