Connections between Different Sports and Ergogenic Aids—Focusing on Salivary Cortisol and Amylase
Abstract
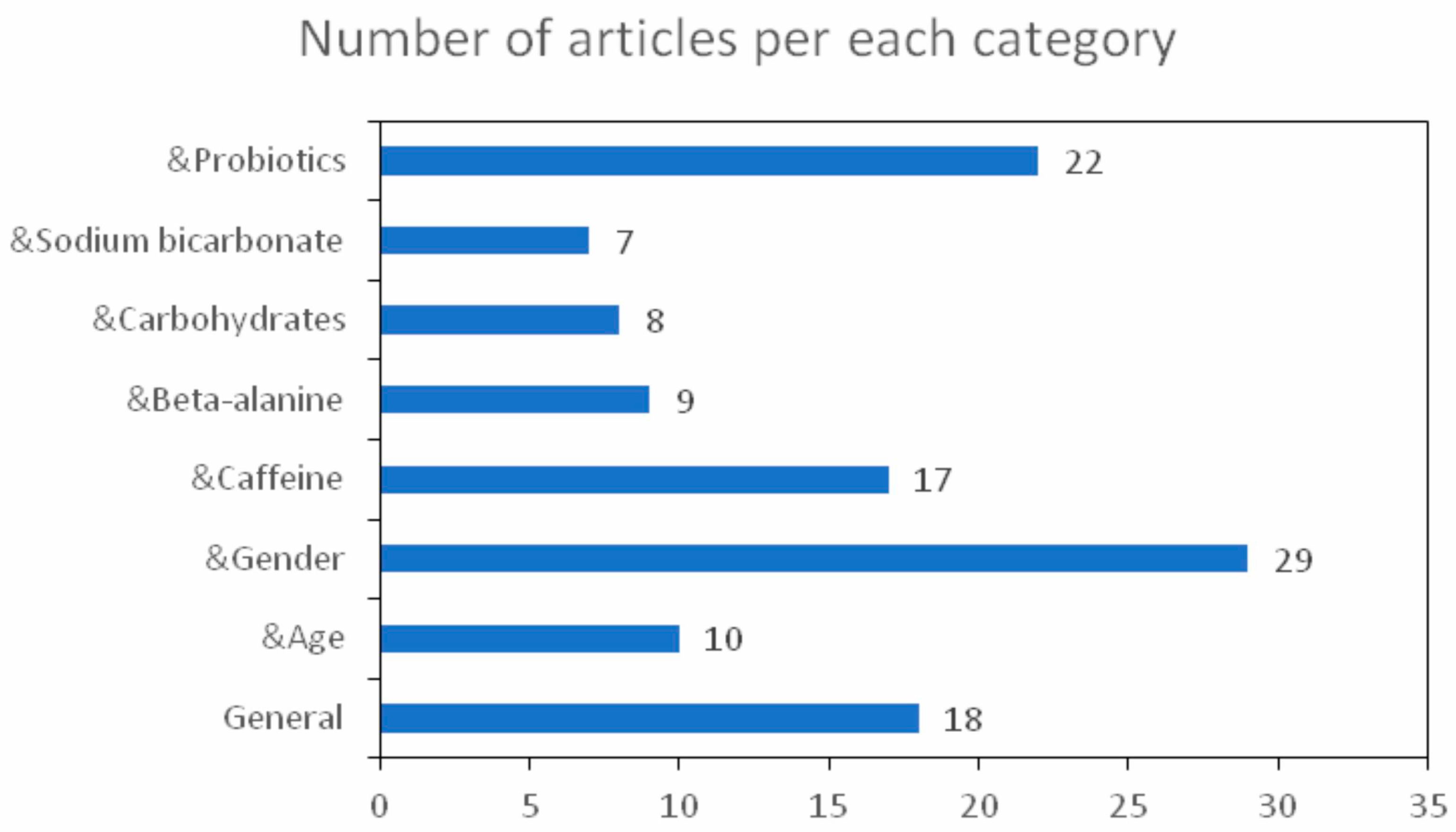
:1. Introduction
2. Methods
3. Results
4. Demographic Groups and Sports
4.1. Age
4.2. Biological Sex
5. Ergogenic Supplements
5.1. Caffeine
5.2. β-Alanine
5.3. Carbohydrates
5.4. Sodium Bicarbonate
5.5. Probiotics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rutherfurd-Markwick, K.; Starck, C.; Dulson, D.K.; Ali, A. Salivary diagnostic markers in males and females during rest and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azarbayjani, M.A.; Fatolahi, H.; Rasaee, M.J.; Peeri, M.; Babaei, R. The effect of exercise mode and intensity of sub-maximal physical activities on salivary testosterone to cortisol ratio and α-amylase in young active males. Int. J. Exerc. Sci. 2011, 4, 283–293. [Google Scholar]
- Cozma, S.; Dima-Cozma, L.; Ghiciuc, C.; Pasquali, V.; Saponaro, A.; Patacchioli, F. Salivary cortisol and α-amylase: Subclinical indicators of stress as cardiometabolic risk. Braz. J. Med. Biol. Res. 2017, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, B.T.; Stanton, W.; Lord, R.; Petocz, P.; Pepping, G.-J. Factors Affecting Measurement of Salivary Cortisol and Secretory Immunoglobulin A in Field Studies of Athletes. Front. Endocrinol. 2017, 8, 168. [Google Scholar] [CrossRef] [Green Version]
- Ducker, K.J.; Lines, R.L.; Chapman, M.T.; Peeling, P.; McKay, A.K.; Gucciardi, D.F. Validity and reliability evidence of a point of care assessment of salivary cortisol and α-amylase: A pre-registered study. PeerJ 2020, 8, e8366. [Google Scholar] [CrossRef] [Green Version]
- Backes, T.; Horvath, P.; Kazial, K. Salivary alpha amylase and salivary cortisol response to fluid consumption in exercising athletes. Biol. Sport 2015, 32, 275–280. [Google Scholar] [CrossRef]
- West, D.W.D.; Phillips, S.M. Associations of exercise-induced hormone profiles and gains in strength and hypertrophy in a large cohort after weight training. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 112, 2693–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porrini, M.; Del Bo, C.; Lanfranco, F.; Strasburger, C. Ergogenic Aids and Supplements. Front. Horm. Res. 2016, 47, 128–152. [Google Scholar] [CrossRef]
- Garthe, I.; Maughan, R.J. Athletes and Supplements: Prevalence and Perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peeling, P.; Binnie, M.; Goods, P.; Sim, M.; Burke, L.M. Evidence-Based Supplements for the Enhancement of Athletic Performance. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Pickering, C.; Grgic, J. Caffeine and Exercise: What Next? Sports Med. 2019, 49, 1007–1030. [Google Scholar] [CrossRef] [Green Version]
- Furst, T.; Massaro, A.; Miller, C.; Williams, B.T.; Lamacchia, Z.M.; Horvath, P.J. β-Alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J. Int. Soc. Sports Nutr. 2018, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, A.; Kayacan, Y.; Ipekoglu, G.; Arslanoglu, E. Effect of carbohydrate–electrolyte consumption on insulin, cortisol hormones and blood glucose after high-intensity exercise. Arch. Physiol. Biochem. 2019, 125, 344–350. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Harris, R.C.; Swain, J.; Corbett, J.; Collard, K.; Dyson, R.J.; Dye, L.; Hodgson, C.; Draper, N. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology 2006, 185, 93–103. [Google Scholar] [CrossRef]
- McMorris, T.; Harris, R.; Howard, A.; Langridge, G.; Hall, B.; Corbett, J.; Dicks, M.; Hodgson, C. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol. Behav. 2007, 90, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.Y.; Garcia, A.B.O.; Rogero, M.M.; Tirapegui, J. Probiotic supplementation in sports and physical exercise: Does it present any ergogenic effect? Nutr. Health 2017, 23, 239–249. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, L.R.; Siegler, J.; Midgley, A. Ergogenic Effects of Sodium Bicarbonate. Curr. Sports Med. Rep. 2008, 7, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Sellami, M.; Bragazzi, N.L.; Slimani, M.; Hayes, L.; Jabbour, G.; de Giorgio, A.; Dugué, B. The Effect of Exercise on Glucoregulatory Hormones: A Countermeasure to Human Aging: Insights from a Comprehensive Review of the Literature. Int. J. Environ. Res. Public Health 2019, 16, 1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadore, E.L.; Lhullier, F.L.R.; Brentano, M.A.; da Silva, E.M.; Ambrosini, M.B.; Spinelli, R.; Silva, R.F.; Kruel, L.F.M. Hormonal Responses to Resistance Exercise in Long-Term Trained and Untrained Middle-Aged Men. J. Strength Cond. Res. 2008, 22, 1617–1624. [Google Scholar] [CrossRef]
- Monje, C.; Rada, I.; Castro-Sepulveda, M.; Peñailillo, L.; Deldicque, L.; Zbinden-Foncea, H. Effects of A High Intensity Interval Session on Mucosal Immune Function and Salivary Hormones in Male and Female Endurance Athletes. J. Sport. Sci. Med. 2020, 19, 436–443. [Google Scholar]
- Silva, R.P.; Vilaça, A.; Guerra, F.D.; Mundim, A.V.; De Agostini, G.G.; De Abreu, L.C.; Zhiguo, Z.; Sorpreso, I.C.E.; Valenti, V.; Silva, N.P. Sex Differences in Physiological Stress Induced by a Long-Lasting Adventure Race: A Prospective Observational Analytical Study. Sportverletz. Sportschaden 2020, 34, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Hsu, G.-S.; Suzuki, K.; Ko, M.-H.; Fang, S.-H. Salivary Immuno Factors, Cortisol and Testosterone Responses in Athletes of a Competitive 5000 m Race. Chin. J. Physiol. 2015, 58, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potgieter, S.; Wright, H.H.; Smith, C. Caffeine Improves Triathlon Performance: A Field Study in Males and Females. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 228–237. [Google Scholar] [CrossRef]
- Beaven, C.M.; Hopkins, W.G.; Hansen, K.T.; Wood, M.R.; Cronin, J.B.; Lowe, T.E. Dose Effect of Caffeine on Testosterone and Cortisol Responses to Resistance Exercise. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 131–141. [Google Scholar] [CrossRef]
- Russell, M.; Reynolds, N.A.; Crewther, B.T.; Cook, C.; Kilduff, L.P. Physiological and Performance Effects of Caffeine Gum Consumed During a Simulated Half-Time by Professional Academy Rugby Union Players. J. Strength Cond. Res. 2020, 34, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Rodas, L.; Martinez, S.; Aguilo, A.; Tauler, P. Caffeine supplementation induces higher IL-6 and IL-10 plasma levels in response to a treadmill exercise test. J. Int. Soc. Sports Nutr. 2020, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Kechribari, I.; Sotirakoglou, Κ.; Tarantilis, P.; Gourdomichali, T.; Michas, G.; Kravvariti, V.; Voumvourakis, K.; Zampelas, A. Acute effects of coffee consumption on self-reported gastrointestinal symptoms, blood pressure and stress indices in healthy individuals. Nutr. J. 2015, 15, 26. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-P.; Li, C.-Y.; Suzuki, K.; Chang, C.-K.; Chou, K.-M.; Fang, S.-H. Green Tea Consumption after Intense Taekwondo Training Enhances Salivary Defense Factors and Antibacterial Capacity. PLoS ONE 2014, 9, e87580. [Google Scholar] [CrossRef] [Green Version]
- Allgrove, J.; Oliveira, M.; Silver, B.; Gleeson, M. Effect of caffeine ingestion during prolonged exhaustive exercise on salivary immunoglobulin A, alpha-amylase and cortisol. In Book of Abtracts of the 14th Annual Congress of the European College of Sport Science; Loland, S., Bo, K., Fasting, K., Hallen, J., Ommundsen, Y., Roberts, G., Tsolakidis, E., Eds.; European College of Sport Science: Oslo, Norway, 2009; 503p. [Google Scholar]
- Cook, C.; Beaven, C.; Kilduff, L.P.; Drawer, S. Acute Caffeine Ingestion’s Increase of Voluntarily Chosen Resistance-Training Load After Limited Sleep. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 157–164. [Google Scholar] [CrossRef]
- Cook, C.J.; Crewther, B.T.; Kilduff, L.P.; Drawer, S.; Gaviglio, C.M. Skill execution and sleep deprivation: Effects of acute caffeine or creatine supplementation—A randomized placebo-controlled trial. J. Int. Soc. Sports Nutr. 2011, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Ganio, M.S.; Klau, J.F.; Casa, D.J.; Armstrong, L.E.; Maresh, C.M. Effect of Caffeine on Sport-Specific Endurance Performance: A Systematic Review. J. Strength Cond. Res. 2009, 23, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Schneiker, K.T.; Bishop, D.; Dawson, B.; Hackett, L.P. Effects of Caffeine on Prolonged Intermittent-Sprint Ability in Team-Sport Athletes. Med. Sci. Sports Exerc. 2006, 38, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Dawson, B.; Schneiker, K.; Goodman, C.; Lay, B. Effect of caffeine supplementation on repeated sprint running performance. J. Sports Med. Phys. Fit. 2008, 48, 472–478. [Google Scholar]
- Klein, C.S.; Clawson, A.; Martin, M.; Saunders, M.J.; Flohr, J.A.; Bechtel, M.K.; Dunham, W.; Hancock, M.; Womack, C.J. The Effect of Caffeine on Performance in Collegiate Tennis Players. J. Caffeine Res. 2012, 2, 111–116. [Google Scholar] [CrossRef]
- Del Coso, J.; Portillo, J.; Muñoz, G.; Abian-Vicen, J.; González-Millán, C.; Muñoz-Guerra, J. Caffeine-containing energy drink improves sprint performance during an international rugby sevens competition. Amino Acids 2013, 44, 1511–1519. [Google Scholar] [CrossRef]
- Skinner, T.; Desbrow, B.; Arapova, J.; Schaumberg, M.A.; Osborne, J.; Grant, G.D.; Anoopkumar-Dukie, S.; Leveritt, M.D. Women Experience the Same Ergogenic Response to Caffeine as Men. Med. Sci. Sports Exerc. 2019, 51, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.K.; Green, J.M. Caffeine and Anaerobic Performance. Sports Med. 2009, 39, 813–832. [Google Scholar] [CrossRef] [PubMed]
- Varanoske, A.N.; Wells, A.; Kozlowski, G.J.; Gepner, Y.; Frosti, C.L.; Boffey, D.; Coker, N.; Harat, I.; Hoffman, J.R. Effects of β-alanine supplementation on physical performance, cognition, endocrine function, and inflammation during a 24 h simulated military operation. Physiol. Rep. 2018, 6, e13938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durkalec-Michalski, K.; Kusy, K.; Ciekot-Sołtysiak, M.; Zieliński, J. The Effect of Beta-Alanine versus Alkaline Agent Supplementation Combined with Branched-Chain Amino Acids and Creatine Malate in Highly-Trained Sprinters and Endurance Athletes: A Randomized Double-Blind Crossover Study. Nutrients 2019, 11, 1961. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.H.; Coakley, J.J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 2006, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.; Harris, R.C.; Kim, H.J.; Harris, B.D.; Sale, C.; Boobis, L.H.; Kim, C.K.; Wise, J.A. Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 2006, 32, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Faigenbaum, A.D.; Ross, R.; Kang, J.; Stout, J.R.; Wise, J.A. Short-duration β-alanine supplementation increases training volume and reduces subjective feelings of fatigue in college football players. Nutr. Res. 2008, 28, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Zanella, P.B.; Alves, F.D.; de Souza, C.G. Effects of beta-alanine supplementation on performance and muscle fatigue in athletes and non-athletes of different sports: A systematic review. J. Sports Med. Phys. Fit. 2017, 57, 1132–1141. [Google Scholar] [CrossRef]
- Wang, R. The Effect of Repeated Sprint Training in Hypoxia and Beta-Alanine Supplementation on Exercise Performance. Ph.D. Thesis, University of Central Florida, Orlando, FL, USA, 2017. [Google Scholar]
- Hoffman, J.; Ratamess, N.; Ross, R.; Kang, J.; Magrelli, J.; Neese, K.; Faigenbaum, A.; Wise, J. β-Alanine and the Hormonal Response to Exercise. Int. J. Sports Med. 2008, 29, 952–958. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Landau, G.; Stout, J.R.; Dabora, M.; Moran, D.S.; Sharvit, N.; Hoffman, M.W.; Ben Moshe, Y.; McCormack, W.P.; Hirschhorn, G.; et al. β-alanine supplementation improves tactical performance but not cognitive function in combat soldiers. J. Int. Soc. Sports Nutr. 2014, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, M.; Nieman, D.C.; Pedersen, B.K. Exercise, nutrition and immune function. J. Sports Sci. 2004, 22, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.; Blannin, A.; Walsh, N.; Castell, L.; Gleeson, M. Effects of Exercise Intensity, Duration and Recovery onin vitroNeutrophil Function in Male Athletes. Int. J. Sports Med. 1999, 20, 128–130. [Google Scholar] [CrossRef]
- Tsintzas, O.-K.; Williams, C.; Wilson, W.; Burrin, J. Influence of carbohydrate supplementation early in exercise on endurance running capacity. Med. Sci. Sports Exerc. 1996, 28, 1373–1379. [Google Scholar] [CrossRef]
- Costa, R.J.S.; Jones, G.E.; Lamb, K.L.; Coleman, R.C.; Williams, J.H. The Effects of a High Carbohydrate Diet on Cortisol and Salivary Immunoglobulin A (s-IgA) During a Period of Increase Exercise Workload Amongst Olympic and Ironman Triathletes. Endoscopy 2005, 26, 880–885. [Google Scholar] [CrossRef]
- Soltani, H.; Keim, N.L.; Laugero, K.D. Increasing Dietary Carbohydrate as Part of a Healthy Whole Food Diet Intervention Dampens Eight Week Changes in Salivary Cortisol and Cortisol Responsiveness. Nutrients 2019, 11, 2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemmens, S.G.; Born, J.M.; Martens, E.A.; Martens, M.J.; Westerterp-Plantenga, M.S. Influence of Consumption of a High-Protein vs. High-Carbohydrate Meal on the Physiological Cortisol and Psychological Mood Response in Men and Women. PLoS ONE 2011, 6, e16826. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Earnest, C.P.; Lowery, R.P.; Wilson, J.M.; Willems, M.E.T. Co-ingestion of Nutritional Ergogenic Aids and High-Intensity Exercise Performance. Sports Med. 2016, 46, 1407–1418. [Google Scholar] [CrossRef]
- Boegman, S.; Stellingwerff, T.; Shaw, G.; Clarke, N.; Graham, K.; Cross, R.; Siegler, J.C. The Impact of Individualizing Sodium Bicarbonate Supplementation Strategies on World-Class Rowing Performance. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 1–44. [Google Scholar] [CrossRef] [Green Version]
- Leite, G.S.; Student, A.S.R.M.; West, N.P.; Lancha, A.H. Probiotics and sports: A new magic bullet? Nutrients 2019, 60, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Effect of Probiotics Supplementations on Health Status of Athletes. Int. J. Environ. Res. Public Health 2019, 16, 4469. [Google Scholar] [CrossRef] [Green Version]
- Cox, A.J.; Pyne, D.; Saunders, P.U.; Fricker, P.A. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sports Med. 2008, 44, 222–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Hopkins, W.G.; Eskesen, D.C.; Jairath, A.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: A randomised control trial in athletes. Nutr. J. 2011, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shing, C.M.; Peake, J.M.; Lim, C.L.; Briskey, D.; Walsh, N.P.; Fortes, M.B.; Ahuja, K.; Vitetta, L. Effects of probiotics supplementation on gastrointestinal permeability, inflammation and exercise performance in the heat. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 114, 93–103. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Stone, J.D.; Turner, S.M.; Anzalone, A.J.; Eimerbrink, M.J.; Pane, M.; Amoruso, A.; Rowlands, D.S.; Oliver, J.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 Supplementation Attenuates Performance and Range-of-Motion Decrements Following Muscle Damaging Exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, B.-E.; Jeon, Y. Effect of Treadmill Exercise and Probiotic Ingestion on Motor Coordination and Brain Activity in Adolescent Mice. Health 2020, 9, 7. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lee, M.-C.; Lee, C.-C.; Ng, K.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C. Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.-C.; Wei, C.-C.; Huang, C.-C.; Chen, W.-L.; Huang, H.-Y. The Beneficial Effects of Lactobacillus plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-C.; Hsu, Y.-J.; Chuang, H.-L.; Hsieh, P.-S.; Ho, H.-H.; Chen, W.-L.; Chiu, Y.-S. In Vivo Ergogenic Properties of the Bifidobacterium longum OLP-01 Isolated from a Weightlifting Gold Medalist. Nutrients 2019, 11, 2003. [Google Scholar] [CrossRef] [Green Version]
- Jäger, R.; Shields, K.A.; Lowery, R.P.; De Souza, E.O.; Partl, J.M.; Hollmer, C.; Purpura, M.; Wilson, J.M. ProbioticBacillus coagulansGBI-30, 6086 reduces exercise-induced muscle damage and increases recovery. PeerJ 2016, 4, e2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galloza, J.; Castillo, B.; Micheo, W. Benefits of Exercise in the Older Population. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Budde, H.; Voelcker-Rehage, C.; Pietrassyk-Kendziorra, S.; Machado, S.; Ribeiro, P.; Arafat, A.M. Steroid hormones in the saliva of adolescents after different exercise intensities and their influence on working memory in a school setting. Psychoneuroendocrinology 2010, 35, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.E.; Leyshon, A.; Hughes, M.G.; Davies, B.; Graham, M.; Baker, J.S. The effect of anaerobic exercise on salivary cortisol, testosterone and immunoglobulin (A) in boys aged 15–16 years. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 107, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Chiodo, S.; Tessitore, A.; Cortis, C.; Cibelli, G.; Lupo, C.; Ammendolia, A.; De Rosas, M.; Capranica, L. Stress-related hormonal and psychological changes to official youth Taekwondo competitions. Scand. J. Med. Sci. Sports 2011, 21, 111–119. [Google Scholar] [CrossRef]
- Park, S.-H.; Park, I.-H.; Lim, S.-T.; Lee, E. Changes in Psychological Anxiety and Physiological Stress Hormones in Korea National Shooters. Brain Sci. 2020, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.J.; Finlay, O.; Griffiths, H.; Sylvester, J.; Williams, M.; Bishop, N.C. Immunoendocrine Response to Cycling following Ingestion of Caffeine and Carbohydrate. Med. Sci. Sports Exerc. 2007, 39, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; Roberson, D.W. Efficacy of Acute Caffeine Ingestion for Short-term High-Intensity Exercise Performance: A Systematic Review. J. Strength Cond. Res. 2010, 24, 257–265. [Google Scholar] [CrossRef]
- Portillo, J.; Del Coso, J.; Abián-Vicén, J. Effects of Caffeine Ingestion on Skill Performance During an International Female Rugby Sevens Competition. J. Strength Cond. Res. 2017, 31, 3351–3357. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Jesus, A.A.; Giglio, B.M.; Marini, A.C.; Lobo, P.C.B.; Mota, J.F.; Pimentel, G.D. Acute Caffeinated Coffee Consumption Does not Improve Time Trial Performance in an 800-m Run: A Randomized, Double-Blind, Crossover, Placebo-Controlled Study. Nutrients 2018, 10, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, H.R.; Tharion, W.J.; Shukitt-Hale, B.; Speckman, K.L.; Tulley, R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Psychopharmacology 2002, 164, 250–261. [Google Scholar] [CrossRef]
- PatonTimothy, C.D.; Lowe, T.; Irvine, A. Caffeinated chewing gum increases repeated sprint performance and augments increases in testosterone in competitive cyclists. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 110, 1243–1250. [Google Scholar] [CrossRef]
- Bunsawat, K.; White, D.; Kappus, R.M.; Baynard, T. Caffeine delays autonomic recovery following acute exercise. Eur. J. Prev. Cardiol. 2015, 22, 1473–1479. [Google Scholar] [CrossRef]
- Gonzaga, L.A.; Vanderlei, L.C.M.; Gomes, R.L.; Valenti, V.E. Caffeine affects autonomic control of heart rate and blood pressure recovery after aerobic exercise in young adults: A crossover study. Sci. Rep. 2017, 7, 14091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Silva, J.P.; Santos, J.F.D.S.; Franchini, E. Can caffeine supplementation reverse the effect of time of day on repeated-sprint exercise performance? Appl. Physiol. Nutr. Metab. 2019, 44, 187–193. [Google Scholar] [CrossRef]
- Klein, L.C.; Whetzel, C.A.; Bennett, J.M.; Ritter, F.E.; Nater, U.M.; Schoelles, M. Caffeine administration does not alter salivary α-amylase activity in young male daily caffeine consumers. BMC Res. Notes 2014, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Dsamou, M.; Palicki, O.; Septier, C.; Chabanet, C.; Lucchi, G.; Ducoroy, P.; Chagnon, M.-C.; Morzel, M. Salivary Protein Profiles and Sensitivity to the Bitter Taste of Caffeine. Chem. Senses 2011, 37, 87–95. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.; Wilborn, C.D.; Sale, C.; Kreider, R.; Jäger, R.; Earnest, C.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.M.; Helms, E.R.; Trexler, E.T.; Fitschen, P.J. Nutritional Recommendations for Physique Athletes. J. Hum. Kinet. 2020, 71, 79–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Church, D.D.; Hoffman, J.R.; Varanoske, A.N.; Wang, R.; Baker, K.M.; La Monica, M.B.; Beyer, K.S.; Dodd, S.J.; Oliveira, L.; Harris, R.C.; et al. Comparison of Two β-Alanine Dosing Protocols on Muscle Carnosine Elevations. J. Am. Coll. Nutr. 2017, 36, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.; Landau, G.; Stout, J.R.; Hoffman, M.W.; Shavit, N.; Rosen, P.; Moran, D.S.; Fukuda, D.; Shelef, I.; Carmom, E.; et al. β-Alanine ingestion increases muscle carnosine content and combat specific performance in soldiers. Amino Acids 2015, 47, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Solis, M.Y.; Cooper, S.; Hobson, R.M.; Artioli, G.; Otaduy, M.C.; Roschel, H.; Robertson, J.; Martin, D.; Painelli, V.S.; Harris, R.C.; et al. Effects of Beta-Alanine Supplementation on Brain Homocarnosine/Carnosine Signal and Cognitive Function: An Exploratory Study. PLoS ONE 2015, 10, e0123857. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Furuse, M. The impact of taurine- and beta-alanine-supplemented diets on behavioral and neurochemical parameters in mice: Antidepressant versus anxiolytic-like effects. Amino Acids 2010, 39, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, M.; Lebiedzińska, A. The effect of diet components on the level of cortisol. Eur. Food Res. Technol. 2016, 242, 2001–2009. [Google Scholar] [CrossRef] [Green Version]
- Slivka, D.; Hailes, W.; Cuddy, J.; Ruby, B. Caffeine and carbohydrate supplementation during exercise when in negative energy balance: Effects on performance, metabolism, and salivary cortisol. Appl. Physiol. Nutr. Metab. 2008, 33, 1079–1085. [Google Scholar] [CrossRef]
- Junior, A.H.L.; Painelli, V.D.S.; Saunders, B.; Artioli, G. Nutritional Strategies to Modulate Intracellular and Extracellular Buffering Capacity During High-Intensity Exercise. Sports Med. 2015, 45, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Baranauskas, M.; Jablonskienė, V.; Abaravičius, J.A.; Samsonienė, L.; Stukas, R. Dietary Acid-Base Balance in High-Performance Athletes. Int. J. Environ. Res. Public Health 2020, 17, 5332. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Candow, D.G.; Smith-Ryan, A.E.; Hirsch, K.R.; Roberts, M.D.; VanDusseldorp, T.A.; Stratton, M.T.; Kaviani, M.; Little, J.P. Supplements and Nutritional Interventions to Augment High-Intensity Interval Training Physiological and Performance Adaptations—A Narrative Review. Nutrients 2020, 12, 390. [Google Scholar] [CrossRef] [Green Version]
- Calvo, J.L.; Xu, H.; Mon-López, D.; Pareja-Galeano, H.; Jiménez, S.L. Effect of sodium bicarbonate contribution on energy metabolism during exercise: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2021, 18, 1–17. [Google Scholar] [CrossRef]
- Hilton, N.P.; Leach, N.K.; Sparks, S.A.; Gough, L.A.; Craig, M.M.; Deb, S.K.; McNaughton, L.R. A Novel Ingestion Strategy for Sodium Bicarbonate Supplementation in a Delayed-Release Form: A Randomised Crossover Study in Trained Males. Sports Med. Open 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise influence on the microbiome–gut–brain axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Wosinska, L.; Cotter, P.D.; O’Sullivan, O.; Guinane, C. The Potential Impact of Probiotics on the Gut Microbiome of Athletes. Nutrients 2019, 11, 2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, N.P.; Horn, P.L.; Pyne, D.; Gebski, V.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin. Nutr. 2014, 33, 581–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haywood, B.A.; Black, K.E.; Baker, D.; McGarvey, J.; Healey, P.; Brown, R. Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. J. Sci. Med. Sport 2014, 17, 356–360. [Google Scholar] [CrossRef]
- Pugh, J.N.; Sparks, A.S.; Doran, D.A.; Fleming, S.C.; Langan-Evans, C.; Kirk, B.; Fearn, R.; Morton, J.P.; Close, G.L. Four weeks of probiotic supplementation reduces GI symptoms during a marathon race. Eur. J. Appl. Phys. 2019, 119, 1491–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a Probiotic Intake on Oxidant and Antioxidant Parameters in Plasma of Athletes During Intense Exercise Training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Geiger, D.; Schauer, M.; Gostner, J.M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Probiotic Supplements Beneficially Affect Tryptophan–Kynurenine Metabolism and Reduce the Incidence of Upper Respiratory Tract Infections in Trained Athletes: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients 2016, 8, 752. [Google Scholar] [CrossRef] [Green Version]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef] [PubMed]
| Demographic Groups/Ergogenic Substance | Effect on Cortisol and Amylase Pre- and Post-Exercise | Effect on Performance |
|---|---|---|
| Age | ↑ Cortisol, ↓ amylase in elliptical and cycle ergo meter in young males [2] ↓ cortisol, amylase in treadmill sessions in young males [2] ↑ salivary cortisol post resistance in middle-aged man [20] in superset strength training protocol | |
| Gender | ↑ amylase activity post exercise in females in cycling [1] ↑ 1.5× amylase activity in males at rest, but similar cortisol levels in high intensity interval training [21] ↑ amylase and cortisol in females in the Ecomotion/ProAdventure Race World [22] -basal amylase levels higher in females in 5000 m race [23] | |
| Caffeine | ↑ post-triathlon cortisol levels–microencalsulated caffeine [24] ↓ testosterone:cortisol ratio in resistance exercise [25] -caffeine gum–no changes in salivary cortisol after simulated half time by professional academy rugby union [26] ↓ salivary cortisol in repeated, high-intensity sprint exercise in competitive cyclists [26] ↑ adrenaline and cortisol levels after recovery leading to increased levels of IL-6 and IL-10 after treadmill exercise [27] -acute coffee consumption activates salivary amylase, but not salivary cortisol [28] -consumption of green tea after a taekwondo training session ↑ salivary amylase activity [29] -caffeine consumed as a cereal bar during exhaustive cycling ↑ endurance and salivary cortisol, but did not affect the salivary amylase increase post-exercise [30] ↑ salivary cortisol after caffeine administration in acute sleep deprived athletes and altered performance [31,32] | -performance improved in endurance athletes [24,33] -significant performance improvement in competitive intermittent-sprint [34,35], tennis performance [36], women’s rugby seven competition [37]. -enhanced endurance exercise performance in women [38] -hypoalgesic effect by diminishing pain [39] ↓ fatigue during repeated, high-intensity sprint exercise in competitive cyclists [26] |
| Beta-alanine | ↑ cortisol level and ↓ testosterone:cortisol ratio at 24 h in a 24 h simulated military operation [40] | -better exercise performance and adaptation [41] ↑ muscle carnosine proportionally with the time of use [42,43] -no significant improvements in fatigue rates during high-intensity anaerobic exercise in highly trained athletes [44] -improved biochemical parameters in regards to muscle fatigue [45] ↑ exercise capacity [46] -elimination of executive function decline post-recovery [12] -improved performance, marksmanship and target engagement speed, as well as muscle endurance [47], nut no impact on cognitive performance [48] |
| Carbohydrates | -attenuate higher plasma cortisol levels and greater immune disturbances [49,50,51] -salivary cortisol has been observed to be similar in the self-selected diet group and carbohydrate group, but increased post-exercise in the self-selected diet group [52] ↓ salivary cortisol levels as a result of increased dietary carbohydrate as part of a Dietary Guideline for Americans-based diet [53] -a high-carbohydrate diet–higher salivary cortisol response in men [54] | |
| Sodium bicarbonate | -sodium bicarbonate in combination with caffeine led to a longer total distance of rowing compared to sodium bicarbonate alone or a possible additional benefit in 2000-m rowing performance when administered together with beta-alanine [55] -a small, but significant performance effect [56] | |
| Probiotics | - strains have been reported to have an influence on exercise recovery [57,58] -beneficial effects in terms of intestinal permeability, immunity, microbiota, inflammation, GIs and URTIs [59] -Lb. fermentum in elite male distance runners ↓ risk and severity of URTIs [60], ↓ severity of GIT in cyclists [61] +Lb. acidophilus, Lb. rhamnosus, Lb. casei, Lb. plantarum, B. lactis, B. breve, B.bifidum and Streptococcus thermophilus ↑ run time to fatigue [62] -B. breve BR03 and S. thermophilus FP4 led to a positive effect on the reduced performance and range of motion followed by intense muscle damaging exercise [63] -an improvement in moderate-intensity exercise [64] -Lb. plantarum TWK10 reported significantly increased exercise performance in a dose-dependent manner [65] -L. plantarum PS128 as a potential for better training management [66] -B. longum OLP-01 for performance-improving and health-promoting [67] -beneficial effects of probiotics in recovery with significantly increases at 24 and 72 h, as well as decreased soreness at 72 h post exercise [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honceriu, C.; Curpan, A.-S.; Ciobica, A.; Ciobica, A.; Trus, C.; Timofte, D. Connections between Different Sports and Ergogenic Aids—Focusing on Salivary Cortisol and Amylase. Medicina 2021, 57, 753. https://doi.org/10.3390/medicina57080753
Honceriu C, Curpan A-S, Ciobica A, Ciobica A, Trus C, Timofte D. Connections between Different Sports and Ergogenic Aids—Focusing on Salivary Cortisol and Amylase. Medicina. 2021; 57(8):753. https://doi.org/10.3390/medicina57080753
Chicago/Turabian StyleHonceriu, Cezar, Alexandrina-Stefania Curpan, Alin Ciobica, Andrei Ciobica, Constantin Trus, and Daniel Timofte. 2021. "Connections between Different Sports and Ergogenic Aids—Focusing on Salivary Cortisol and Amylase" Medicina 57, no. 8: 753. https://doi.org/10.3390/medicina57080753
APA StyleHonceriu, C., Curpan, A.-S., Ciobica, A., Ciobica, A., Trus, C., & Timofte, D. (2021). Connections between Different Sports and Ergogenic Aids—Focusing on Salivary Cortisol and Amylase. Medicina, 57(8), 753. https://doi.org/10.3390/medicina57080753








