Shedding the Light on the Natural History of Intracranial Aneurysms: An Updated Overview
Abstract
:1. Introduction
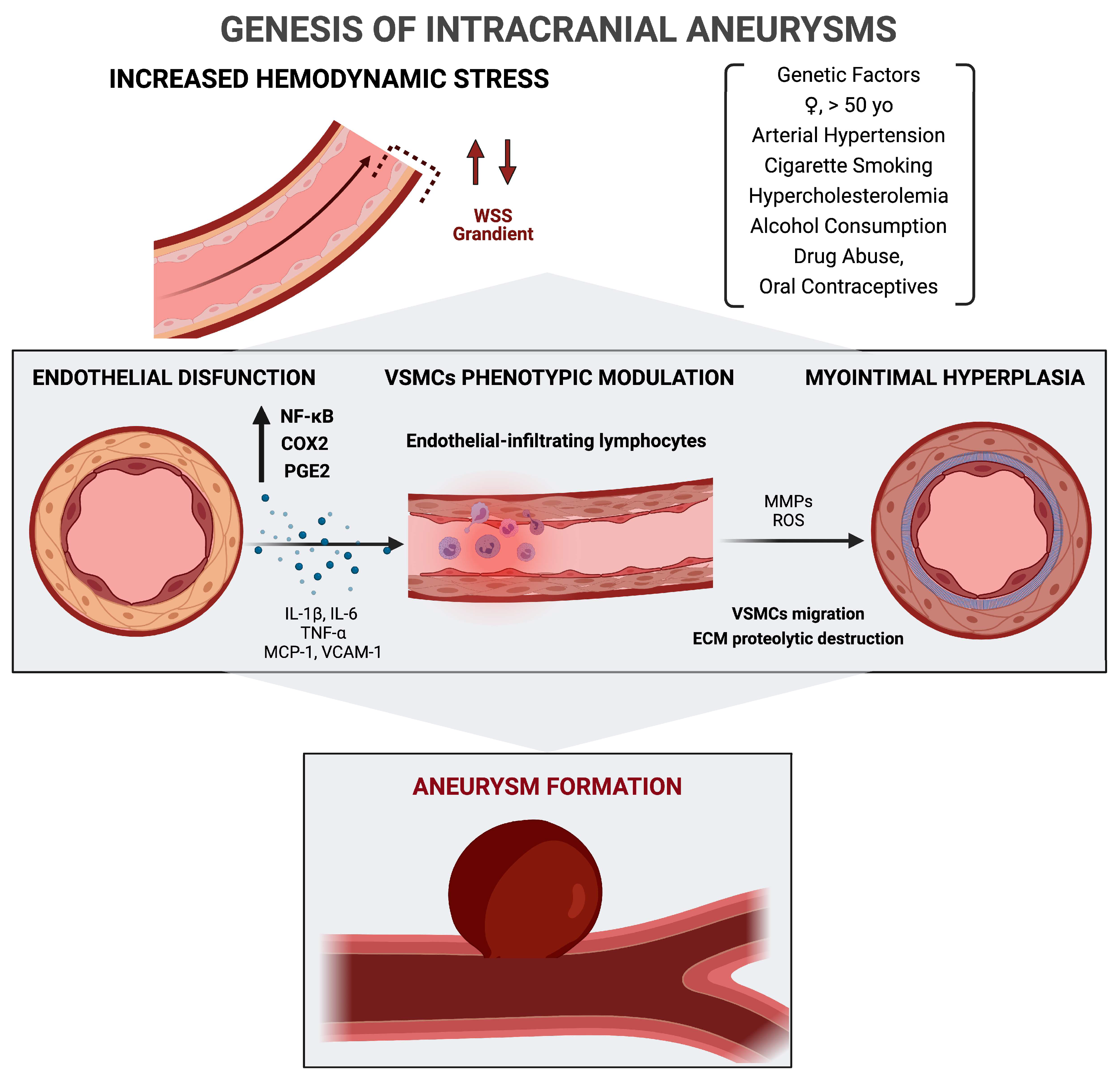
2. Genesis of Intracranial Aneurysms
2.1. Genetic and Extrinsic Risk Factors
2.2. Wall Shear Stress, Endothelial Dysfunction, and Role of Inflammation
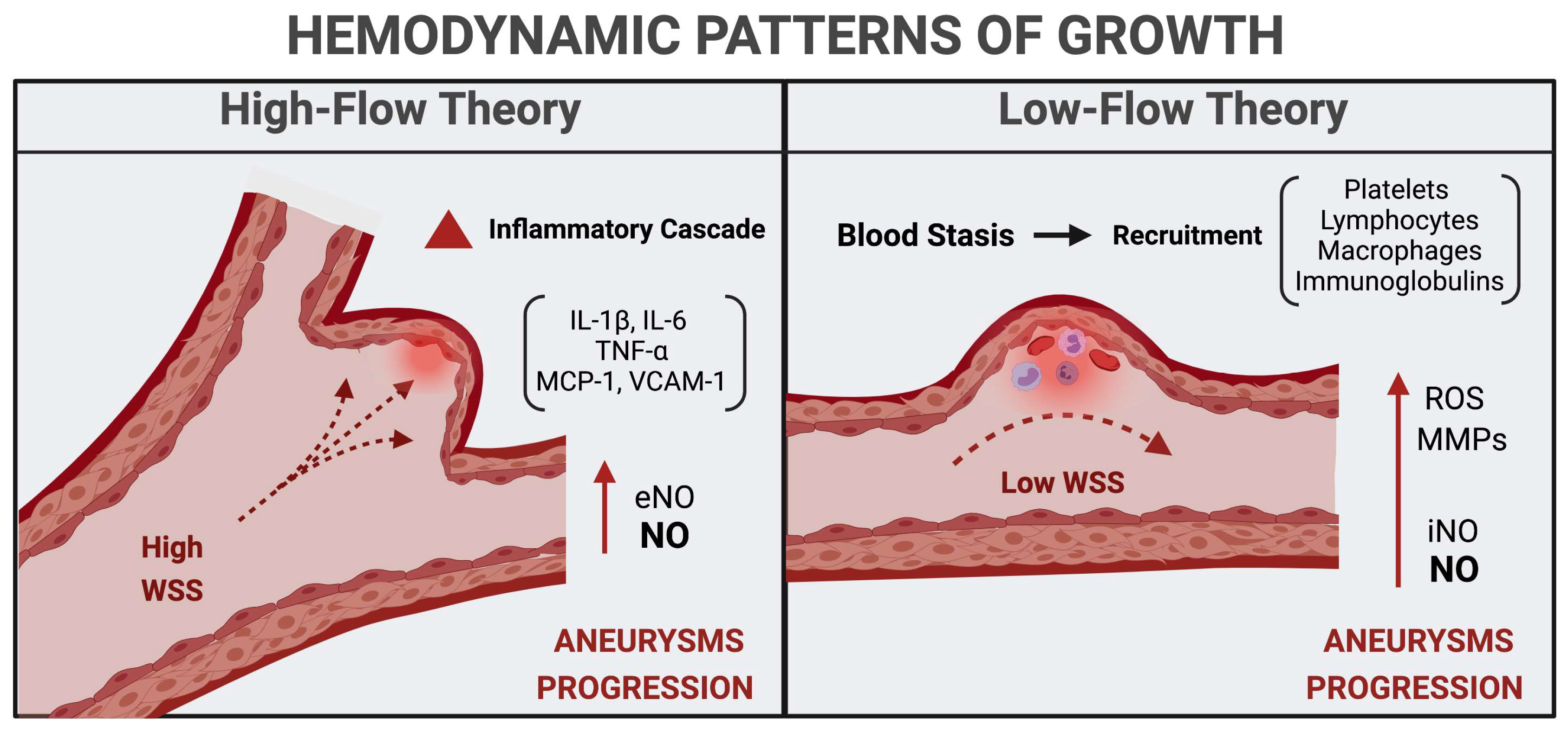
3. Hemodynamic Phenotypes and Patterns of Growth
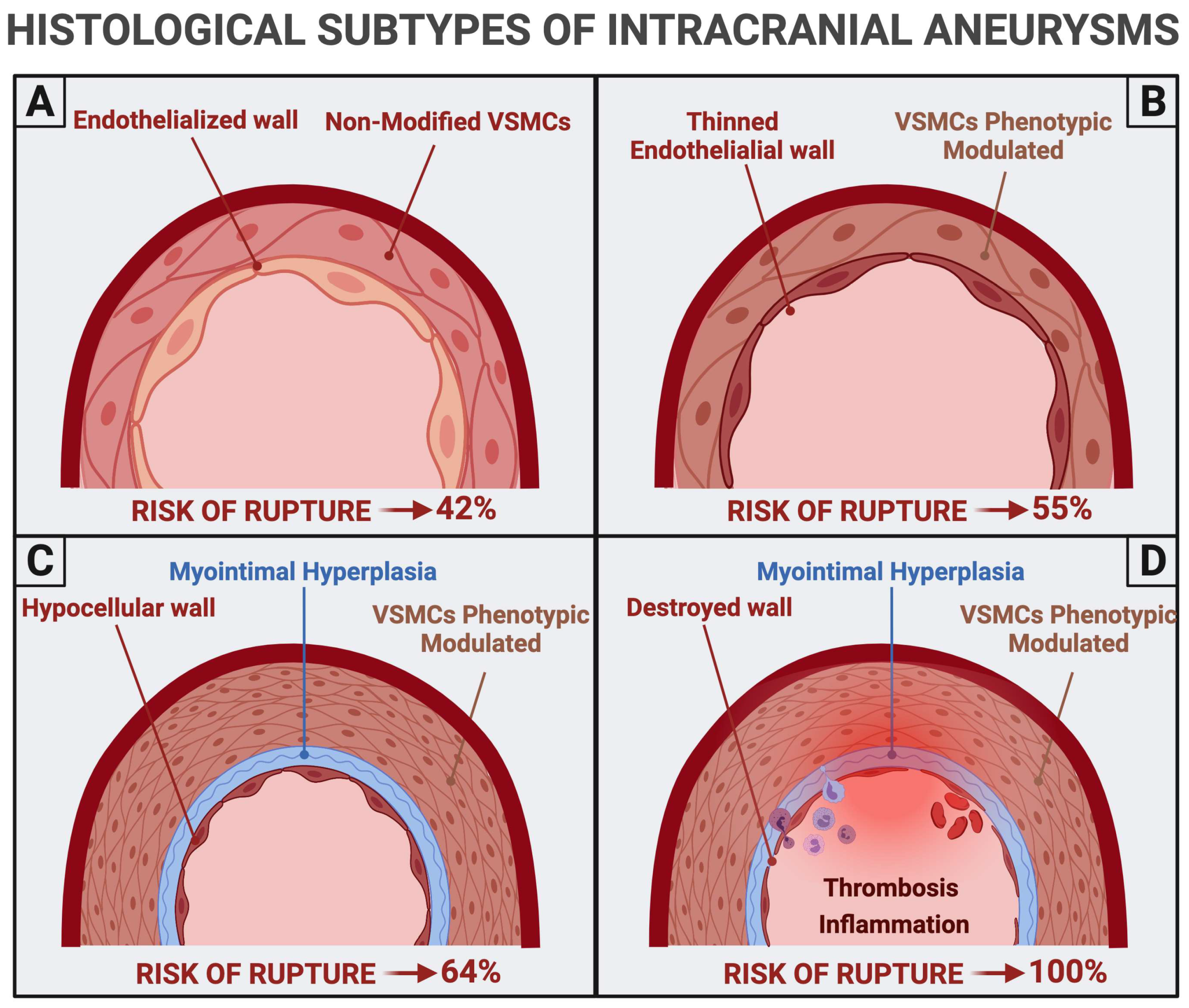
4. Risk of Rupture
4.1. Histopathological Findings
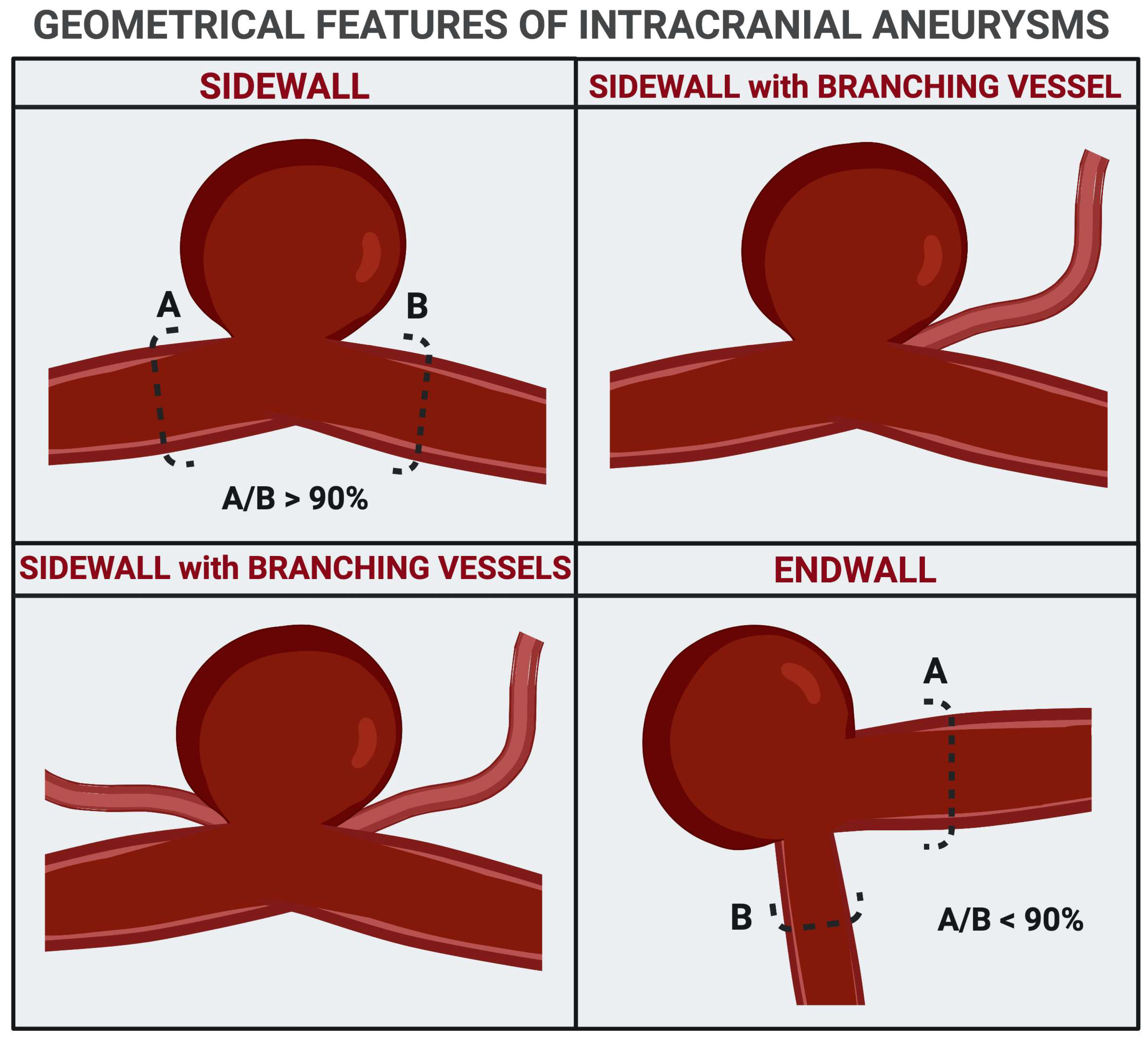
4.2. Size, Angioarchitectural Features, and Intra-Aneurysmal Flow
5. Future Perspectives: Imaging of Inflammation and Target Therapies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juvela, S. Prevalence of and risk factors for intracranial aneurysms. Lancet Neurol. 2011, 10, 595–597. [Google Scholar] [CrossRef]
- Johnston, S.C.; Selvin, S.; Gress, D.R. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology 1998, 50, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J., 3rd; Meissner, I.; Brown, R.D., Jr.; Piepgras, D.G.; Forbes, G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Serrone, J.C.; Maekawa, H.; Tjahjadi, M.; Hernesniemi, J. Aneurysmal subarachnoid hemorrhage: Pathobiology, current treatment and future directions. Expert Rev. Neurother. 2015, 15, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.P.; Sauerbeck, L.R.; Foroud, T.; Huston, J., 3rd; Pankratz, N.; Meissner, I.; Brown, R.D., Jr. The Familial Intracranial Aneurysm (FIA) study protocol. BMC Med. Genet. 2005, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Bromberg, J.E.; Rinkel, G.J.; Algra, A.; van Duyn, C.M.; Greebe, P.; Ramos, L.M.; van Gijn, J. Familial subarachnoid hemorrhage: Distinctive features and patterns of inheritance. Ann. Neurol. 1995, 38, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Struycken, P.M.; Pals, G.; Limburg, M.; Pronk, J.C.; Wijmenga, C.; Pearson, P.L.; Luijten, J.A.; van den Berg, J.S.; Vermeulen, M.; Rinkel, G.J.; et al. Anticipation in familial intracranial aneurysms in consecutive generations. Eur. J. Hum. Genet. 2003, 11, 737–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schievink, W.I. Genetics and aneurysm formation. Neurosurg. Clin. N. Am. 1998, 9, 485–495. [Google Scholar] [CrossRef]
- Schievink, W.I. Genetics of intracranial aneurysms. Neurosurgery 1997, 40, 651–662, discussion 662–653. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, A.; Miettinen, H.; Karkola, K.; Papinaho, S.; Vanninen, R.; Puranen, M.; Hernesniemi, J. Risk of harboring an unruptured intracranial aneurysm. Stroke 1998, 29, 359–362. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Hattori, H.; Yorifuji, T.; Tashiro, Y.; Nakahata, T. Intracranial aneurysms in Ehlers-Danlos syndrome type IV in early childhood. Pediatr. Neurol. 2001, 25, 336–339. [Google Scholar] [CrossRef]
- Olubajo, F.; Kaliaperumal, C.; Choudhari, K.A. Vascular Ehlers-Danlos Syndrome: Literature review and surgical management of intracranial vascular complications. Clin. Neurol. Neurosurg. 2020, 193, 105775. [Google Scholar] [CrossRef]
- Masoumi, A.; Reed-Gitomer, B.; Kelleher, C.; Bekheirnia, M.R.; Schrier, R.W. Developments in the management of autosomal dominant polycystic kidney disease. Ther. Clin. Risk Manag. 2008, 4, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Sanchis, I.M.; Shukoor, S.; Irazabal, M.V.; Madsen, C.D.; Chebib, F.T.; Hogan, M.C.; El-Zoghby, Z.; Harris, P.C.; Huston, J.; Brown, R.D.; et al. Presymptomatic Screening for Intracranial Aneurysms in Patients with Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2019, 14, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Menzies, L.; D’Arco, F.; Ganesan, V.; Hurst, J.A. Intracranial vascular pathology in two further patients with Floating-Harbor syndrome: Proposals for cerebrovascular disease risk management. Eur. J. Med. Genet. 2020, 63, 103785. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, Y.; Kaku, Y.; Nishi, T.; Mukasa, A.; Yamashiro, S. Multiple Cerebral Aneurysms Associated with Neurofibromatosis Type 1. J. Stroke Cerebrovasc. Dis. 2019, 28, e83–e91. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Brinjikji, W.; Kallmes, D.F. Prevalence of Intracranial Aneurysms in Patients with Connective Tissue Diseases: A Retrospective Study. AJNR Am. J. Neuroradiol. 2016, 37, 1422–1426. [Google Scholar] [CrossRef] [Green Version]
- Finney, L.H.; Roberts, T.S.; Anderson, R.E. Giant intracranial aneurysm associated with Marfan’s syndrome. Case report. J. Neurosurg. 1976, 45, 342–347. [Google Scholar] [CrossRef]
- Román, G.; Fisher, M.; Perl, D.P.; Poser, C.M. Neurological manifestations of hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease): Report of 2 cases and review of the literature. Ann. Neurol. 1978, 4, 130–144. [Google Scholar] [CrossRef]
- Schievink, W.I.; Katzmann, J.A.; Piepgras, D.G.; Schaid, D.J. Alpha-1-antitrypsin phenotypes among patients with intracranial aneurysms. J. Neurosurg. 1996, 84, 781–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyesiku, N.M.; Colohan, A.R.; Barrow, D.L.; Reisner, A. Cocaine-induced aneurysmal rupture: An emergent negative factor in the natural history of intracranial aneurysms? Neurosurgery 1993, 32, 518–525, discussion 525–516. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, D.; Zhao, J. The interleukin-6 gene -572G>C promoter polymorphism is related to intracranial aneurysms in Chinese Han nationality. Neurosci. Lett. 2008, 440, 1–3. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Wu, C.; Cao, X.; He, M.; You, C. The interleukin-6-572G/C gene polymorphism and the risk of intracranial aneurysms in a Chinese population. Genet. Test. Mol. Biomark. 2012, 16, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sima, X.; Bai, P.; Zhang, L.; Sun, H.; Liang, W.; Liu, J.; Zhang, L.; Gao, L. Interactions of miR-34b/c and TP53 polymorphisms on the risk of intracranial aneurysm. Clin. Dev. Immunol. 2012, 2012, 567586. [Google Scholar] [CrossRef]
- Alg, V.S.; Sofat, R.; Houlden, H.; Werring, D.J. Genetic risk factors for intracranial aneurysms: A meta-analysis in more than 116,000 individuals. Neurology 2013, 80, 2154–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, E.P.; Kim, B.J.; Kim, C.; Choi, H.J.; Jeon, J.P. Association of SOX17 Gene Polymorphisms and Intracranial Aneurysm: A Case-Control Study and Meta-Analysis. World Neurosurg. 2018, 110, e823–e829. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, I.K.; Ahn, J.S.; Woo, D.C.; Kim, S.T.; Song, S.; Koh, G.Y.; Kim, H.S.; Jeon, B.H.; Kim, I. Deficiency of endothelium-specific transcription factor Sox17 induces intracranial aneurysm. Circulation 2015, 131, 995–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña Silva, R.A.; Kung, D.K.; Mitchell, I.J.; Alenina, N.; Bader, M.; Santos, R.A.; Faraci, F.M.; Heistad, D.D.; Hasan, D.M. Angiotensin 1-7 reduces mortality and rupture of intracranial aneurysms in mice. Hypertension 2014, 64, 362–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caranci, F.; Briganti, F.; Cirillo, L.; Leonardi, M.; Muto, M. Epidemiology and genetics of intracranial aneurysms. Eur. J. Radiol. 2013, 82, 1598–1605. [Google Scholar] [CrossRef]
- Marchese, E.; Vignati, A.; Albanese, A.; Nucci, C.G.; Sabatino, G.; Tirpakova, B.; Lofrese, G.; Zelano, G.; Maira, G. Comparative evaluation of genome-wide gene expression profiles in ruptured and unruptured human intracranial aneurysms. J. Biol. Regul. Homeost. Agents 2010, 24, 185–195. [Google Scholar]
- Pimiento, J.M.; Maloney, S.P.; Tang, P.C.; Muto, A.; Westvik, T.S.; Fitzgerald, T.N.; Fancher, T.T.; Tellides, G.; Dardik, A. Endothelial nitric oxide synthase stimulates aneurysm growth in aged mice. J. Vasc. Res. 2008, 45, 251–258. [Google Scholar] [CrossRef]
- Abruzzo, T.; Kendler, A.; Apkarian, R.; Workman, M.; Khoury, J.C.; Cloft, H.J. Cerebral aneurysm formation in nitric oxide synthase-3 knockout mice. Curr. Neurovasc. Res. 2007, 4, 161–169. [Google Scholar] [CrossRef]
- Katritsis, D.; Kaiktsis, L.; Chaniotis, A.; Pantos, J.; Efstathopoulos, E.P.; Marmarelis, V. Wall shear stress: Theoretical considerations and methods of measurement. Prog. Cardiovasc. Dis. 2007, 49, 307–329. [Google Scholar] [CrossRef]
- Staarmann, B.; Smith, M.; Prestigiacomo, C.J. Shear stress and aneurysms: A review. Neurosurg. Focus 2019, 47, E2. [Google Scholar] [CrossRef] [Green Version]
- Sforza, D.M.; Putman, C.M.; Cebral, J.R. Hemodynamics of Cerebral Aneurysms. Annu. Rev. Fluid Mech. 2009, 41, 91–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frösen, J.; Tulamo, R.; Paetau, A.; Laaksamo, E.; Korja, M.; Laakso, A.; Niemelä, M.; Hernesniemi, J. Saccular intracranial aneurysm: Pathology and mechanisms. Acta Neuropathol. 2012, 123, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Cebral, J.R.; Detmer, F.; Chung, B.J.; Choque-Velasquez, J.; Rezai, B.; Lehto, H.; Tulamo, R.; Hernesniemi, J.; Niemela, M.; Yu, A.; et al. Local Hemodynamic Conditions Associated with Focal Changes in the Intracranial Aneurysm Wall. AJNR Am. J. Neuroradiol. 2019, 40, 510–516. [Google Scholar] [CrossRef]
- Boussel, L.; Rayz, V.; McCulloch, C.; Martin, A.; Acevedo-Bolton, G.; Lawton, M.; Higashida, R.; Smith, W.S.; Young, W.L.; Saloner, D. Aneurysm growth occurs at region of low wall shear stress: Patient-specific correlation of hemodynamics and growth in a longitudinal study. Stroke 2008, 39, 2997–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, N.; Saito, N.; Han, X.; Ohashi, T.; Sato, M. Effect of spatial gradient in fluid shear stress on morphological changes in endothelial cells in response to flow. Biochem. Biophys. Res. Commun. 2010, 395, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Turjman, A.S.; Turjman, F.; Edelman, E.R. Role of fluid dynamics and inflammation in intracranial aneurysm formation. Circulation 2014, 129, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Hasan, D.; Chalouhi, N.; Jabbour, P.; Hashimoto, T. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: Preliminary results. J. Neuroinflamm. 2012, 9, 222. [Google Scholar] [CrossRef] [Green Version]
- Moriwaki, T.; Takagi, Y.; Sadamasa, N.; Aoki, T.; Nozaki, K.; Hashimoto, N. Impaired progression of cerebral aneurysms in interleukin-1beta-deficient mice. Stroke 2006, 37, 900–905. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Morishita, R.; Hashimoto, N. Reduced collagen biosynthesis is the hallmark of cerebral aneurysm: Contribution of interleukin-1beta and nuclear factor-kappaB. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Aoki, T.; Frösen, J.; Fukuda, M.; Bando, K.; Shioi, G.; Tsuji, K.; Ollikainen, E.; Nozaki, K.; Laakkonen, J.; Narumiya, S. Prostaglandin E2-EP2-NF-κB signaling in macrophages as a potential therapeutic target for intracranial aneurysms. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Starke, R.M.; Chalouhi, N.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Wada, K.; Shimada, K.; Hasan, D.M.; Greig, N.H.; et al. Critical role of TNF-α in cerebral aneurysm formation and progression to rupture. J. Neuroinflamm. 2014, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- Mitsui, K.; Ikedo, T.; Kamio, Y.; Furukawa, H.; Lawton, M.T.; Hashimoto, T. TLR4 (Toll-Like Receptor 4) Mediates the Development of Intracranial Aneurysm Rupture. Hypertension 2020, 75, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Starke, R.M.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Owens, G.K.; Koch, W.J.; Greig, N.H.; Dumont, A.S. TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: Implications for cerebral aneurysm pathology. J. Cereb. Blood Flow Metab. 2013, 33, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Frösen, J. Smooth muscle cells and the formation, degeneration, and rupture of saccular intracranial aneurysm wall—A review of current pathophysiological knowledge. Transl. Stroke Res. 2014, 5, 347–356. [Google Scholar] [CrossRef]
- Hao, H.; Gabbiani, G.; Bochaton-Piallat, M.L. Arterial smooth muscle cell heterogeneity: Implications for atherosclerosis and restenosis development. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Tulamo, R.; Frösen, J.; Hernesniemi, J.; Niemelä, M. Inflammatory changes in the aneurysm wall: A review. J. Neurointerv. Surg. 2010, 2, 120–130. [Google Scholar] [CrossRef]
- Song, Y.; Liu, P.; Li, Z.; Shi, Y.; Huang, J.; Li, S.; Liu, Y.; Zhang, Z.; Wang, Y.; Zhu, W.; et al. The Effect of Myosin Light Chain Kinase on the Occurrence and Development of Intracranial Aneurysm. Front. Cell. Neurosci. 2018, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Appelboom, G.; Chapel, D.; Connolly, E.; Goodman, A.; Lopresti, M.; Taylor, B.; Zilinyi, R. Role of the complement cascade in cerebral aneurysm formation, growth, and rupture. Neuroimmunol. Neuroinflamm. 2015, 2, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Chalouhi, N.; Ali, M.S.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. Biology of intracranial aneurysms: Role of inflammation. J. Cereb. Blood Flow Metab. 2012, 32, 1659–1676. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Li, Z.; Song, L.; Han, T.; Feng, Q.; Guo, Y.; Xu, J.; He, M.; You, C. Increased apoptosis and cysteinyl aspartate specific protease-3 gene expression in human intracranial aneurysm. J. Clin. Neurosci. 2007, 14, 550–555. [Google Scholar] [CrossRef]
- Fennell, V.S.; Kalani, M.Y.; Atwal, G.; Martirosyan, N.L.; Spetzler, R.F. Biology of Saccular Cerebral Aneurysms: A Review of Current Understanding and Future Directions. Front. Surg. 2016, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Rui, Y.N.; Hagan, J.P.; Kim, D.H. Intracranial Aneurysms: Pathology, Genetics, and Molecular Mechanisms. Neuromol. Med. 2019, 21, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, H.; Hashimoto, N.; Kang, Y.; Yamazoe, N.; Kikuchi, H.; Yamaguchi, S.; Niimi, H. Cerebral blood flow patterns at major vessel bifurcations and aneurysms in rats. J. Neurosurg. 1991, 74, 258–262. [Google Scholar] [CrossRef]
- Fukuda, S.; Hashimoto, N.; Naritomi, H.; Nagata, I.; Nozaki, K.; Kondo, S.; Kurino, M.; Kikuchi, H. Prevention of rat cerebral aneurysm formation by inhibition of nitric oxide synthase. Circulation 2000, 101, 2532–2538. [Google Scholar] [CrossRef] [Green Version]
- Sho, E.; Sho, M.; Singh, T.M.; Xu, C.; Zarins, C.K.; Masuda, H. Blood flow decrease induces apoptosis of endothelial cells in previously dilated arteries resulting from chronic high blood flow. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1139–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Dehcordi, S.R.; Luzzi, S.; Cimini, A.; Cifone, M.G.; Cinque, B. Involvement of NOS2 Activity on Human Glioma Cell Growth, Clonogenic Potential, and Neurosphere Generation. Int. J. Mol. Sci 2018, 19, 2801. [Google Scholar] [CrossRef] [Green Version]
- Tulamo, R.; Frösen, J.; Junnikkala, S.; Paetau, A.; Pitkäniemi, J.; Kangasniemi, M.; Niemelä, M.; Jääskeläinen, J.; Jokitalo, E.; Karatas, A.; et al. Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery 2006, 59, 1069–1076, discussion 1076–1067. [Google Scholar] [CrossRef]
- Unruptured intracranial aneurysms—Risk of rupture and risks of surgical intervention. N. Engl. J. Med. 1998, 339, 1725–1733. [CrossRef]
- Starke, R.M.; Chalouhi, N.; Ali, M.S.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. The role of oxidative stress in cerebral aneurysm formation and rupture. Curr. Neurovasc. Res. 2013, 10, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, T.; Nishimura, M.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Hashimoto, N. Reactive oxygen species modulate growth of cerebral aneurysms: A study using the free radical scavenger edaravone and p47phox(-/-) mice. Lab. Investig. 2009, 89, 730–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liepsch, D.W. Flow in tubes and arteries—A comparison. Biorheology 1986, 23, 395–433. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.; Bouzourène, K.; Harrison, V.J.; Brunner, H.R.; Hayoz, D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Ollikainen, E.; Tulamo, R.; Kaitainen, S.; Honkanen, P.; Lehti, S.; Liimatainen, T.; Hernesniemi, J.; Niemelä, M.; Kovanen, P.T.; Frösen, J. Macrophage Infiltration in the Saccular Intracranial Aneurysm Wall as a Response to Locally Lysed Erythrocytes That Promote Degeneration. J. Neuropathol. Exp. Neurol. 2018, 77, 890–903. [Google Scholar] [CrossRef]
- Meng, H.; Tutino, V.M.; Xiang, J.; Siddiqui, A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. AJNR Am. J. Neuroradiol. 2014, 35, 1254–1262. [Google Scholar] [CrossRef] [Green Version]
- Texakalidis, P.; Sweid, A.; Mouchtouris, N.; Peterson, E.C.; Sioka, C.; Rangel-Castilla, L.; Reavey-Cantwell, J.; Jabbour, P. Aneurysm Formation, Growth, and Rupture: The Biology and Physics of Cerebral Aneurysms. World Neurosurg. 2019, 130, 277–284. [Google Scholar] [CrossRef]
- Brisman, J.L.; Song, J.K.; Newell, D.W. Cerebral aneurysms. N. Engl. J. Med. 2006, 355, 928–939. [Google Scholar] [CrossRef] [Green Version]
- Niemann, A.; Weigand, S.; Hoffmann, T.; Skalej, M.; Tulamo, R.; Preim, B.; Saalfeld, S. Interactive exploration of a 3D intracranial aneurysm wall model extracted from histologic slices. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 99–107. [Google Scholar] [CrossRef]
- Frösen, J.; Piippo, A.; Paetau, A.; Kangasniemi, M.; Niemelä, M.; Hernesniemi, J.; Jääskeläinen, J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: Histological analysis of 24 unruptured and 42 ruptured cases. Stroke 2004, 35, 2287–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, A.; Kirino, T.; Hashi, K.; Aoki, N.; Fukuhara, S.; Hashimoto, N.; Nakayama, T.; Sakai, M.; Teramoto, A.; Tominari, S.; et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N. Engl. J. Med. 2012, 366, 2474–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, T.; Timofeev, E.V.; Saito, T.; Shimizu, H.; Ezura, M.; Matsumoto, Y.; Takayama, K.; Tominaga, T.; Takahashi, A. A proposed parent vessel geometry-based categorization of saccular intracranial aneurysms: Computational flow dynamics analysis of the risk factors for lesion rupture. J. Neurosurg. 2005, 103, 662–680. [Google Scholar] [CrossRef]
- Ujiie, H.; Tachibana, H.; Hiramatsu, O.; Hazel, A.L.; Matsumoto, T.; Ogasawara, Y.; Nakajima, H.; Hori, T.; Takakura, K.; Kajiya, F. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: A possible index for surgical treatment of intracranial aneurysms. Neurosurgery 1999, 45, 119–129, discussion 129–130. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, S.K.; Humphrey, J.D. Influence of size, shape and properties on the mechanics of axisymmetric saccular aneurysms. J. Biomech. 1996, 29, 1015–1022. [Google Scholar] [CrossRef]
- Weir, B.; Disney, L.; Karrison, T. Sizes of ruptured and unruptured aneurysms in relation to their sites and the ages of patients. J. Neurosurg. 2002, 96, 64–70. [Google Scholar] [CrossRef]
- Peiffer, V.; Sherwin, S.J.; Weinberg, P.D. Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc. Res. 2013, 99, 242–250. [Google Scholar] [CrossRef]
- Samaniego, E.A.; Roa, J.A.; Hasan, D. Vessel wall imaging in intracranial aneurysms. J. Neurointerv. Surg. 2019, 11, 1105–1112. [Google Scholar] [CrossRef] [Green Version]
- Niemelä, M.; Frösen, J.; Hernesniemi, J.; Dashti, R.; Palotie, A. Molecular pathology of aneurysms. Surg. Neurol. 2008, 70, 36–38. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, K.; Taneda, M.; Asai, T.; Kinoshita, A.; Ito, M.; Kuroda, R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke 1999, 30, 1396–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tateshima, S.; Viñuela, F.; Villablanca, J.P.; Murayama, Y.; Morino, T.; Nomura, K.; Tanishita, K. Three-dimensional blood flow analysis in a wide-necked internal carotid artery-ophthalmic artery aneurysm. J. Neurosurg. 2003, 99, 526–533. [Google Scholar] [CrossRef]
- Duan, Z.; Li, Y.; Guan, S.; Ma, C.; Han, Y.; Ren, X.; Wei, L.; Li, W.; Lou, J.; Yang, Z. Morphological parameters and anatomical locations associated with rupture status of small intracranial aneurysms. Sci Rep. 2018, 8, 6440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, A.; Di Vitantonio, H.; De Paulis, D.; Del Maestro, M.; Raysi, S.D.; Murrone, D.; Luzzi, S.; Galzio, R.J. Cortical aneurysms of the middle cerebral artery: A review of the literature. Surg. Neurol. Int. 2017, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S.; Poussa, K.; Lehto, H.; Porras, M. Natural history of unruptured intracranial aneurysms: A long-term follow-up study. Stroke 2013, 44, 2414–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murayama, Y.; Takao, H.; Ishibashi, T.; Saguchi, T.; Ebara, M.; Yuki, I.; Arakawa, H.; Irie, K.; Urashima, M.; Molyneux, A.J. Risk Analysis of Unruptured Intracranial Aneurysms: Prospective 10-Year Cohort Study. Stroke 2016, 47, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Roessler, K.; Cejna, M.; Zachenhofer, I. Aneurysmatic subarachnoidal haemorrhage: Incidence and location of small ruptured cerebral aneurysms—A retrospective population-based study. Wien. Klin. Wochenschr. 2011, 123, 444–449. [Google Scholar] [CrossRef]
- Greving, J.P.; Wermer, M.J.; Brown, R.D., Jr.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Neyazi, B.; Sandalcioglu, I.E.; Maslehaty, H. Evaluation of the risk of rupture of intracranial aneurysms in patients with aneurysmal subarachnoid hemorrhage according to the PHASES score. Neurosurg. Rev. 2019, 42, 489–492. [Google Scholar] [CrossRef]
- Texakalidis, P.; Hilditch, C.A.; Lehman, V.; Lanzino, G.; Pereira, V.M.; Brinjikji, W. Vessel Wall Imaging of Intracranial Aneurysms: Systematic Review and Meta-analysis. World Neurosurg. 2018, 117, 453–458.e451. [Google Scholar] [CrossRef]
- Hasan, D.M.; Mahaney, K.B.; Magnotta, V.A.; Kung, D.K.; Lawton, M.T.; Hashimoto, T.; Winn, H.R.; Saloner, D.; Martin, A.; Gahramanov, S.; et al. Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: A pilot study. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1032–1038. [Google Scholar] [CrossRef] [Green Version]
- Vasanawala, S.S.; Nguyen, K.L.; Hope, M.D.; Bridges, M.D.; Hope, T.A.; Reeder, S.B.; Bashir, M.R. Safety and technique of ferumoxytol administration for MRI. Magn. Reason. Med. 2016, 75, 2107–2111. [Google Scholar] [CrossRef] [Green Version]
- Campanella, R.; Guarnaccia, L.; Caroli, M.; Zarino, B.; Carrabba, G.; La Verde, N.; Gaudino, C.; Rampini, A.; Luzzi, S.; Riboni, L.; et al. Personalized and translational approach for malignant brain tumors in the era of precision medicine: The strategic contribution of an experienced neurosurgery laboratory in a modern neurosurgery and neuro-oncology department. J. Neurol. Sci. 2020, 417, 117083. [Google Scholar] [CrossRef]
- Luzzi, S.; Crovace, A.M.; Del Maestro, M.; Giotta Lucifero, A.; Elbabaa, S.K.; Cinque, B.; Palumbo, P.; Lombardi, F.; Cimini, A.; Cifone, M.G.; et al. The cell-based approach in neurosurgery: Ongoing trends and future perspectives. Heliyon 2019, 5, e02818. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Guarracino, C.; Mosconi, M.; Foiadelli, T.; Savasta, S. Gene therapies for high-grade gliomas: From the bench to the bedside. Acta Biomed. 2020, 91, 32–50. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Schena, L.; Mosconi, M.; Foiadelli, T.; Savasta, S. Potential roads for reaching the summit: An overview on target therapies for high-grade gliomas. Acta Biomed. 2020, 91, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Trabatti, C.; Mosconi, M.; Savasta, S.; Foiadelli, T. Innovative therapies for malignant brain tumors: The road to a tailored cure. Acta Biomed. 2020, 91, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Giotta Lucifero, A.; Brambilla, I.; Magistrali, M.; Mosconi, M.; Savasta, S.; Foiadelli, T. Adoptive immunotherapies in neuro-oncology: Classification, recent advances, and translational challenges. Acta Biomed. 2020, 91, 18–31. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S. Against the Resilience of High-Grade Gliomas: The Immunotherapeutic Approach (Part I). Brain Sci. 2021, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Hasan, D.M.; Mahaney, K.B.; Brown, R.D., Jr.; Meissner, I.; Piepgras, D.G.; Huston, J.; Capuano, A.W.; Torner, J.C. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke 2011, 42, 3156–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, Y.; Kitazato, K.T.; Yagi, K.; Shimada, K.; Matsushita, N.; Kinouchi, T.; Kanematsu, Y.; Satomi, J.; Kageji, T.; Nagahiro, S. Statins promote the growth of experimentally induced cerebral aneurysms in estrogen-deficient rats. Stroke 2011, 42, 2286–2293. [Google Scholar] [CrossRef]
- Aoki, T.; Nishimura, M.; Kataoka, H.; Ishibashi, R.; Miyake, T.; Takagi, Y.; Morishita, R.; Hashimoto, N. Role of angiotensin II type 1 receptor in cerebral aneurysm formation in rats. Int. J. Mol. Med. 2009, 24, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Fisher, C.L.; Demel, S.L. Nonsteroidal Anti-Inflammatory Drugs: A Potential Pharmacological Treatment for Intracranial Aneurysm. Cerebrovasc. Dis. Extra 2019, 9, 31–45. [Google Scholar] [CrossRef]
- Suzuki, T.; Kamio, Y.; Makino, H.; Hokamura, K.; Kimura, T.; Yamasaki, T.; Hiramatsu, H.; Umemura, K.; Namba, H. Prevention Effect of Antiplatelets on Aneurysm Rupture in a Mouse Intracranial Aneurysm Model. Cerebrovasc. Dis. 2018, 45, 180–186. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Shiraya, S.; Miyake, T.; Yamakawa, S.; Aoki, M.; Makino, H.; Nishimura, M.; Morishita, R. Inhibition of experimental abdominal aortic aneurysm in a rat model by the angiotensin receptor blocker valsartan. Int. J. Mol. Med. 2008, 22, 703–708. [Google Scholar]
- Ishibashi, R.; Aoki, T.; Nishimura, M.; Hashimoto, N.; Miyamoto, S. Contribution of mast cells to cerebral aneurysm formation. Curr. Neurovasc. Res. 2010, 7, 113–124. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giotta Lucifero, A.; Baldoncini, M.; Bruno, N.; Galzio, R.; Hernesniemi, J.; Luzzi, S. Shedding the Light on the Natural History of Intracranial Aneurysms: An Updated Overview. Medicina 2021, 57, 742. https://doi.org/10.3390/medicina57080742
Giotta Lucifero A, Baldoncini M, Bruno N, Galzio R, Hernesniemi J, Luzzi S. Shedding the Light on the Natural History of Intracranial Aneurysms: An Updated Overview. Medicina. 2021; 57(8):742. https://doi.org/10.3390/medicina57080742
Chicago/Turabian StyleGiotta Lucifero, Alice, Matías Baldoncini, Nunzio Bruno, Renato Galzio, Juha Hernesniemi, and Sabino Luzzi. 2021. "Shedding the Light on the Natural History of Intracranial Aneurysms: An Updated Overview" Medicina 57, no. 8: 742. https://doi.org/10.3390/medicina57080742
APA StyleGiotta Lucifero, A., Baldoncini, M., Bruno, N., Galzio, R., Hernesniemi, J., & Luzzi, S. (2021). Shedding the Light on the Natural History of Intracranial Aneurysms: An Updated Overview. Medicina, 57(8), 742. https://doi.org/10.3390/medicina57080742