Impact of Bone Cement Augmentation on the Fixation Strength of TFNA Blades and Screws
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens and Study Groups
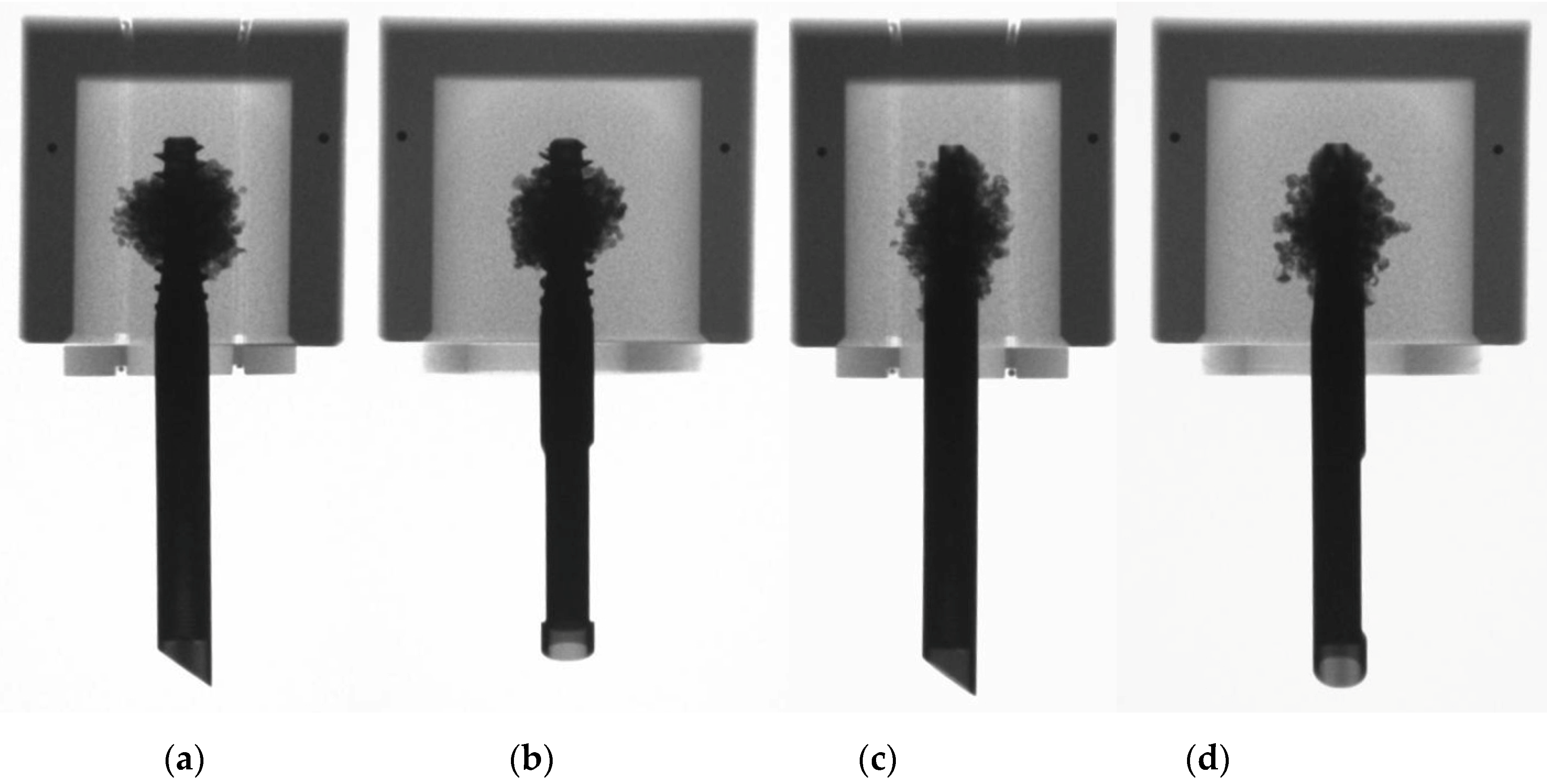
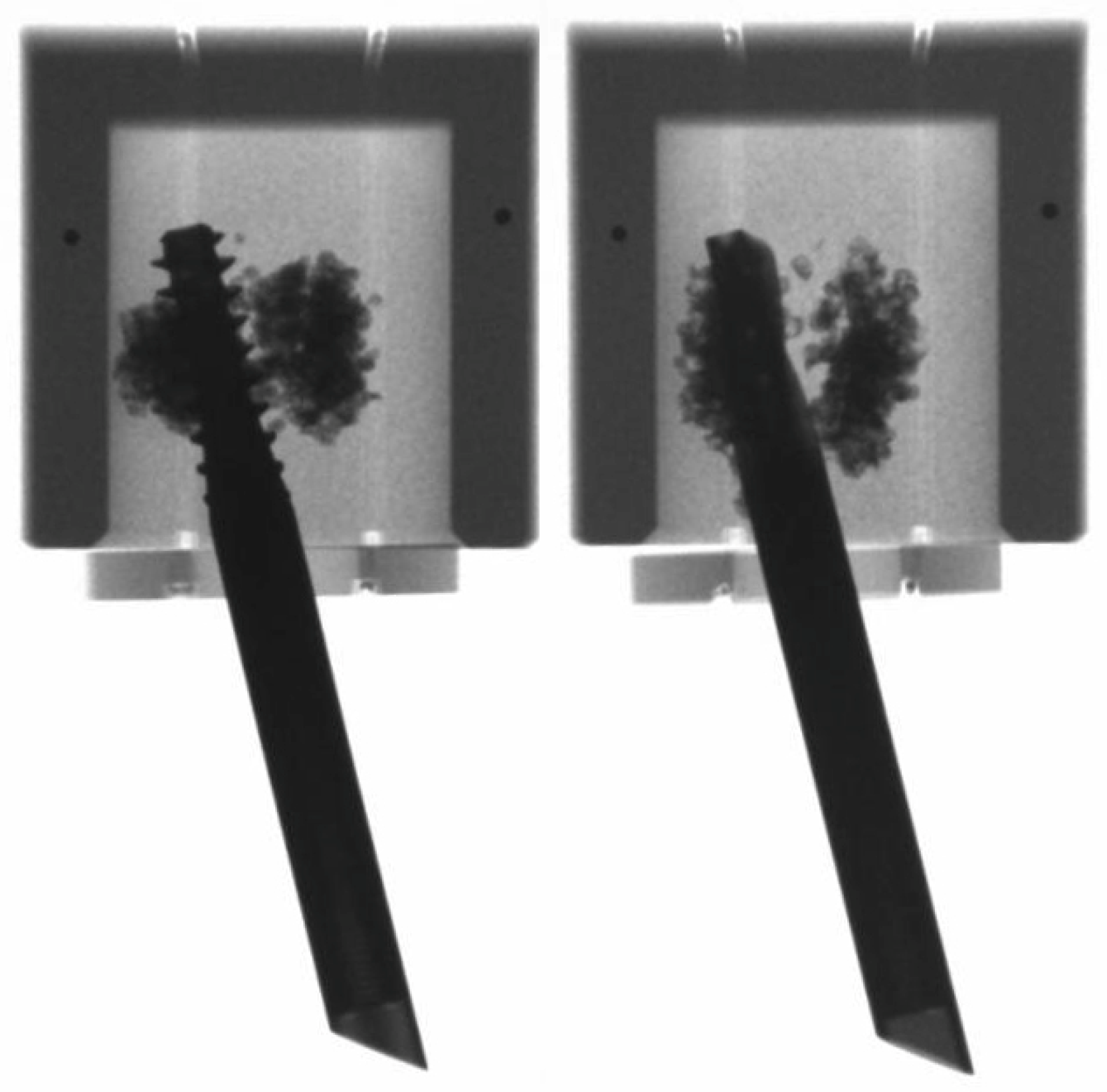
2.2. Preparation
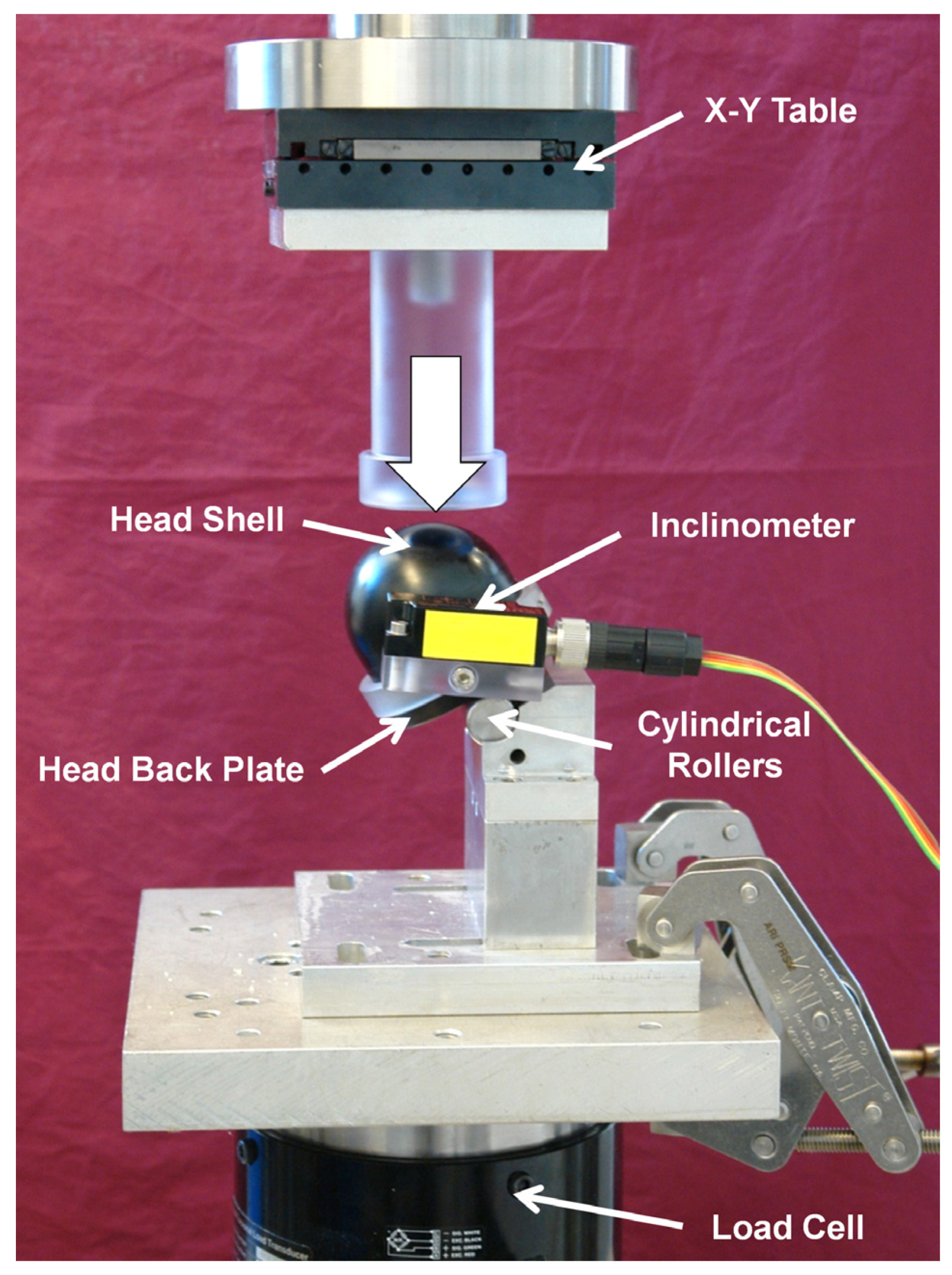
2.3. Mechanical Testing
2.4. Data Acquisition and Evaluation
2.5. Statistical Analysis
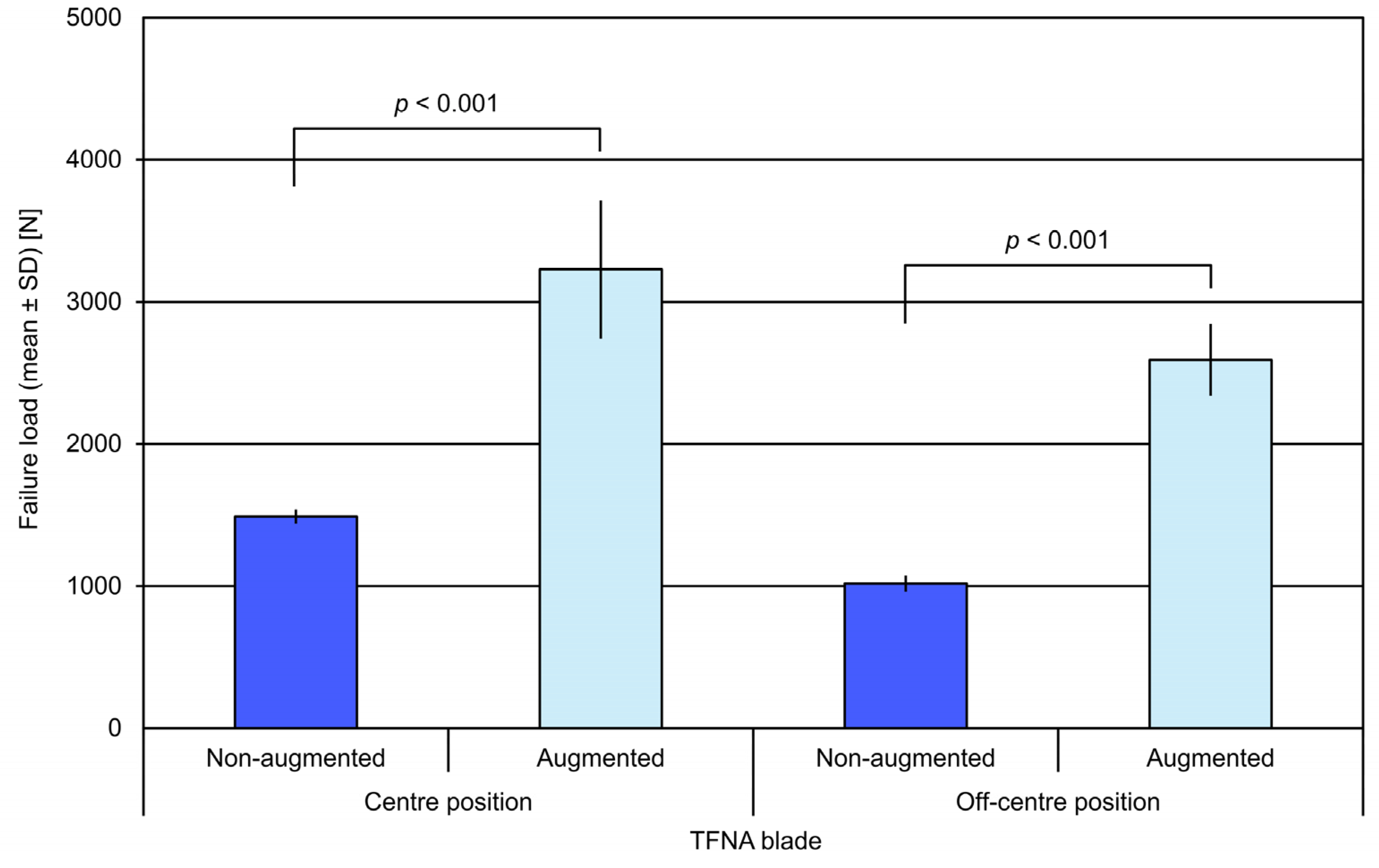
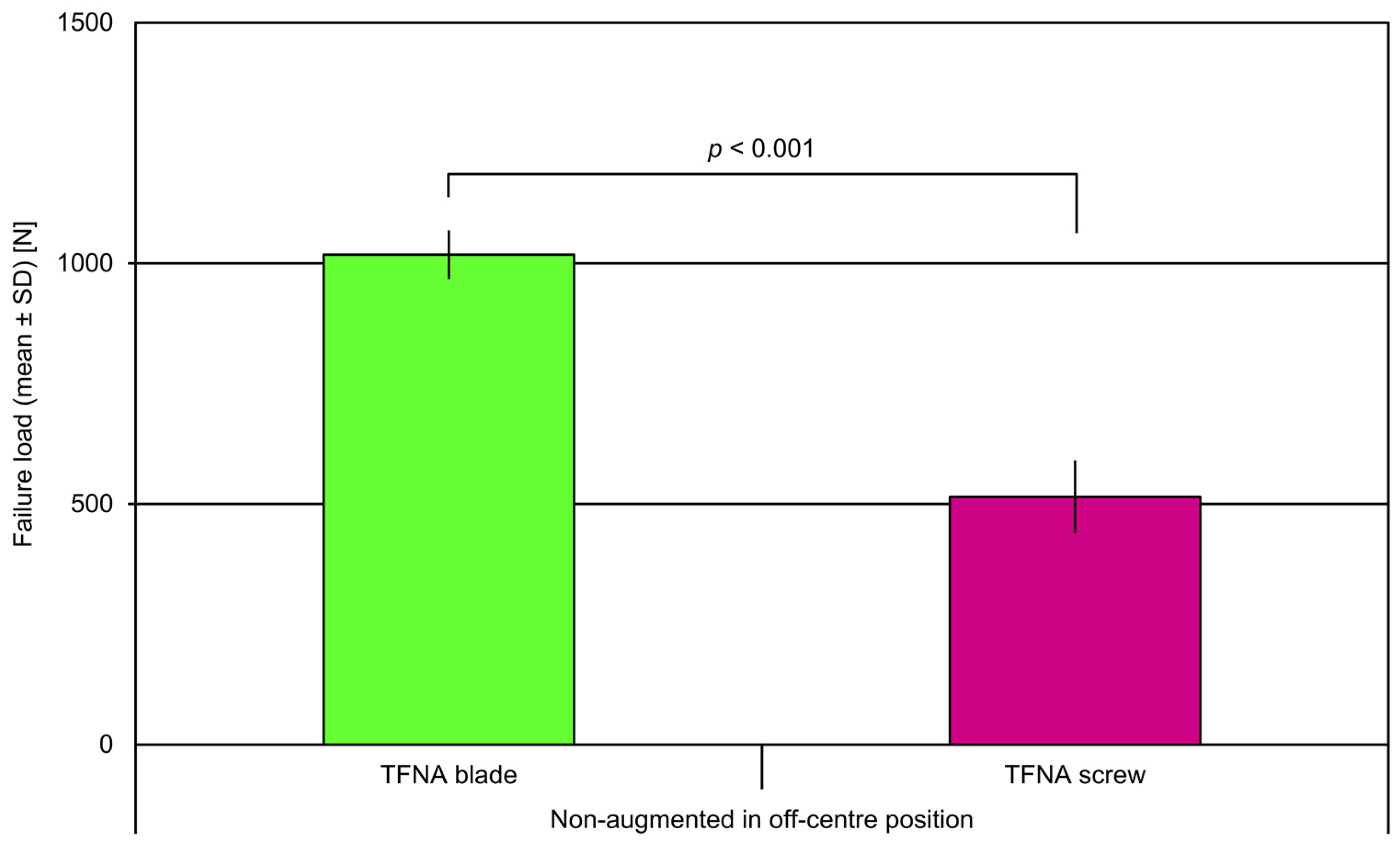
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, A.; Exuzides, A.; Spangler, L.; O’Malley, C.; Colby, C.; Johnston, K.; Agodoa, I.; Baker, J.; Kagan, R. Burden of Illness for Osteoporotic Fractures Compared With Other Serious Diseases Among Postmenopausal Women in the United States. Mayo Clin. Proc. 2015, 90, 53–62. [Google Scholar] [CrossRef]
- Friedman, S.M.; Mendelson, D. Epidemiology of Fragility Fractures. Clin. Geriatr. Med. 2014, 30, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.; Tsilidis, K.K.; Orfanos, P.; Benetou, V.; E Ntzani, E.; Soerjomataram, I.; Künn-Nelen, A.; Kymmer, U.P.; Eriksson, S.; Brenner, H.; et al. Burden of hip fracture using disability-adjusted life-years: A pooled analysis of prospective cohorts in the CHANCES consortium. Lancet Public Health 2017, 2, e239–e246. [Google Scholar] [CrossRef]
- Sermon, A.; Zderic, I.; Khatchadourian, R.; Scherrer, S.; Knobe, M.; Stoffel, K.; Gueorguiev, B. Bone cement augmentation of femoral nail head elements increases their cut-out resistance in poor bone quality– A biomechanical study. J. Biomech. 2021, 118, 110301. [Google Scholar] [CrossRef] [PubMed]
- Svedbom, A.; EU Review Panel of IOF; Hernlund, E.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: A compendium of country-specific reports. Arch. Osteoporos. 2013, 8, 1–218. [Google Scholar] [CrossRef]
- Vanhaecht, K.; Sermeus, W.; Peers, J.; Lodewijckx, C.; Deneckere, S.; Leigheb, F.; Boonen, S.; Sermon, A.; Boto, P.; Mendes, R.V.; et al. The impact of care pathways for patients with proximal femur fracture: Rationale and design of a cluster-randomized controlled trial. BMC Health Serv. Res. 2012, 12, 124. [Google Scholar] [CrossRef]
- Parker, M.J.; Handoll, H. Gamma and other cephalocondylic intramedullary nails versus extramedullary implants for extracapsular hip fractures in adults. Cochrane Database Syst. Rev. 2010, CD000093. [Google Scholar] [CrossRef]
- Szita, J.; Cserháti, P.; Bosch, U.; Manninger, J.; Bodzay, T.; Fekete, K. Intracapsular femoral neck fractures: The importance of early reduction and stable osteosynthesis. Injury 2002, 33 (Suppl. 3), 41–46. [Google Scholar] [CrossRef]
- Bonnaire, F.; Zenker, H.; Lill, C.; Weber, A.T.; Linke, B. Treatment strategies for proximal femur fractures in osteoporotic patients. Osteoporos. Int. 2004, 16 (Suppl. 2), S93–S102. [Google Scholar] [CrossRef]
- Brunner, A.; Büttler, M.; Lehmann, U.; Curd Frei, H.; Kratter, R.; Di Lazzaro, M.; Scola, A.; Sermon, A.; Attal, R. What is the optimal salvage procedure for cut-out after surgical fixation of trochanteric fractures with the PFNA or TFN? A multicentre study. Injury 2016, 47, 432–438. [Google Scholar] [CrossRef]
- Baumgaertner, M.R.; Curtin, S.L.; Lindskog, D.M.; Keggi, J.M. The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J. Bone Joint. Surg. Am. 1995, 77, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, P.R.T.; Zdero, R.; Shah, S.; Olsen, M.; Waddell, J.P.; Schemitsch, E.H. Femoral head lag screw position for cephalomedullary nails: A biomechanical analysis. J. Orthop. Trauma 2012, 26, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Puthezhath, K.; Jayaprakash, C. Is calcar referenced tip-apex distance a better predicting factor for cutting out in biaxial cephalomedullary nails than tip-apex distance? J. Orthop. Surg. 2017, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tosounidis, T.H.; Castillo, R.; Kanakaris, N.K.; Giannoudis, P.V. Common complications in hip fracture surgery: Tips/tricks and solutions to avoid them. Injury 2015, 46 (Suppl. 5), S3–S11. [Google Scholar] [CrossRef]
- Kammerlander, C.; Doshi, H.; Gebhard, F.; Scola, A.; Meier, C.; Linhart, W.; Garcia-Alonso, M.; Nistal, J.; Blauth, M. Long-term results of the augmented PFNA: A prospective multicenter trial. Arch. Orthop. Trauma Surg. 2013, 134, 343–349. [Google Scholar] [CrossRef]
- Kammerlander, C.; Hem, E.S.; Klopfer, T.; Gebhard, F.; Sermon, A.; Dietrich, M.; Bach, O.; Weil, Y.; Babst, R.; Blauth, M. Cement augmentation of the Proximal Femoral Nail Antirotation (PFNA)—A multicentre randomized controlled trial. Injury 2018, 49, 1436–1444. [Google Scholar] [CrossRef]
- Röderer, G.; Scola, A.; Schmoelz, W.; Gebhard, F.; Windolf, M.; Hofmann-Fliri, L. Biomechanical in vitro assessment of screw augmentation in locked plating of proximal humerus fractures. Injury 2013, 44, 1327–1332. [Google Scholar] [CrossRef]
- Wähnert, D.; Hofmann-Fliri, L.; Richards, R.G.; Gueorguiev, B.; Raschke, M.J.; Windolf, M. Implant augmentation: Adding bone cement to improve the treatment of osteoporotic distal femur fractures: A biomechanical study using human cadaver bones. Medicine 2014, 93, e166. [Google Scholar] [CrossRef]
- Sermon, A.; Boner, V.; Schwieger, K.; Boger, A.; Boonen, S.; Broos, P.; Richards, R.; Windolf, M. Biomechanical evaluation of bone-cement augmented Proximal Femoral Nail Antirotation blades in a polyurethane foam model with low density. Clin. Biomech. 2011, 27, 71–76. [Google Scholar] [CrossRef]
- Sermon, A.; Hofmann-Fliri, L.; Richards, R.G.; Flamaing, J.; Windolf, M. Cement augmentation of hip implants in osteoporotic bone: How much cement is needed and where should it go? J. Orthop. Res. 2014, 32, 362–368. [Google Scholar] [CrossRef]
- Kammerlander, C.; Gebhard, F.; Meier, C.; Lenich, A.; Linhart, W.; Clasbrummel, B.; Neubauer-Gartzke, T.; Garcia-Alonso, M.; Pavelka, T.; Blauth, M. Standardised cement augmentation of the PFNA using a perforated blade: A new technique and preliminary clinical results. A prospective multicentre trial. Injury 2011, 42, 1484–1490. [Google Scholar] [CrossRef]
- Bergmann, G.; Deuretzbacher, G.; Heller, M.; Graichen, F.; Rohlmann, A.; Strauss, J.; Duda, G. Hip contact forces and gait patterns from routine activities. J. Biomech. 2001, 34, 859–871. [Google Scholar] [CrossRef]
- Sommers, M.B.; Roth, C.; Hall, H.; Kam, B.C.C.; Ehmke, L.W.; Krieg, J.C.; Madey, S.M.; Bottlang, M. A Laboratory Model to Evaluate Cutout Resistance of Implants for Pertrochanteric Fracture Fixation. J. Orthop. Trauma 2004, 18, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Sermon, A.; Boner, V.; Boger, A.; Schwieger, K.; Boonen, S.; Broos, P.L.; Richards, R.; Windolf, M. Potential of polymethylmethacrylate cement-augmented helical proximal femoral nail antirotation blades to improve implant stability—A biomechanical investigation in human cadaveric femoral heads. J. Trauma Inj. Infect. Crit. Care 2012, 72, E54–E59. [Google Scholar] [CrossRef] [PubMed]
- Erhart, S.; Schmoelz, W.; Blauth, M.; Lenich, A. Biomechanical effect of bone cement augmentation on rotational stability and pull-out strength of the Proximal Femur Nail Antirotation™. Injury 2011, 42, 1322–1327. [Google Scholar] [CrossRef]
- Bartucci, E.J.; Gonzalez, M.H.; Cooperman, D.R.; I Freedberg, H.; Barmada, R.; Laros, G.S. The effect of adjunctive methylmethacrylate on failures of fixation and function in patients with intertrochanteric fractures and osteoporosis. J. Bone Jt. Surg. Am. 1985, 67, 1094–1107. [Google Scholar] [CrossRef]
- Von Der Linden, P.; Gisep, A.; Boner, V.; Windolf, M.; Appelt, A.; Suhm, N. Biomechanical evaluation of a new augmentation method for enhanced screw fixation in osteoporotic proximal femoral fractures. J. Orthop. Res. 2006, 24, 2230–2237. [Google Scholar] [CrossRef]
- Knobe, M.; Bettag, S.; Kammerlander, C.; Altgassen, S.; Maier, K.-J.; Nebelung, S.; Prescher, A.; Horst, K.; Pishnamaz, M.; Herren, C.; et al. Is bone-cement augmentation of screw-anchor fixation systems superior in unstable femoral neck fractures? A biomechanical cadaveric study. Injury 2018, 50, 292–300. [Google Scholar] [CrossRef]
- Knobe, M.; Altgassen, S.; Maier, K.-J.; Gradl-Dietsch, G.; Kaczmarek, C.; Nebelung, S.; Klos, K.; Kim, B.-S.; Gueorguiev, B.; Horst, K.; et al. Screw-blade fixation systems in Pauwels three femoral neck fractures: A biomechanical evaluation. Int. Orthop. 2017, 42, 409–418. [Google Scholar] [CrossRef]
- Cleveland, M.; Bosworth, D.M.; Thompson, F.R.; Wilson, H.J., Jr.; Ishizuka, T. A ten-year analysis of intertrochanteric fractures of the femur. J. Bone Jt. Surg. Am. 1959, 41-A, 1399–1408. [Google Scholar] [CrossRef]
- Goffin, J.M.; Pankaj, P.; Simpson, A.H.R.W.; Seil, R.; Gerich, T.G. Does bone compaction around the helical blade of a proximal femoral nail anti-rotation (PFNA) decrease the risk of cut-out?: A subject-specific computational study. Bone Jt. Res. 2013, 2, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wähnert, D.; Lange, J.; Schulze, M.; Lenschow, S.; Stange, R.; Raschke, M. The potential of implant augmentation in the treatment of osteoporotic distal femur fractures: A biomechanical study. Injury 2013, 44, 808–812. [Google Scholar] [CrossRef] [PubMed]


| Group Number | Implant Type | Position | Augmentation |
|---|---|---|---|
| 1 | Screw | Centre | No |
| 2 | Screw | Centre | Yes |
| 3 | Screw | Off-centre | No |
| 4 | Screw | Off-centre | Yes |
| 5 | Blade | Centre | No |
| 6 | Blade | Centre | Yes |
| 7 | Blade | Off-centre | No |
| 8 | Blade | Off-centre | Yes |
| TFNA HE | Failure Load Increase after Cement Augmentation [%] | |
|---|---|---|
| Centre Position | Off-Centre Position | |
| Blade | 117 | 155 |
| Screw | 138 | 420 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sermon, A.; Hofmann-Fliri, L.; Zderic, I.; Agarwal, Y.; Scherrer, S.; Weber, A.; Altmann, M.; Knobe, M.; Windolf, M.; Gueorguiev, B. Impact of Bone Cement Augmentation on the Fixation Strength of TFNA Blades and Screws. Medicina 2021, 57, 899. https://doi.org/10.3390/medicina57090899
Sermon A, Hofmann-Fliri L, Zderic I, Agarwal Y, Scherrer S, Weber A, Altmann M, Knobe M, Windolf M, Gueorguiev B. Impact of Bone Cement Augmentation on the Fixation Strength of TFNA Blades and Screws. Medicina. 2021; 57(9):899. https://doi.org/10.3390/medicina57090899
Chicago/Turabian StyleSermon, An, Ladina Hofmann-Fliri, Ivan Zderic, Yash Agarwal, Simon Scherrer, André Weber, Martin Altmann, Matthias Knobe, Markus Windolf, and Boyko Gueorguiev. 2021. "Impact of Bone Cement Augmentation on the Fixation Strength of TFNA Blades and Screws" Medicina 57, no. 9: 899. https://doi.org/10.3390/medicina57090899
APA StyleSermon, A., Hofmann-Fliri, L., Zderic, I., Agarwal, Y., Scherrer, S., Weber, A., Altmann, M., Knobe, M., Windolf, M., & Gueorguiev, B. (2021). Impact of Bone Cement Augmentation on the Fixation Strength of TFNA Blades and Screws. Medicina, 57(9), 899. https://doi.org/10.3390/medicina57090899








