Real-Life Experience with Pomalidomide plus Low-Dose Dexamethasone in Patients with Relapsed and Refractory Multiple Myeloma: A Retrospective and Prospective Study
Abstract
:1. Introduction
2. Methods
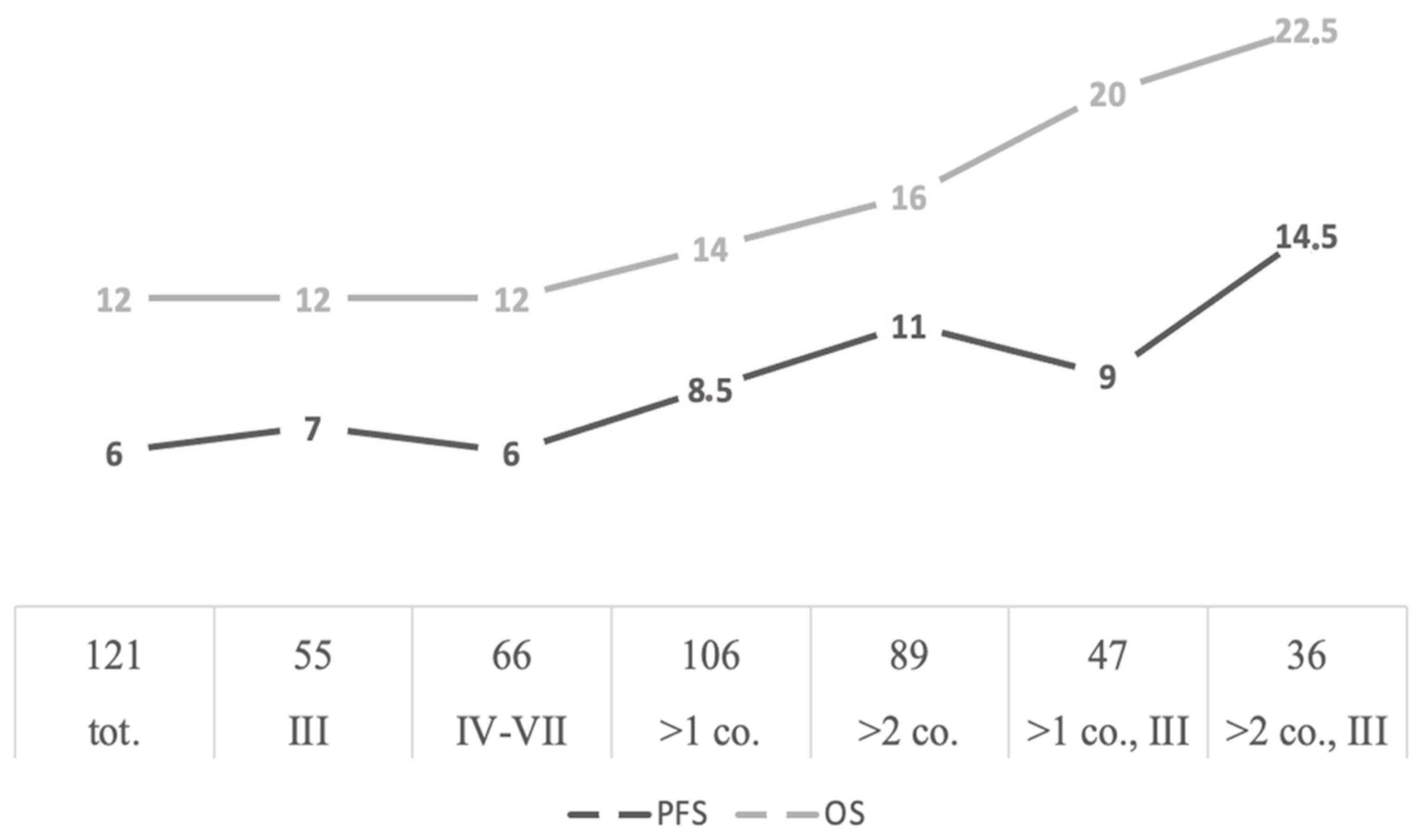
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyle, R.A.; Rajkumar, S.V. Multiple myeloma. Blood 2008, 111, 2962–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastritis, E.; Zervas, K.; Symeonidis, A.; Terpos, E.; Delimbassi, S.; Anagnostopoulos, N.; Michali, E.; Zomas, A.; Katodritou, E.; Gika, D.; et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): An analysis of the Greek Myeloma Study Group (GMSG). Leukemia 2009, 23, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Harousseau, J.L.; Stoppa, A.M.; Sotto, J.J.; Fuzibet, J.G.; Rossi, J.F.; Casassus, P.; Maisonneuve, H.; Facon, T.; Ifrah, N.; et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N. Engl. J. Med. 1996, 335, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larocca, A.; Mina, R.; Gay, F.; Bringhen, S.; Boccadoro, M. Emerging drugs and combinations to treat multiple myeloma. Oncotarget 2017, 8, 60656–60672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, M.; Spencer, A.; Attal, M.; Prince, H.M.; Harousseau, J.L.; Dmoszynska, A.; San Miguel, J.; Hellmann, A.; Facon, T.; Foà, R.; et al. Study Investigators. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N. Engl. J. Med. 2007, 357, 2123–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quach, H.; Ritchie, D.; Stewart, A.K.; Neeson, P.; Harrison, S.; Smyth, M.J.; Prince, H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010, 24, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagannah, S.; Hofmeister, C.C.; Siegel, D.S.; Vij, R.; Lonial, S.; Anderson, K.C.; Chen, M.; Zaki, M.; Richardson, P.G. Pomalidomide (POM) with Low-Dose Dexamethasone (LoDex) in Patients (Pts) with Relapsed and Refractory Multiple Myeloma Who Have Received Prior Therapy with Lenalidomide (LEN) and Bortezomib (BORT): Updated Phase 2 Results and Age Subgroup Analysis. Blood 2012, 120, 450. [Google Scholar] [CrossRef]
- Chim, C.S.; Kumar, S.K.; Orlowski, R.Z.; Cook, G.; Richardson, P.G.; Gertz, M.A.; Giralt, S.; Mateos, M.V.; Leleu, X.; Anderson, K.C. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 2018, 32, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Yee, G.C.; Somerfield, M.R.; Flynn, P.J.; Halabi, S.; Jagannath, S.; Orlowski, R.Z.; Roodman, D.G.; Twilde, P.; Anderson, K. American Society of Clinical Oncology. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J. Clin. Oncol. 2007, 25, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Avet-Loiseau, H. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Matous, J.; Siegel, D.S.D.; Duong, H.K.; et Siegel, D.S.D.; Duong, H.K.; Kasserra, C.; Sternas, L.; Jacques, C.; Klesczewski, K.; Zaki, M.H.; et al. MM-008 trial: Pharmacokinetics (PK) and tolerability of pomalidomide plus low-dose dexamethasone (POM plus LoDEX) in relapsed/refractory multiple myeloma (RRMM) patients with renal impairment (RI). J. Clin. Oncol. 2013, 31, 8585. [Google Scholar] [CrossRef]
- Lopez-Girona, A.; Mendy, D.; Ito, T.; Miller, K.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.; Abbasian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335, Erratum in: Leukemia, 2012, 26, 2445. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; de Wit, E. Recent progress in relapsed multiple myeloma therapy: Implications for treatment decisions. Br. J. Haematol. 2017, 179, 198–218. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Broijl, A. Treatment of relapsed and refractory multiple myeloma. Haematologica 2016, 101, 396–406, Erratum in: Haematologica 2016, 101, 995. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.; Oriol, A. Pomilidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): A randomized, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 781–794. [Google Scholar] [CrossRef]
| Age—yr (range) <65 yr 65–87 yr 75–87 yr | 65 (38–87) 55 (45.5) 66 (54.5) 16 (13.2) |
| Gender Male Female | 57 (47.1) 64 (52.9) |
| ECOG 0 1 2 3 ne | 43 (35.5) 57 (47.1) 15 (12.4) 2 (1.7) 4 (3.3) |
| Type of myeloma IgG κ | 45 (37.2) |
| IgG λ | 24 (19.8) |
| IgA κ | 9 (7.5) |
| IgA λ | 14 (11.6) |
| Light chain κ | 13 (10.7) |
| Light chain λ | 13 (10.7) |
| NS/SP | 3 (2.5) |
| ISS stage I II III ne | 40 (33) 27 (22.3) 25 (20.7) 29 (24) |
| Cytogenetic High risk Standard risk | 55 (45.5) 9 (7.5) 46 (38) |
| Previous lines th. II III IV V VI | 55 (45.5) 36 (29.7) 22 (18.2) 5 (4.1) 3 (2.5) |
| Transplantation Autologous (single or tandem) Allogeneic | 56 (46.3) 2 (1.7) |
| Refractory myeloma Relapsed myeloma na | 57 (47.1) 58 (47.9) 6 (5) |
| Regimen † | Total Patients |
|---|---|
| BVD | 10 |
| CVD | 1 |
| Ctx | 4 |
| Dara | 1 |
| DaraRD | 2 |
| DaraVD | 3 |
| DPACE | 2 |
| EloRD | 1 |
| IxaRD | 1 |
| KD | 6 |
| KCD | 1 |
| KRD | 9 |
| KTD | 1 |
| RD | 60 |
| RMD | 1 |
| TD | 1 |
| VD | 7 |
| VDMy | 6 |
| VDPACE | 2 |
| VMP | 1 |
| VRD | 1 |
| Category † | Total Patients 106 |
|---|---|
| CR VGPR PR MD SD PD ne | 2 (1.9) 8 (7.5) 36 (34) 9 (8.5) 19 (17.9) 25 (23.6) 7 (6.6) |
| ORR (≥PR) CRR (sCR/CR) ORR, III ORR, IV-VII | 46 (43.4) 2 (1.9) 22 (46.8) 24 (40.6) |
| Neutropenia | 31 |
| Anemia | 16 |
| Worsening renal function | 5 |
| Haematological toxicity nos | 3 |
| Pneumonia | 4 |
| Skin changes | 3 |
| Diarrhea | 3 |
| Worsening of cardiac function | 3 |
| Leukopenia | 3 |
| Thrombocytopenia | 3 |
| Dizziness | 2 |
| Deep vein thrombosis | 2 |
| Pyrexia | 1 |
| Sepsis | 2 |
| Trilinear cytopenia | 2 |
| Atrial fibrillation | 1 |
| Decreased appetite | 1 |
| Fatigue | 1 |
| Muscle spasms | 1 |
| Myocardial infarction | 1 |
| Secondary neoplasm | 1 |
| Worsening of vision | 1 |
| Transient ischemic attack | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Giudice, M.L.; Gozzetti, A.; Antonioli, E.; Orciuolo, E.; Ghio, F.; Ciofini, S.; Candi, V.; Fontanelli, G.; Attucci, I.; Formica, G.; et al. Real-Life Experience with Pomalidomide plus Low-Dose Dexamethasone in Patients with Relapsed and Refractory Multiple Myeloma: A Retrospective and Prospective Study. Medicina 2021, 57, 900. https://doi.org/10.3390/medicina57090900
Del Giudice ML, Gozzetti A, Antonioli E, Orciuolo E, Ghio F, Ciofini S, Candi V, Fontanelli G, Attucci I, Formica G, et al. Real-Life Experience with Pomalidomide plus Low-Dose Dexamethasone in Patients with Relapsed and Refractory Multiple Myeloma: A Retrospective and Prospective Study. Medicina. 2021; 57(9):900. https://doi.org/10.3390/medicina57090900
Chicago/Turabian StyleDel Giudice, Maria Livia, Alessandro Gozzetti, Elisabetta Antonioli, Enrico Orciuolo, Francesco Ghio, Sara Ciofini, Veronica Candi, Giulia Fontanelli, Irene Attucci, Giuseppe Formica, and et al. 2021. "Real-Life Experience with Pomalidomide plus Low-Dose Dexamethasone in Patients with Relapsed and Refractory Multiple Myeloma: A Retrospective and Prospective Study" Medicina 57, no. 9: 900. https://doi.org/10.3390/medicina57090900
APA StyleDel Giudice, M. L., Gozzetti, A., Antonioli, E., Orciuolo, E., Ghio, F., Ciofini, S., Candi, V., Fontanelli, G., Attucci, I., Formica, G., Bocchia, M., Galimberti, S., Petrini, M., & Buda, G. (2021). Real-Life Experience with Pomalidomide plus Low-Dose Dexamethasone in Patients with Relapsed and Refractory Multiple Myeloma: A Retrospective and Prospective Study. Medicina, 57(9), 900. https://doi.org/10.3390/medicina57090900







