Gray Ramus Communicans Nerve Block for Acute Pain Control in Vertebral Compression Fracture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Patient Population
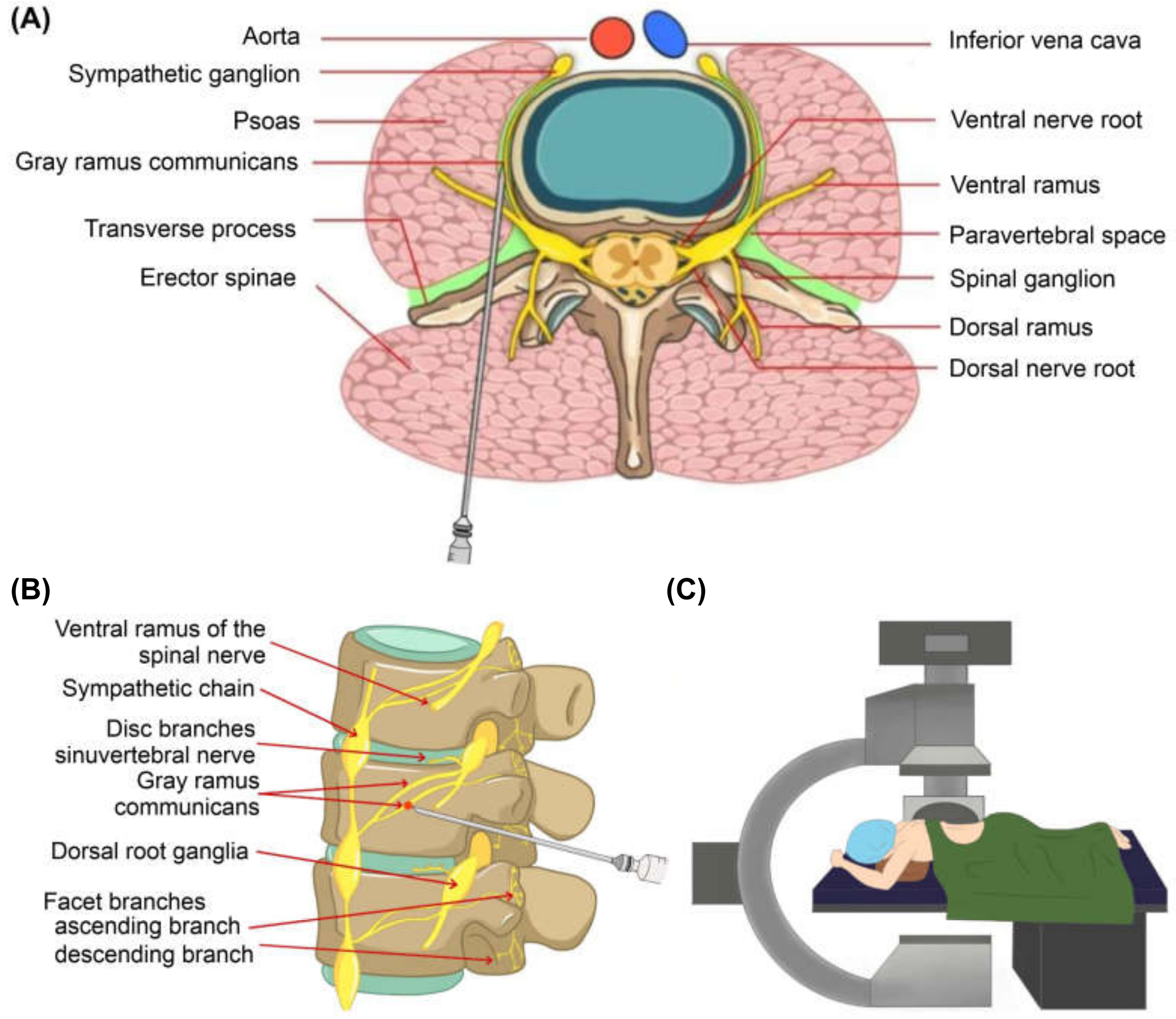
2.2. Gray Ramus Nerve Block
2.3. Outcome Assessment and Evaluation of Risk Factors for Pain Control Failure
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. The Changes in Pain and Functional Outcome Overtime after GRNB
3.3. Risk Factor Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Melton, L.J., III; Thamer, M.; Ray, N.F.; Chan, J.K.; Chesnut, C.H., III; Einhorn, T.A.; Johnston, C.C.; Raisz, L.G.; Silverman, S.L.; Siris, E.S. Fractures attributable to osteoporosis: Report from the National Osteoporosis Foundation. J. Bone Miner. Res. 1997, 12, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P. Vertebral Fracture: Diagnostic Difficulties of a Major Medical Problem. J. Bone Miner. Res. 2018, 33, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballane, G.; Cauley, J.A.; Luckey, M.M.; Fuleihan, G.E.H. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos. Int. 2017, 28, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J. The burden of osteoporosis. J. Endocrinol. Investig. 1999, 22, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, R.; Osborne, R.H.; Ebeling, P.R.; Wark, J.D.; Mitchell, P.; Wriedt, C.; Graves, S.; Staples, M.P.; Murphy, B. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N. Engl. J. Med. 2009, 361, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, I.; Dudeney, S.; Reinhardt, M.-K.; Bell, G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine 2001, 26, 1631–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, N.B.; Harris, S.; Genant, H.K. Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos. Int. 2001, 12, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.; Gomersall, T.; Lloyd Jones, M.; Rawdin, A.; Hernández, M.; Dias, S.; Wilson, D.; Rees, A. Percutaneous vertebroplasty and percutaneous balloon kyphoplasty for the treatment of osteoporotic vertebral fractures: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2014, 18, 1–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Ju, C.I.; Lee, S.M.; Shin, H. Radiofrequency Neurotomy of the Gray Ramus Communicans for Lumbar Osteoporotic Compression Fracture. J. Korean Neurosurg. Soc. 2007, 41, 7–10. [Google Scholar]
- Jang, J.S.; Kwon, H.K.; Lee, J.J.; Hwang, S.M.; Lim, S.Y. Rami communicans nerve block for the treatment of symptomatic schmorl’s nodes—A case report. Korean J. Pain 2010, 23, 262. [Google Scholar] [CrossRef]
- Chandler, G.; Dalley, G.; Hemmer, J.; Seely, T. Gray ramus communicans nerve block: Novel treatment approach for painful osteoporotic vertebral compression fracture. South. Med. J. 2001, 94, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Suseki, K.; Takahashi, Y.; Takahashi, K.; Chiba, T.; Yamagata, M.; Moriya, H. Sensory nerve fibres from lumbar intervertebral discs pass through rami communicantes: A possible pathway for discogenic low back pain. J. Bone Jt. Surg. Br. Vol. 1998, 80, 737–742. [Google Scholar] [CrossRef]
- Vanaclocha, V.; Saiz Sapena, N.; Rivera, M.; Herrera, J.M.; Ortiz-Criado, J.M.; Monzó-Blasco, A.; Guijarro-Jorge, R.; Vanaclocha, L. Selective block of grey communicantes in upper thoracic sympathectomy. A feasibility study on human cadaveric specimens. Br. J. Neurosurg. 2020, 34, 362–369. [Google Scholar] [CrossRef]
- Tae, H.S.; Kim, S.D.; Park, J.Y.; Kim, S.H.; Lim, D.J.; Suh, J.K. Gray ramus communicans nerve block: A useful therapeutic adjuvant for painful osteoporotic vertebral compression fracture. J. Korean Neurosurg. Soc. 2003, 34, 505–508. [Google Scholar]
- Chandler, G.; Dalley, G.; Hemmer, J.; Seely, T. Comparison of Thoracic versus Lumbar Gray Ramus Communicans Nerve Block in the treatment of painful osteoporotic vertebral compression fracture. Pain Physician 2000, 3, 240. [Google Scholar] [CrossRef]
- Choi, S.E.; Suh, J.W.; Hwang, S.M.; Lim, S.Y.; Shin, K.M. Clinical Efficacy of Ramus Communicans Nerve Block in Patient with Vertebral Compression Fracture. J. Korean Pain Soc. 2004, 17, 175–179. [Google Scholar] [CrossRef]


| Total | Failure Group | Success Group | |||||
|---|---|---|---|---|---|---|---|
| n = 63 | n = 14 | n = 49 | p-Value | ||||
| Gender (male) | 24 | 38% | 3 | 21% | 21 | 43% | 0.1454 |
| Age | 66.19 | 15.17 | 72.86 | 7.77 | 64.29 | 16.41 | 0.0088 |
| Job, yes | 20 | 31% | 1 | 7% | 19 | 39% | 0.0267 |
| Post-secondary education | 21 | 33% | 2 | 14% | 19 | 39% | 0.0879 |
| Smoke, yes | 7 | 11% | 1 | 7% | 6 | 12% | 1.0000 |
| Alcoholism, yes | 16 | 25% | 3 | 21% | 13 | 26% | 0.6989 |
| HTN | 27 | 42% | 8 | 57% | 19 | 39% | 0. 2207 |
| DM | 8 | 13% | 1 | 7% | 7 | 14% | 0.6714 |
| Kummell | 2 | 3% | 0 | 0% | 2 | 4% | 1.0000 |
| BMI | 24.06 | 3.61 | 23.64 | 2.26 | 24.18 | 3.95 | 0.5275 |
| BMD | −2.12 | 1.42 | −3.31 | 0.93 | −1.79 | 1.38 | 0.0003 |
| Duration to GRNB after fracture, days | 4.6 | 4.15 | 4.79 | 2.81 | 4.55 | 4.51 | 0.8559 |
| Back pain duration, days | 4.04 | 4.33 | 3.64 | 3.15 | 4.16 | 4.66 | 0.6976 |
| TLICS | 2.92 | 3.15 | 3.29 | 3.28 | 2.82 | 1.48 | 0.2932 |
| VAS n = 49 | VAS at Rest n = 49 | ODI n = 49 | RDQ n = 49 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Mean | SE | p-Value a | p-Value b | Mean | SE | p-Value a | p-Value b | Mean | SE | p-Value a | p-Value b | Mean | SE | p-Value a | p-Value b |
| 0 | 7.88 | 0.31 | <0.0001 | 4.37 | 0.38 | <0.0001 | 77.95% | 18.28% | <0.0001 | 19.59 | 3.57 | <0.0001 | ||||
| 3 | 4.94 | 0.36 | 2.27 | 0.32 | 60.80% | 18.65% | 18.24 | 3.70 | ||||||||
| 14 | 4.06 | 0.35 | 1.73 | 0.29 | 48.41% | 21.10% | 15.59 | 4.34 | ||||||||
| 30 | 2.86 | 0.29 | 1.24 | 0.21 | 40.37% | 20.27% | 13.35 | 5.06 | ||||||||
| 90 | 2.39 | 0.28 | 1.24 | 0.19 | 32.49% | 22.82% | 10.69 | 5.92 | ||||||||
| 180 | 2.12 | 0.31 | 1.06 | 0.22 | 22.73% | 16.08% | 9.18 | 5.45 | ||||||||
| diff 3 vs. 0 | 2.94 | 0.38 | <0.0001 | 2.10 | 0.40 | <0.0001 | 17.15% | 2.58 | <0.0001 | 1.35 | 0.68 | 0.05 | ||||
| diff 14 vs. 0 | 3.82 | 0.41 | <0.0001 | 2.63 | 0.42 | <0.0001 | 29.54% | 3.96 | <0.0001 | 4.00 | 0.79 | <0.0001 | ||||
| diff 30 vs. 0 | 5.02 | 0.41 | <0.0001 | 3.12 | 0.42 | <0.0001 | 37.58% | 3.98 | <0.0001 | 6.25 | 0.84 | <0.0001 | ||||
| diff 90 vs. 0 | 5.49 | 0.42 | <0.0001 | 3.12 | 0.43 | <0.0001 | 45.46% | 4.23 | <0.0001 | 8.90 | 0.91 | <0.0001 | ||||
| diff 180 vs. 0 | 5.76 | 0.44 | <0.0001 | 3.31 | 0.45 | <0.0001 | 55.21% | 3.46 | <0.0001 | 10.41 | 0.87 | <0.0001 | ||||
| diff 14 vs. 3 | 0.88 | 0.42 | 0.041 | 0.53 | 0.45 | 0.24 | 12.39% | 3.27 | <0.0001 | 2.65 | 0.73 | 0.00 | ||||
| diff 30 vs. 3 | 2.08 | 0.40 | <0.0001 | 1.02 | 0.40 | 0.01 | 20.43% | 3.29 | <0.0001 | 4.90 | 0.81 | <0.0001 | ||||
| diff 90 vs. 3 | 2.55 | 0.42 | <0.0001 | 1.02 | 0.34 | 0.00 | 28.31% | 3.51 | <0.0001 | 7.55 | 0.96 | <0.0001 | ||||
| diff 180 vs. 3 | 2.82 | 0.46 | <0.0001 | 1.20 | 0.37 | 0.00 | 38.06% | 2.99 | <0.0001 | 9.06 | 0.92 | <0.0001 | ||||
| diff 30 vs. 14 | 1.20 | 0.27 | <0.0001 | 0.49 | 0.25 | 0.05 | 8.04% | 2.62 | 0.00 | 2.25 | 0.58 | <0.0001 | ||||
| diff 90 vs.14 | 1.67 | 0.28 | <0.0001 | 0.49 | 0.29 | 0.10 | 15.92% | 2.59 | <0.0001 | 4.90 | 0.69 | <0.0001 | ||||
| diff 180 vs. 14 | 1.94 | 0.35 | <0.0001 | 0.67 | 0.32 | 0.04 | 25.67% | 2.56 | <0.0001 | 6.41 | 0.72 | <0.0001 | ||||
| diff 90 vs. 30 | 0.47 | 0.20 | 0.02 | 0.00 | 0.15 | 1.00 | 7.88% | 2.41 | 0.00 | 2.65 | 0.53 | <0.0001 | ||||
| diff 180 vs. 30 | 0.74 | 0.27 | 0.01 | 0.18 | 0.20 | 0.36 | 17.63% | 2.69 | <0.0001 | 4.16 | 0.65 | <0.0001 | ||||
| diff 180 vs. 90 | 0.27 | 0.19 | 0.17 | 0.18 | 0.12 | 0.14 | 9.76% | 2.19 | <0.0001 | 1.51 | 0.44 | 0.00 | ||||
| Univariate Model | Multivariate Model + Variable Selection | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||||||||
| Failure Group n = 14 | Success Group n = 49 | OR | Lower | Upper | p-Value | OR | Lower | Upper | p-Value | ||||
| Gender | M | 3 | 78.57 | 21 | 57.14 | 2.75 | 0.68 | 11.11 | 0.1556 | ||||
| F | 11 | 21.43 | 28 | 42.86 | |||||||||
| Smoke | No | 13 | 92.86 | 43 | 87.76 | ||||||||
| Yes | 1 | 7.14 | 6 | 12.24 | 1.81 | 0.20 | 16.47 | 0.5967 | |||||
| Alcoholism | No | 11 | 78.57 | 36 | 73.47 | ||||||||
| Yes | 3 | 21.43 | 13 | 26.53 | 1.32 | 0.32 | 5.51 | 0.6996 | |||||
| HTN | Yes | 8 | 57.14 | 19 | 38.78 | 0.48 | 0.14 | 1.58 | 0.2257 | ||||
| DM | Yes | 1 | 7.14 | 7 | 14.29 | 2.17 | 0.24 | 19.28 | 0.4881 | ||||
| History of osteoporosis | Yes | 2 | 14.29 | 3 | 6.12 | 0.39 | 0.06 | 2.61 | 0.3328 | ||||
| Steroid use | Yes | 0 | 0.00 | 0 | 0.00 | - | |||||||
| Kummell | Yes | 0 | 0.00 | 2 | 4.08 | 1.53 | 0.04 | 66.01 | 0.8256 | ||||
| Age | 72.86 | 7.77 | 64.29 | 16.41 | 0.96 | 0.91 | 1.00 | 0.0721 | 0.97 | 0.94 | 1.01 | 0.0972 | |
| BMI | 23.64 | 2.35 | 24.18 | 3.95 | 1.04 | 0.88 | 1.24 | 0.6243 | |||||
| BMD | −3.31 | 0.97 | −1.79 | 1.38 | 2.80 | 1.46 | 5.39 | 0.0020 | 2.67 | 1.37 | 5.18 | 0.0038 | |
| TLICS | 3.29 | 1.38 | 2.82 | 1.48 | 0.81 | 0.54 | 1.20 | 0.2907 | |||||
| Number | Patient | Intervention Regimen | Intervention | Control | Outcome | Follow Up Period | Complication | |
|---|---|---|---|---|---|---|---|---|
| Our study | 63 | acute fracture, within 3 d after fracture (T10~L5) | 2% bupivacaine 5 mg Dexamethasone | GNRB | None | VAS, motion 7.88 at pre 4.93 at 3 days 4.61 at 14 days 2.857 at 90 day sResting VAS ODI RDQ | Before And after 3,14, 30, 90, 180 days | No infection No vessel leak |
| Chandler 2001 [12] | 52 | After conservative analgesic therapy (TL) | 2% lidocaine 2% triamcinolone | GNRB | None | VAS 10 92% at least 1 63% at least 4 | Before And after | No report |
| SW kim 2007 [10] | 22 | less 2 weeks after trauma (L1-4) | RF | RF | None | VAS 7.8 at pre 2.6 at 48 h 2.8 at 90 d (p < 0.005) Modified Macnab 12-6-2-2 at E-G-F-P | At least, 4 month | No significant complication |
| HS Tae 2003 [15] | 36 | after failure conservative treatment for 4 weeks or vertebroplasty | 2% lidocaine 5 mg Dexamethasone 40 mg Methylprednisolone acetate | GNRB | None | VAS 9.2 at pre 0~3 (80.5%), 4~6 (13,9%) 6< (5.6%) at 24 h0~3 (52.9%) 4~6 (35.3%) 6< (11.8%) at from 4 to 12.5 months. | At least, 4 month | No procedure related complication |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.-Y.; Choi, I.; Kim, T.-G.; Kim, W.-J.; Shin, I.-Y.; Khil, E.-K. Gray Ramus Communicans Nerve Block for Acute Pain Control in Vertebral Compression Fracture. Medicina 2021, 57, 744. https://doi.org/10.3390/medicina57080744
Park D-Y, Choi I, Kim T-G, Kim W-J, Shin I-Y, Khil E-K. Gray Ramus Communicans Nerve Block for Acute Pain Control in Vertebral Compression Fracture. Medicina. 2021; 57(8):744. https://doi.org/10.3390/medicina57080744
Chicago/Turabian StylePark, Dou-Young, Il Choi, Tae-Gyum Kim, Woo-Jae Kim, Il-Young Shin, and Eun-Kyung Khil. 2021. "Gray Ramus Communicans Nerve Block for Acute Pain Control in Vertebral Compression Fracture" Medicina 57, no. 8: 744. https://doi.org/10.3390/medicina57080744
APA StylePark, D.-Y., Choi, I., Kim, T.-G., Kim, W.-J., Shin, I.-Y., & Khil, E.-K. (2021). Gray Ramus Communicans Nerve Block for Acute Pain Control in Vertebral Compression Fracture. Medicina, 57(8), 744. https://doi.org/10.3390/medicina57080744






