Assessment of the Impact of Alcohol Consumption Patterns on Heart Rate Variability by Machine Learning in Healthy Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
- Casual drinkers: who usually drink only occasionally low quantities of alcohol (e.g., a toast at a celebration), corresponding to a consumption of 4 units or less in the past week. These subjects scored ≤3 points on the consumption sub-scale of the AUDIT questionnaire.
- Binge drinkers: who consume at least 6 units of alcohol on repeated occasions at least once per month. These subjects scored >3 points on the consumption sub-scale of the AUDIT questionnaire.
- Heavy drinkers: who consume weekly at least 16 units in men and 10 units in women [25].
2.3. HRV Methods
2.4. Statistical Analysis
2.5. Machine Learning Method
3. Results
3.1. General Characteristics
3.2. HRV Time-Domain Alterations
3.3. HRV Frequency-Domain Alterations
3.4. Machine Learning Algorithms
4. Discussion
4.1. Assessment of Alcohol Intake
4.2. Assessment of HRV
4.3. Effects of Cardiovascular Risk Factors on HRV
4.3.1. Age and Gender
4.3.2. Smoking and Body Mass Index
4.3.3. Anxiety and Depression
4.4. Effects of Drinking Patterns on HRV
4.4.1. Casual Drinking
4.4.2. Binge Drinking
4.4.3. Heavy Drinking
4.5. Benefits of Machine Learning Algorithms
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Status Report on Alcohol and Health 2018. Available online: http://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/ (accessed on 27 March 2020).
- Mostofsky, E.; Chahal, H.S.; Mukamal, K.J.; Rimm, E.B.; Mittleman, M.A. Alcohol and Immediate Risk of Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis. Circulation 2016, 133, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashiyama, A.; Okamura, T.; Watanabe, M.; Kokubo, Y.; Wakabayashi, I.; Okayama, A.; Miyamoto, Y. Alcohol Consumption and Cardiovascular Disease Incidence in Men with and without Hypertension: The Suita Study. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2013, 36, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehm, J.; Baliunas, D.; Borges, G.L.G.; Graham, K.; Irving, H.; Kehoe, T.; Parry, C.D.; Patra, J.; Popova, S.; Poznyak, V.; et al. The Relation between Different Dimensions of Alcohol Consumption and Burden of Disease: An Overview. Addict. (Abingdon Engl.) 2010, 105, 817–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanowicz, M.; Schmidt, J.E.; Bostwick, J.M.; Mrazek, D.A.; Karpyak, V.M. Changes in Heart Rate Variability Associated With Acute Alcohol Consumption: Current Knowledge and Implications for Practice and Research. Alcohol. Clin. Exp. Res. 2011, 35, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- van de Borne, P.; Mark, A.L.; Montano, N.; Mion, D.; Somers, V.K. Effects of Alcohol on Sympathetic Activity, Hemodynamics, and Chemoreflex Sensitivity. Hypertension 1997, 29, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Zaza, A.; Lombardi, F. Autonomic Indexes Based on the Analysis of Heart Rate Variability: A View from the Sinus Node. Cardiovasc. Res. 2001, 50, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Rocchetti, M.; Malfatto, G.; Lombardi, F.; Zaza, A. Role of the Input/Output Relation of Sinoatrial Myocytes in Cholinergic Modulation of Heart Rate Variability. J. Cardiovasc. Electrophysiol. 2000, 11, 522–530. [Google Scholar] [CrossRef]
- Monfredi, O.; Lyashkov, A.E.; Johnsen, A.B.; Inada, S.; Schneider, H.; Wang, R.; Nirmalan, M.; Wisloff, U.; Maltsev, V.A.; Lakatta, E.G.; et al. Biophysical Characterization of the Underappreciated and Important Relationship Between Heart Rate Variability and Heart Rate. Hypertension 2014, 64, 1334–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, A.; Camm, A.J.; Cerutti, S.; Guzik, P.; Huikuri, H.; Lombardi, F.; Malik, M.; Peng, C.-K.; Porta, A.; Sassi, R.; et al. Reference Values of Heart Rate Variability. Heart Rhythm 2017, 14, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenks, S.; Peacock, W.F.; Cornelius, A.P.; Shafer, S.; Pillow, M.T.; Rayasam, S.S. Heart Rate and Heart Rate Variability in Emergency Medicine. Am. J. Emerg. Med. 2020, 38, 1335–1339. [Google Scholar] [CrossRef]
- Cozma, D.; Streian, C.G.; Petrescu, L.; Mornos, C. Subclinical Left Atrium Remodelling in Patients with Frequent Premature Ventricular Contractions. Kardiol. Pol. 2014, 72, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Huang, Y.-C.; Huang, W.-L. Heart Rate Variability as a Potential Biomarker for Alcohol Use Disorders: A Systematic Review and Meta-Analysis. Drug Alcohol Depend. 2019, 204, 107502. [Google Scholar] [CrossRef] [PubMed]
- Weise, F.; Krell, D.; Brinkhoff, N. Acute Alcohol Ingestion Reduces Heart Rate Variability. Drug Alcohol Depend. 1986, 17, 89–91. [Google Scholar] [CrossRef]
- Koskinen, P.; Virolainen, J.; Kupari, M. Acute Alcohol Intake Decreases Short-Term Heart Rate Variability in Healthy Subjects. Clin. Sci. 1994, 87, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, F.T.; Fukunaga, T.; Kajiura, H.; Umeno, K.; Takakura, H.; Ono, T.; Nishijo, H. Effects of Aldehyde Dehydrogenase-2 Genotype on Cardiovascular and Endocrine Responses to Alcohol in Young Japanese Subjects. Auton. Neurosci. 2002, 102, 60–70. [Google Scholar] [CrossRef]
- Ryan, J.M.; Howes, L.G. Relations between Alcohol Consumption, Heart Rate, and Heart Rate Variability in Men. Heart 2002, 88, 641–642. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.R.; Valladares, E.M.; Motivala, S.; Thayer, J.F.; Ehlers, C.L. Association between Nocturnal Vagal Tone and Sleep Depth, Sleep Quality, and Fatigue in Alcohol Dependence. Psychosom. Med. 2006, 68, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Araki, S.; Yokoyama, K.; Sata, F.; Yamashita, K.; Ono, Y. Autonomic Neurotoxicity of Alcohol Assessed by Heart Rate Variability. J. Auton. Nerv. Syst. 1994, 48, 105–111. [Google Scholar] [CrossRef]
- Bzdok, D.; Altman, N.; Krzywinski, M. Statistics versus Machine Learning. Nat. Methods 2018, 15, 233–234. [Google Scholar] [CrossRef]
- Sunagawa, K.; Kawada, T.; Nakahara, T. Dynamic Nonlinear Vago-Sympathetic Interaction in Regulating Heart Rate. Heart Vessels 1998, 13, 157–174. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. In Screening and Brief Intervention for Alcohol Problems in Primary Care; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Romanian National Institute of Public Health (INSP). Luna Naţională A Informării Despre Efectele Consumului De Alcool. Available online: http://insp.gov.ro/sites/cnepss/wp-content/uploads/2016/01/ANALIZA-DE-SITUATIE-2019.pdf (accessed on 20 April 2020).
- Aalto, M.; Alho, H.; Halme, J.T.; Seppä, K. AUDIT and Its Abbreviated Versions in Detecting Heavy and Binge Drinking in a General Population Survey. Drug Alcohol Depend. 2009, 103, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; McDonnell, K.; Kris, M.G.; Shen, R.; Sima, C.S.; Bach, P.B.; Rizvi, N.A.; Riely, G.J. Pack-Years of Cigarette Smoking as a Prognostic Factor in Patients with Stage IIIB/IV Nonsmall Cell Lung Cancer. Cancer 2010, 116, 670–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Denver, J.W.; Reed, S.F.; Porges, S.W. Methodological Issues in the Quantification of Respiratory Sinus Arrhythmia. Biol. Psychol. 2007, 74, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV--Heart Rate Variability Analysis Software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.A. Alcohol and Malnutrition in the Pathogenesis of Experimental Alcoholic Cardiomyopathy. J. Pathol. 1980, 130, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Pop, G.N.; Faur, C.; Goldis, A.; Beceanu, A.; Abu-Awwad, A.; Ciocarlie, T. Alterations of Heart Rate Variability and Heart Rate Turbulence in Patients with Dilated Alcoholic and Non-Alcoholic Cardiomyopathy. Rev. Chim. 2020. [Google Scholar] [CrossRef]
- Lopes, C.; Andreozzi, V.L.; Ramos, E.; Sá Carvalho, M. Modelling over Week Patterns of Alcohol Consumption. Alcohol Alcohol. 2008, 43, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinner, H.A.; Sheu, W.J. Reliability of Alcohol Use Indices. The Lifetime Drinking History and the MAST. J. Stud. Alcohol 1982, 43, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Chaikelson, J.S.; Arbuckle, T.Y.; Lapidus, S.; Gold, D.P. Measurement of Lifetime Alcohol Consumption. J. Stud. Alcohol 1994, 55, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.; Marshall, J.R.; Trevisan, M.; Freudenheim, J.L.; Chan, A.W.; Markovic, N.; Vána, J.E.; Priore, R.L. Test-Retest Reliability of the Cognitive Lifetime Drinking History. Am. J. Epidemiol. 1997, 146, 975–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townshend, J.M.; Duka, T. Patterns Of Alcohol Drinking In A Population Of Young Social Drinkers: A Comparison Of Questionnaire And Diary Measures. Alcohol Alcohol. 2002, 37, 187–192. [Google Scholar] [CrossRef] [Green Version]
- van Ravenswaaij-Arts, C.M.A.; Kollee, L.A.A.; Hopman, J.C.W.; Stoelinga, G.B.A.; van Geijn, H.P. Heart Rate Variability. Ann. Intern. Med. 1993, 118, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Camm, A.J. Heart Rate Variability. Clin. Cardiol. 1990, 13, 570–576. [Google Scholar] [CrossRef]
- Porges, S.W. The Polyvagal Perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef]
- Palatini, P. Elevated Heart Rate as a Predictor of Increased Cardiovascular Morbidity. J. Hypertens. Suppl. Off. J. Int. Soc. Hypertens. 1999, 17, S3–S10. [Google Scholar]
- Berntson, G.G.; Cacioppo, J.T. Heart Rate Variability: Stress and Psychiatric Conditions. In Dynamic Electrocardiography; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 57–64. ISBN 978-0-470-98748-3. [Google Scholar]
- Porter, T.R.; Eckberg, D.L.; Fritsch, J.M.; Rea, R.F.; Beightol, L.A.; Schmedtje, J.F.; Mohanty, P.K. Autonomic Pathophysiology in Heart Failure Patients. Sympathetic-Cholinergic Interrelations. J. Clin. Investig. 1990, 85, 1362–1371. [Google Scholar] [CrossRef] [Green Version]
- Weippert, M.; Kumar, M.; Kreuzfeld, S.; Arndt, D.; Rieger, A.; Stoll, R. Comparison of Three Mobile Devices for Measuring R–R Intervals and Heart Rate Variability: Polar S810i, Suunto T6 and an Ambulatory ECG System. Eur. J. Appl. Physiol. 2010, 109, 779–786. [Google Scholar] [CrossRef]
- Zhang, J. Effect of Age and Sex on Heart Rate Variability in Healthy Subjects. J. Manip. Physiol. Ther. 2007, 30, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.; Malik, M. Changes in Heart Rate Variability with Age. Pacing Clin. Electrophysiol. 1996, 19, 1863–1866. [Google Scholar] [CrossRef]
- Antelmi, I.; de Paula, R.S.; Shinzato, A.R.; Peres, C.A.; Mansur, A.J.; Grupi, C.J. Influence of Age, Gender, Body Mass Index, and Functional Capacity on Heart Rate Variability in a Cohort of Subjects without Heart Disease. Am. J. Cardiol. 2004, 93, 381–385. [Google Scholar] [CrossRef]
- Abhishekh, H.A.; Nisarga, P.; Kisan, R.; Meghana, A.; Chandran, S.; Raju, T.; Sathyaprabha, T.N. Influence of Age and Gender on Autonomic Regulation of Heart. J. Clin. Monit. Comput. 2013, 27, 259–264. [Google Scholar] [CrossRef]
- Murgia, F.; Melotti, R.; Foco, L.; Gögele, M.; Meraviglia, V.; Motta, B.; Steger, A.; Toifl, M.; Sinnecker, D.; Müller, A.; et al. Effects of Smoking Status, History and Intensity on Heart Rate Variability in the General Population: The CHRIS Study. PLoS ONE 2019, 14, e0215053. [Google Scholar] [CrossRef]
- Koenig, J.; Jarczok, M.N.; Warth, M.; Ellis, R.J.; Bach, C.; Hillecke, T.K.; Thayer, J.F. Body Mass Index Is Related to Autonomic Nervous System Activity as Measured by Heart Rate Variability—A Replication Using Short Term Measurements. J. Nutr. Health Aging 2014, 18, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Schmidt, F.M.; Sander, C.; Hegerl, U. Heart Rate Variability as Indicator of Clinical State in Depression. Front. Psychiatry 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giurgi-Oncu, C.; Tudoran, C.; Enatescu, V.R.; Tudoran, M.; Pop, G.N.; Bredicean, C. Evolution of Heart Rate Variability and Heart Rate Turbulence in Patients with Depressive Illness Treated with Selective Serotonin Reuptake Inhibitors. Medicina 2020, 56, 590. [Google Scholar] [CrossRef]
- Pah, A.-M.; Bucuras, P.; Buleu, F.; Tudor, A.; Iurciuc, S.; Velimirovici, D.; Streian, C.G.; Badalica-Petrescu, M.; Christodorescu, R.; Dragan, S. The Importance of DS-14 and HADS Questionnaires in Quantifying Psychological Stress in Type 2 Diabetes Mellitus. Medicina 2019, 55, 569. [Google Scholar] [CrossRef] [Green Version]
- Tudoran, M.; Tudoran, C.; Ciocarlie, T.; Giurgi-Oncu, C. Aspects of Diastolic Dysfunction in Patients with New and Recurrent Depression. PLoS ONE 2020, 15, e0228449. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, J.A.; Quintana, D.S.; Abbott, M.J.-A.; Kemp, A.H. Anxiety Disorders Are Associated with Reduced Heart Rate Variability: A Meta-Analysis. Front. Psychiatry 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Nunan, D.; Sandercock, G.R.H.; Brodie, D.A. A Quantitative Systematic Review of Normal Values for Short-Term Heart Rate Variability in Healthy Adults. Pacing Clin. Electrophysiol. PACE 2010, 33, 1407–1417. [Google Scholar] [CrossRef]
- DePetrillo, P.B.; White, K.V.; Liu, M.; Hommer, D.; Goldman, D. Effects of Alcohol Use and Gender on the Dynamics of EKG Time-Series Data. Alcohol. Clin. Exp. Res. 1999, 23, 745–750. [Google Scholar] [CrossRef]
- Thayer, J.F.; Hall, M.; Sollers, J.J., III; Fischer, J.E. Alcohol Use, Urinary Cortisol, and Heart Rate Variability in Apparently Healthy Men: Evidence for Impaired Inhibitory Control of the HPA Axis in Heavy Drinkers. Int. J. Psychophysiol. 2006, 59, 244–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupari, M.; Virolainen, J.; Koskinen, P.; Tikkanen, M.J. Short-Term Heart Rate Variability and Factors Modifying the Risk of Coronary Artery Disease in a Population Sample. Am. J. Cardiol. 1993, 72, 897–903. [Google Scholar] [CrossRef]
- Pomeranz, B.; Macaulay, R.J.; Caudill, M.A.; Kutz, I.; Adam, D.; Gordon, D.; Kilborn, K.M.; Barger, A.C.; Shannon, D.C.; Cohen, R.J. Assessment of Autonomic Function in Humans by Heart Rate Spectral Analysis. Am. J. Physiol. 1985, 248, H151–H153. [Google Scholar] [CrossRef] [PubMed]
- Akselrod, S.; Gordon, D.; Ubel, F.A.; Shannon, D.C.; Berger, A.C.; Cohen, R.J. Power Spectrum Analysis of Heart Rate Fluctuation: A Quantitative Probe of Beat-to-Beat Cardiovascular Control. Science 1981, 213, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular Neural Regulation Explored in the Frequency Domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willems, J.L.; Abreu-Lima, C.; Arnaud, P.; van Bemmel, J.H.; Brohet, C.; Degani, R.; Denis, B.; Gehring, J.; Graham, I.; van Herpen, G. The Diagnostic Performance of Computer Programs for the Interpretation of Electrocardiograms. N. Engl. J. Med. 1991, 325, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Agliari, E.; Barra, A.; Barra, O.A.; Fachechi, A.; Franceschi Vento, L.; Moretti, L. Detecting Cardiac Pathologies via Machine Learning on Heart-Rate Variability Time Series and Related Markers. Sci. Rep. 2020, 10, 8845. [Google Scholar] [CrossRef] [PubMed]
- Chiew, C.J.; Liu, N.; Tagami, T.; Wong, T.H.; Koh, Z.X.; Ong, M.E.H. Heart Rate Variability Based Machine Learning Models for Risk Prediction of Suspected Sepsis Patients in the Emergency Department. Medicine 2019, 98, e14197. [Google Scholar] [CrossRef]
- Krittanawong, C.; Virk, H.U.H.; Kumar, A.; Aydar, M.; Wang, Z.; Stewart, M.P.; Halperin, J.L. Machine Learning and Deep Learning to Predict Mortality in Patients with Spontaneous Coronary Artery Dissection. Sci. Rep. 2021, 11, 8992. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef]
- Shameer, K.; Johnson, K.W.; Glicksberg, B.S.; Dudley, J.T.; Sengupta, P.P. Machine Learning in Cardiovascular Medicine: Are We There Yet? Heart 2018, 104, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, S.; Gast, K.B.; de Mutsert, R.; Swenne, C.A.; Jukema, J.W.; Middeldorp, S.; Rosendaal, F.R.; Dekkers, O.M. Heart Rate Variability and First Cardiovascular Event in Populations without Known Cardiovascular Disease: Meta-Analysis and Dose-Response Meta-Regression. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2013, 15, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Larson, M.G.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Feldman, C.L.; Levy, D. Impact of Reduced Heart Rate Variability on Risk for Cardiac Events. The Framingham Heart Study. Circulation 1996, 94, 2850–2855. [Google Scholar] [CrossRef] [PubMed]
- Migliaro, E.R.; Canetti, R.; Contreras, P.; Hakas, M. Heart Rate Variability: Short-Term Studies Are as Useful as Holter to Differentiate Diabetic Patients from Healthy Subjects. Ann. Noninvasive Electrocardiol. 2003, 8, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nussinovitch, U.; Elishkevitz, K.P.; Katz, K.; Nussinovitch, M.; Segev, S.; Volovitz, B.; Nussinovitch, N. Reliability of Ultra-Short ECG Indices for Heart Rate Variability. Ann. Noninvasive Electrocardiol. 2011, 16, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Min, K.B.; Min, J.-Y.; Paek, D.; Cho, S.-I.; Son, M. Is 5-Minute Heart Rate Variability a Useful Measure for Monitoring the Autonomic Nervous System of Workers? Int. Heart J. 2008, 49, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter | Units | Description |
|---|---|---|
| Time Domain | ||
| RR | ms | The mean of RR intervals |
| SDNN | ms | Standard deviation of RR intervals |
| HR | /min | The mean heart rate |
| STD HR | /min | Standard deviation of instantaneous heart rate values |
| RMSSD | ms | Square root of the mean squared differences between successive RR intervals |
| NN50 | count | Number of successive RR interval pairs that differ more than 50 ms |
| pNN50 | % | NN50 divided by the total number of RR intervals |
| RRTI | − | The integral of the RR interval histogram divided by the height of the histogram (triangular index) |
| TINN | ms | Baseline width of the RR interval histogram |
| Frequency Domain | ||
| VLF, LF, and HF peaks | Hz | Peak frequencies for VLF, LF and HF bands |
| VLF, LF, and HF powers | ms2 | Absolute powers of VLF, LF and HF bands |
| VLF, LF, and HF powers | % | Relative powers of VLF, LF and HF bands |
| VLF [%] = VLF [ms2]/total power [ms2] × 100% | ||
| LF [%] = LF [ms2]/total power [ms2] × 100% | ||
| HF [%] = HF [ms2]/total power [ms2] × 100% | ||
| LF and HF powers | n.u. | Powers of LF and HF bands in normalized units |
| LF [n.u.] = LF [ms2]/(total power [ms2] − VLF [ms2]) | ||
| HF [n.u.] = HF [ms2]/(total power [ms2] − VLF [ms2]) | ||
| LF/HF | − | Ratio between LF and HF band powers |
| Total power | ms2 | Total spectral power |
| Casual Drinkers n = 45 | Binge Drinkers n = 62 | Heavy Drinkers n = 35 | p | |
|---|---|---|---|---|
| Age (years) | 28 [27–32] | 30 [27–33] | 27 [24–29] | 0.001 |
| Male/Female gender | 24/21 | 46/16 | 26/9 | 0.057 |
| Urban area | 36 (80%) | 40 (64.5%) | 24 (68.6%) | 0.215 |
| BMI (kg/m2) | 26.5 [21.5–30] | 25 [23.5–28.5] | 25 [23–28.5] | 0.920 |
| Physical activity > 4 h/week | 9 (20%) | 32 (51.6%) | 10 (28.6%) | 0.002 |
| HADS questionnaire | ||||
| Anxiety | 6 [4–9] | 4 [2–7] | 6 [4–8] | 0.009 |
| Depression | 3 [1–4] | 3 [1.75–6] | 2 [1–6] | 0.740 |
| Alcohol consumption | ||||
| AUDIT questionnaire | 3 [1–5] | 6 [5–8] | 13 [10–15] | <0.001 |
| Weekly intake (units) | 2 [0–4.5] | 7 [5–9] | 19 [16–21] | <0.001 |
| Drinking start age | 20 [17.75–25.5] | 18 [17–21] | 18 [16–20] | 0.016 |
| YAI index | 19 [0–38.5] | 72 [31.5–91.75] | 151 [105–198] | <0.001 |
| Alcohol type (%) | ||||
| Beer | 80 [70–83.75] | 70 [20–80] | 50 [20–70] | 0.002 |
| Wine | 10 [0–20] | 10 [5–50] | 30 [15–40] | 0.001 |
| Distilled Drinks | 10 [8.75–15] | 15 [8–30] | 10 [0–35] | 0.311 |
| Smoking | ||||
| Incidence | 23 (51.1%) | 23 (37.1%) | 32 (91.4%) | <0.001 |
| Pack Year index | 4 [2.75–11.75] | 10 [4–18] | 8 [3–14.25] | 0.133 |
| Casual Drinkers n = 45 | Binge Drinkers n = 62 | Heavy Drinkers n = 35 | p | |
|---|---|---|---|---|
| RR (ms) | 825 [767–852] | 856 [758–940] | 771 [705–883] | 0.160 |
| Min HR (/min) | 68 [57–77] | 61.5 [56.5–65] | 65.9 [56.5–73.3] | 0.127 |
| Max HR (/min) | 87 [81.6–99.3] | 85 [73.7–95.7] | 92 [74.6–99.4] | 0.313 |
| Mean HR (/min) | 73.5 [67.8–82] | 68 [65–78.8] | 75.7 [64–82.7] | 0.228 |
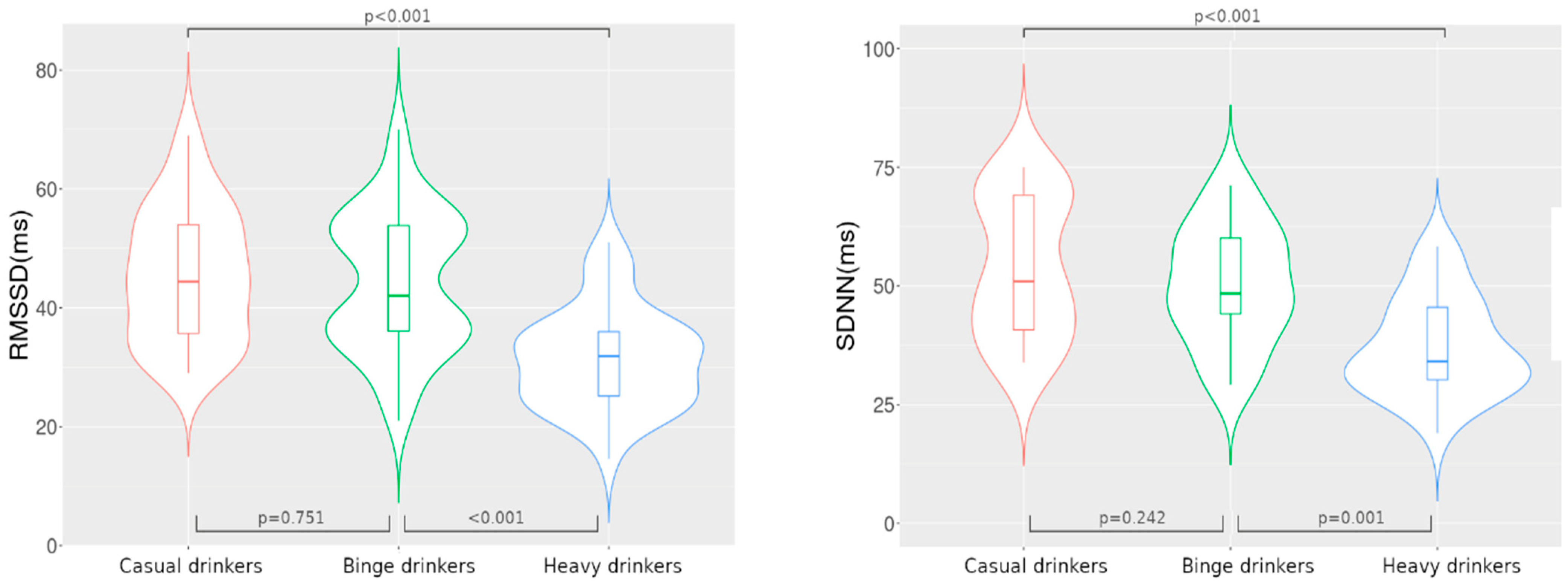
| SDNN (ms) | 48.5 [40.7–69.8] | 46.8 [39.5–59.6] | 35.5 [31–47.9] | <0.001 * |
| RMSSD (ms) | 44.4 [35.1–54.5] | 42 [35.9–54] | 31.9 [24.6–36.1] | <0.001 * |
| lnRMMSD | 3.79 [3.55–3.99] | 3.73 [3.58–3.98] | 3.46 [3.20–3.58] | <0.001 * |
| NN50 (count) | 79.8 [44.7–98.1]] | 53.3 [8.5–97.5] | 39.9 [19.3–48.5] | 0.049 * |
| pNN50 (%) | 15.6 [12.2–28.6] | 15.7 [11.5–32.1] | 9.5 [7.8–26] | 0.043 * |
| RRTI | 11.1 [7.4–14.9] | 9.7 [6.5–12.1] | 10.1 [6.7–15.3] | 0.424 |
| TINN (ms) | 285.2 [183–331.6] | 215.1 [151.7–322.5] | 249 [157–292.4] | 0.248 |
| Casual Drinkers n = 45 | Binge Drinkers n = 62 | Heavy Drinkers n = 35 | p | |
|---|---|---|---|---|
| VLF peak (Hz) | 0.037 [0.029–0.040] | 0.037 [0.029–0.039] | 0.037 [0.029–0.040] | 0.706 |
| VLF (ms2) | 72 [29.26–132.66] | 111 [53.29–173.89] | 125 [49.2–401.9] | 0.096 |
| LF peak (Hz) | 0.101 [0.080–0.106] | 0.087 [0.067–0.114] | 0.097 [0.078–0.121] | 0.598 |
| LF (ms2) | 715.1 [437.6–1779] | 1226 [954.7–1621.6] | 815 [299.8–2878.7] | 0.284 |
| LF (n.u.) | 59.9 [55.2–72.3] | 56.7 [50.8–69.8] | 65.6 [55.1–75.5] | 0.180 |
| HF peak (Hz) | 0.219 [0.193–0.248] | 0.212 [0.177–0.279] | 0.163 [0.153–0.289] | 0.042 * |
| HF (ms2) | 726.1 [369.5–1015] | 864.9 [674–1290.8] | 392.9 [206.6–519] | 0.002 * |
| HF (n.u.) | 40.1 [28.8–43.7] | 43.2 [30.8–49.1] | 32.2 [25.8–42.8] | 0.048 * |
| LF/HF ratio | 1.53 [1.27–2.71] | 1.31 [1.03–2.26] | 1.97 [1.27–2.89] | 0.165 |
| Total power (ms) | 1042 [982–3068] | 2329 [1781–3173] | 1064 [932–1692] | 0.002 * |
| Algorithm | Performance * |
|---|---|
| Gradient Boosting | 0.885 |
| Neural Net (MLP) | 0.877 |
| XGBoost Tree | 0.876 |
| Random Forrest | 0.812 |
| Support-Vector Machine | 0.801 |
| Linear-AS | 0.737 |
| Variables | Time Domain | Frequency Domain | Total Scaled Impact | ||||
|---|---|---|---|---|---|---|---|
| RMSSD | SDNN | pNN50 | LF | HF | LF/HF Ratio | ||
| Age | 0.631 | 1 | 1 | 1 | 1 | 1 | 1 |
| Alcohol a | 1 | 0.741 | 0.504 | 0.451 | 0.246 | 0.767 | 0.66 |
| Smoking b | 0.263 | 0.217 | 0.52 | 0.471 | 0.188 | 0.461 | 0.38 |
| Anxiety c | 0.091 | 0.201 | 0.307 | 0.294 | 0.114 | 0.997 | 0.36 |
| Depression c | 0.151 | 0.245 | 0.137 | 0.288 | 0.204 | 0.909 | 0.34 |
| BMI | 0.335 | 0.223 | 0.348 | 0.148 | 0.213 | 0.436 | 0.30 |
| Gender | 0.143 | 0.079 | 0.024 | 0.081 | 0.057 | 0.029 | 0.07 |
| Physical activity | 0.144 | 0.071 | 0.053 | 0.042 | 0.033 | 0.014 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, G.N.; Christodorescu, R.; Velimirovici, D.E.; Sosdean, R.; Corbu, M.; Bodea, O.; Valcovici, M.; Dragan, S. Assessment of the Impact of Alcohol Consumption Patterns on Heart Rate Variability by Machine Learning in Healthy Young Adults. Medicina 2021, 57, 956. https://doi.org/10.3390/medicina57090956
Pop GN, Christodorescu R, Velimirovici DE, Sosdean R, Corbu M, Bodea O, Valcovici M, Dragan S. Assessment of the Impact of Alcohol Consumption Patterns on Heart Rate Variability by Machine Learning in Healthy Young Adults. Medicina. 2021; 57(9):956. https://doi.org/10.3390/medicina57090956
Chicago/Turabian StylePop, Gheorghe Nicusor, Ruxandra Christodorescu, Dana Emilia Velimirovici, Raluca Sosdean, Miruna Corbu, Olivia Bodea, Mihaela Valcovici, and Simona Dragan. 2021. "Assessment of the Impact of Alcohol Consumption Patterns on Heart Rate Variability by Machine Learning in Healthy Young Adults" Medicina 57, no. 9: 956. https://doi.org/10.3390/medicina57090956
APA StylePop, G. N., Christodorescu, R., Velimirovici, D. E., Sosdean, R., Corbu, M., Bodea, O., Valcovici, M., & Dragan, S. (2021). Assessment of the Impact of Alcohol Consumption Patterns on Heart Rate Variability by Machine Learning in Healthy Young Adults. Medicina, 57(9), 956. https://doi.org/10.3390/medicina57090956








