Usefulness of KL-6 for Predicting Clinical Outcomes in Hospitalized COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. KL-6 Assay
2.3. Statistical Analysis
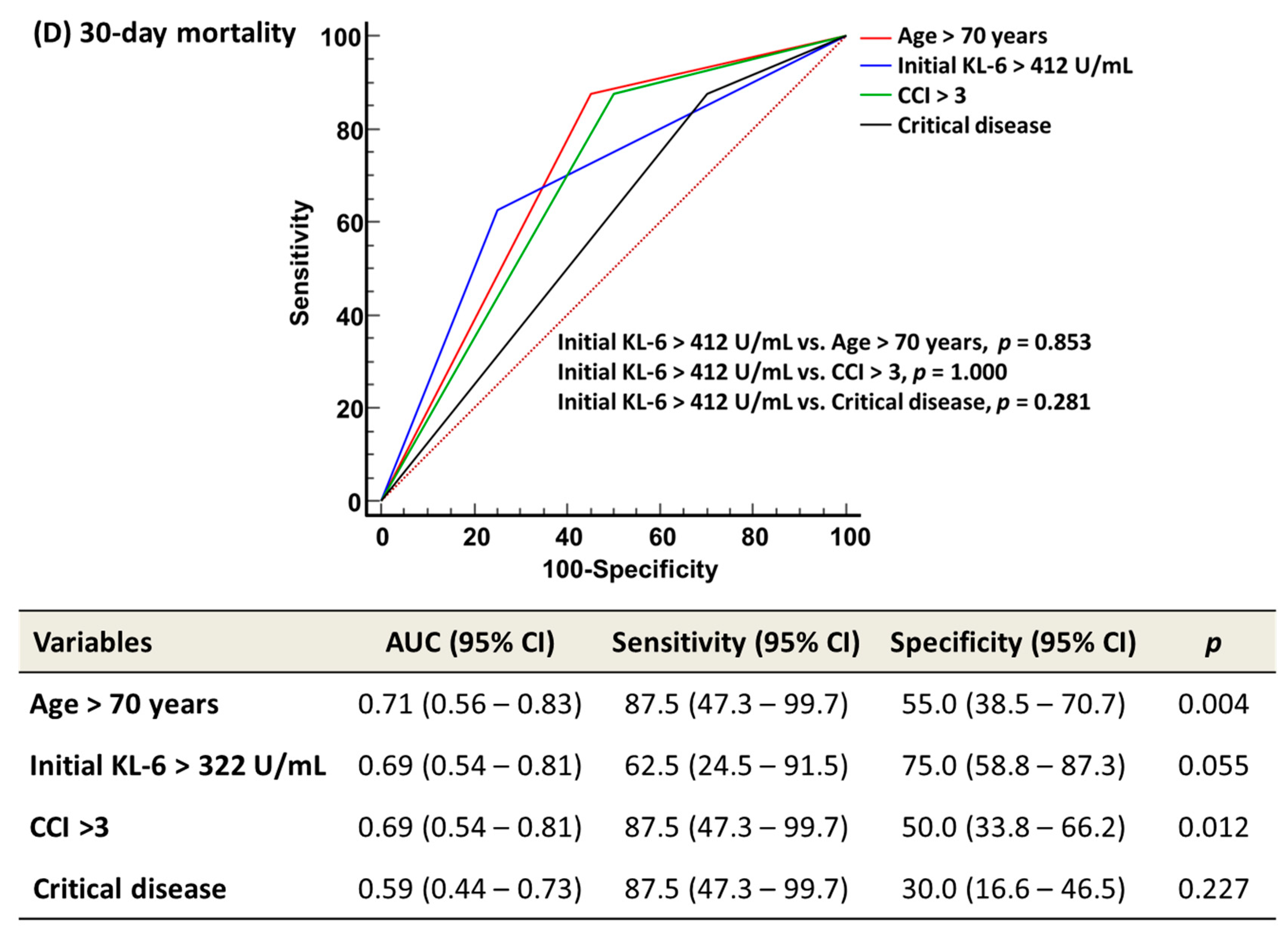
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, K.H.; Lee, S.W.; Kim, T.S.; Huh, H.J.; Lee, J.; Kim, S.Y.; Park, J.S.; Kim, G.J.; Sung, H.; Roh, K.H.; et al. Guidelines for Laboratory Diagnosis of Coronavirus Disease 2019 (COVID-19) in Korea. Ann. Lab. Med. 2020, 40, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Hur, M.; Kim, H.; Lee, C.H.; Lee, J.H.; Kim, H.W.; Nam, M. Prognostic Utility of Procalcitonin, Presepsin, and the VACO Index for Predicting 30-day Mortality in Hospitalized COVID-19 Patients. Ann. Lab. Med. 2022, 42, 406–414. [Google Scholar] [CrossRef]
- Word Health Organization: Living Guidance for Clinical Management of COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 4 July 2022).
- Supady, A.; Badulak, J.; Evans, L.; Curtis, J.R.; Brodie, D. Should We Ration Extracorporeal Membrane Oxygenation during the COVID-19 Pandemic? Lancet Respir. Med. 2021, 9, 326–328. [Google Scholar] [CrossRef]
- Kondili, E.; Makris, D.; Georgopoulos, D.; Rovina, N.; Kotanidou, A.; Koutsoukou, A. COVID-19 ARDS: Points to Be Considered in Mechanical Ventilation and Weaning. J. Pers. Med. 2021, 11, 1109. [Google Scholar] [CrossRef]
- Griffiths, M.J.D.; McAuley, D.F.; Perkins, G.D.; Barrett, N.; Blackwood, B.; Boyle, A.; Chee, N.; Connolly, B.; Dark, P.; Finney, S.; et al. Guidelines on the Management of Acute Respiratory Distress Syndrome. BMJ Open Respir. Res. 2019, 6, e000420. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Guarnieri, G.; Braccioni, F.; Lococo, S.; Molena, B.; Cecchetto, A.; Giraudo, C.; Bertagna De Marchi, L.; Caminati, M.; Senna, G. The Pathogenesis, Epidemiology and Biomarkers of Susceptibility of Pulmonary Fibrosis in COVID-19 Survivors. Clin. Chem. Lab. Med. 2022, 60, 307–316. [Google Scholar] [CrossRef]
- Iwasaki, M.; Saito, J.; Zhao, H.; Sakamoto, A.; Hirota, K.; Ma, D. Inflammation Triggered by SARS-CoV-2 and ACE2 Augment Drives Multiple Organ Failure of Severe COVID-19: Molecular Mechanisms and Implications. Inflammation 2021, 44, 13–34. [Google Scholar] [CrossRef]
- Yim, J.; Lim, H.H.; Kwon, Y. COVID-19 and Pulmonary Fibrosis: Therapeutics in Clinical Trials, Repurposing, and Potential Development. Arch. Pharm. Res. 2021, 44, 499–513. [Google Scholar] [CrossRef]
- Shaw, R.J.; Bradbury, C.; Abrams, S.T.; Wang, G.; Toh, C. COVID-19 and Immunothrombosis: Emerging Understanding and Clinical Management. Br. J. Haematol. 2021, 194, 518–529. [Google Scholar] [CrossRef]
- Wigén, J.; Löfdahl, A.; Bjermer, L.; Elowsson-Rendin, L.; Westergren-Thorsson, G. Converging Pathways in Pulmonary Fibrosis and COVID-19—The Fibrotic Link to Disease Severity. Respir. Med. X 2020, 2, 100023. [Google Scholar] [CrossRef]
- Hajjar, L.A.; Costa, I.; Rizk, S.I.; Biselli, B.; Gomes, B.R.; Bittar, C.S.; de Oliveira, G.Q.; de Almeida, J.P.; de Oliveira Bello, M.V.; Garzillo, C.; et al. Intensive Care Management of Patients with COVID-19: A Practical Approach. Ann. Intensive Care 2021, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Kostopanagiotou, K.; Schuurmans, M.M.; Inci, I.; Hage, R. COVID-19-Related End Stage Lung Disease: Two Distinct Phenotypes. Ann. Med. 2022, 54, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Juan Guardela, B.M.; Sun, J.; Zhang, T.; Xu, B.; Balnis, J.; Huang, Y.; Ma, S.F.; Molyneaux, P.L.; Maher, T.M.; Noth, I.; et al. 50-Gene Risk Profiles in Peripheral Blood Predict COVID-19 Outcomes: A Retrospective, Multicenter Cohort Study. EBioMedicine 2021, 69, 103439. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, P.; Oldham, J.M. Fibrotic Lung Disease: A Molecular Glimpse into Severe COVID-19? EBioMedicine 2021, 69, 103470. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Kim, K.; Kang, E.K.; Yang, H.J.; Lee, E. Prediction of COVID-19-Related Mortality and 30-Day and 60-Day Survival Probabilities Using a Nomogram. J. Korean Med. Sci. 2021, 36, e248. [Google Scholar] [CrossRef]
- Salaffi, F.; Carotti, M.; Di Carlo, M.; Ceccarelli, L.; Galli, M.; Sarzi-Puttini, P.; Giovagnoni, A. Predicting Severe/Critical Outcomes in Patients With SARS-CoV2 Pneumonia: Development of the PrediCtion SeveRe/CrItical ouTcome in COVID-19 (CRITIC) Model. Front. Med. 2021, 8, 695195. [Google Scholar] [CrossRef]
- Ishikawa, N.; Hattori, N.; Yokoyama, A.; Kohno, N. Utility of KL-6/MUC1 in the Clinical Management of Interstitial Lung Diseases. Respir. Investig. 2012, 50, 3–13. [Google Scholar] [CrossRef]
- Stainer, A.; Faverio, P.; Busnelli, S.; Catalano, M.; Della Zoppa, M.; Marruchella, A.; Pesci, A.; Luppi, F. Molecular Biomarkers in Idiopathic Pulmonary Fibrosis: State of the Art and Future Directions. Int. J. Mol. Sci. 2021, 22, 6255. [Google Scholar] [CrossRef]
- Frix, A.N.; Schoneveld, L.; Ladang, A.; Henket, M.; Duysinx, B.; Vaillant, F.; Misset, B.; Moutschen, M.; Louis, R.; Cavalier, E.; et al. Could KL-6 Levels in COVID-19 Help to Predict Lung Disease? Respir. Res. 2020, 21, 309. [Google Scholar] [CrossRef]
- Deng, K.; Fan, Q.; Yang, Y.; Deng, X.; He, R.; Tan, Y.; Lan, Y.; Deng, X.; Pan, Y.; Wang, Y.; et al. Prognostic Roles of KL-6 in Disease Severity and Lung Injury in COVID-19 Patients: A Longitudinal Retrospective Analysis. J. Med. Virol. 2021, 93, 2505–2512. [Google Scholar] [CrossRef]
- Arnold, D.T.; Donald, C.; Lyon, M.; Hamilton, F.W.; Morley, A.J.; Attwood, M.; Dipper, A.; Barratt, S.L. Krebs von den Lungen 6 (KL-6) as a Marker for Disease Severity and Persistent Radiological Abnormalities Following COVID-19 Infection at 12 Weeks. PLoS ONE 2021, 16, e0249607. [Google Scholar] [CrossRef] [PubMed]
- Scarpati, G.; Polverino, B.; Boffardi, M.; Piazza, O. KL-6 levels in COVID-19. Transl. Med. UniSa 2020, 23, 67. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, M.; Cameli, P.; Refini, R.M.; Bergantini, L.; Alonzi, V.; Lanzarone, N.; Bennett, D.; Rana, G.D.; Montagnani, F.; Scolletta, S.; et al. Serum KL-6 Concentrations as a Novel biomarker of Severe COVID-19. J. Med. Virol. 2020, 92, 2216–2220. [Google Scholar] [CrossRef] [PubMed]
- Suryananda, T.D.; Yudhawati, R. Association of Serum KL-6 Levels on COVID-19 Severity: A Cross-Sectional Study Design with Purposive Sampling. Ann. Med. Surg. 2021, 69, 102673. [Google Scholar] [CrossRef] [PubMed]
- Awano, N.; Inomata, M.; Kuse, N.; Tone, M.; Takada, K.; Muto, Y.; Fujimoto, K.; Akagi, Y.; Mawatari, M.; Ueda, A.; et al. Serum KL-6 Level Is a Useful Biomarker for Evaluating the Severity of Coronavirus Disease 2019. Respir. Investig. 2020, 58, 440–447. [Google Scholar] [CrossRef]
- Chen, H.; Qin, R.; Huang, Z.; Luo, W.; Zheng, P.; Huang, H.; Hu, H.; Wang, H.; Sun, B. Clinical Relevance of Serum Krebs von den Lungen-6 Levels in Patients with Coronavirus Disease 2019. Cytokine 2021, 148, 155513. [Google Scholar] [CrossRef]
- Yamaya, T.; Hagiwara, E.; Baba, T.; Kitayama, T.; Murohashi, K.; Higa, K.; Sato, Y.; Otoshi, R.; Tabata, E.; Shintani, R.; et al. Serum Krebs von den Lungen-6 Levels Are Associated with Mortality and Severity in Patients with Coronavirus Disease 2019. Respir. Investig. 2021, 59, 596–601. [Google Scholar] [CrossRef]
- Maruyama, S.; Nakamori, Y.; Nakano, H.; Tsuyumu, K.; Kanayama, S.; Iwamura, H.; Wada, D.; Yoshihara, T.; Saito, F.; Yoshiya, K.; et al. Peak Value of Serum KL-6 May Be Useful for Predicting Poor Prognosis of Severe COVID-19 Patients. Eur. J. Med. Res. 2022, 27, 69. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Bergantini, L.; Cameli, P.; Curatola, G.; Remediani, L.; Bennett, D.; Bianchi, F.; Perillo, F.; Volterrani, L.; Mazzei, M.A.; et al. Serial KL-6 Measurements in COVID-19 Patients. Intern Emerg. Med. 2021, 16, 1541–1545. [Google Scholar] [CrossRef]
- D’Agnano, V.; Scialo, F.; Perna, F.; Atripaldi, L.; Sanduzzi, S.; Allocca, V.; Vitale, M.; Pastore, L.; Bianco, A.; Perrotta, F. Exploring the Role of Krebs von den Lungen-6 in Severe to Critical COVID-19 Patients. Life 2022, 12, 1141. [Google Scholar] [CrossRef]
- Castellví, I.; Castillo, D.; Corominas, H.; Mariscal, A.; Orozco, S.; Benito, N.; Pomar, V.; Baucells, A.; Mur, I.; de la Rosa-Carrillo, D.; et al. Krebs von Den Lungen-6 Glycoprotein Circulating Levels Are Not Useful as Prognostic Marker in COVID-19 Pneumonia: A Large Prospective Cohort Study. Front. Med. 2022, 9, 973918. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Fujii, Y.; Hirota, K. Critical Care Demand and Intensive Care Supply for Patients in Japan with COVID-19 at the Time of the State of Emergency Declaration in April 2020: A Descriptive Analysis. Medicina 2020, 56, 530. [Google Scholar] [CrossRef]
- Byrne, M.; Scott, T.E.; Sinclair, J.; Chockalingam, N. COVID-19 and Critical Care Capacity: Can We Mitigate Demand? Respirology 2022, 27, 107–108. [Google Scholar] [CrossRef] [PubMed]








| Variable | Total (n = 48) | Q1 (n = 12) | Q2 (n = 12) | Q3 (n = 12) | Q4 (n = 12) | p * |
|---|---|---|---|---|---|---|
| Age, years | 72.0 (63.0–79.0) | 68.0 (57.0–74.5) | 73.5 (63.0–83.0) | 75.0 (70.0–77.0) | 71.0 (64.5–79.5) | 0.658 |
| Male | 28 (58.3) | 7 (58.3) | 9 (75.0) | 6 (50.0) | 6 (50.0) | 0.561 |
| Comorbidities | ||||||
| HTN | 25 (52.1) | 6 (50.0) | 5 (41.7) | 6 (50.0) | 8 (66.7) | 0.663 |
| DM | 16 (33.3) | 4 (33.3) | 3 (25.0) | 3 (25.0) | 6 (50.0) | 0.522 |
| Solid cancer | 7 (14.6) | 1 (8.3) | 3 (25.0) | 2 (16.7) | 1 (8.3) | 0.606 |
| Pulmonary disease † | 4 (8.3) | 1 (8.3) | 0 (0.0) | 1 (8.3) | 2 (16.7) | 0.350 |
| CHF | 4 (8.3) | 1 (8.3) | 2 (16.7) | 1 (8.3) | 0 (0.0) | 0.535 |
| Dementia | 3 (6.2) | 1 (8.3) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 0.270 |
| CVD | 3 (6.2) | 2 (16.7) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0.271 |
| CAD | 3 (6.2) | 1 (8.3) | 1 (8.3) | 0 (0.0) | 1 (8.3) | 0.785 |
| CKD | 2 (4.2) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 1 (8.3) | 0.555 |
| PAD | 2 (4.2) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 1 (8.3) | 0.555 |
| Liver disease | 2 (4.2) | 0 (0.0) | 1 (8.3) | 1 (8.3) | 0 (0.0) | 0.555 |
| Rheumatic disease | 1 (2.1) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.382 |
| Symptoms | ||||||
| Fever (≥37.5 °C) | 33 (68.7) | 7 (58.3) | 9 (75.0) | 9 (75.0) | 8 (66.7) | 0.785 |
| Dyspnea | 25 (52.1) | 5 (41.7) | 5 (41.7) | 7 (58.3) | 8 (66.7) | 0.521 |
| General weakness | 23 (47.9) | 7 (58.3) | 6 (50.0) | 6 (50.0) | 4 (33.3) | 0.663 |
| Cough | 18 (37.5) | 5 (41.7) | 6 (50.0) | 4 (33.3) | 3 (25.0) | 0.619 |
| Sputum | 11 (22.9) | 5 (41.7) | 4 (33.3) | 1 (8.3) | 1 (8.3) | 0.111 |
| Fatigue | 10 (20.8) | 4 (33.3) | 3 (25.0) | 1 (8.3) | 2 (16.7) | 0.471 |
| Gastrointestinal ‡ | 6 (12.5) | 2 (16.7) | 1 (8.3) | 2 (16.7) | 1 (8.3) | 0.858 |
| Chilling | 5 (10.4) | 2 (16.7) | 1 (8.3) | 1 (8.3) | 1 (8.3) | 0.880 |
| Myalgia | 5 (10.4) | 2 (16.7) | 2 (16.7) | 0 (0.0) | 1 (8.3) | 0.483 |
| Headache | 5 (10.4) | 2 (16.7) | 1 (8.3) | 2 (16.7) | 0 (0.0) | 0.483 |
| Sore throat | 2 (4.2) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0.555 |
| Hemoptysis | 1 (2.1) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0.382 |
| Rhinorrhea | 1 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0.382 |
| Nasal obstruction | 1 (2.1) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.382 |
| Chest pain | 1 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0.382 |
| COVID-19 Dx. to enrollment, days | 4.0 (0.5–9.0) | 3.5 (0.5–12.0) | 3.5 (1.0–8.5) | 7.0 (2.0–12.0) | 4.5 (0.0–8.0) | 0.813 |
| Vital signs | ||||||
| SBP, mm Hg | 130.0 (112.0–142.5) | 136.0 (125.0–145.0) | 135.5 (122.5–140.0) | 120.0 (114.0–160.0) | 114.0 (104.0–138.5) | 0.294 |
| DBP, mm Hg | 77.0 (70.0–80.5) | 80.0 (70.0–90.0) | 74.0 (70.0–80.0) | 74.5 (65.0–80.5) | 74.5 (69.5–85.0) | 0.834 |
| HR, beats/min | 83.5 (76.5–93.0) | 84.5 (81.0–93.5) | 79.5 (72.0–88.0) | 85.5 (76.0–96.5) | 83.5 (75.0–98.5) | 0.692 |
| RR, breaths/min | 20.0 (20.0–23.5) | 20.0 (20.0–20.5) | 21.0 (20.0–22.5) | 20.0 (20.0–22.5) | 24.0 (17.0–28.5) | 0.397 |
| BT, °C | 37.2 (36.9–37.8) | 37.0 (36.9–37.9) | 37.5 (37.1–37.9) | 37.2 (36.8–37.6) | 37.0 (37.7) | 0.335 |
| SpO2, % | 95.9 (93.8–97.1) | 97.0 (94.4–98.8) | 96.0 (95.0–96.9) | 94.0 (93.0–97.5) | 94.9 (90.0–97.0) | 0.481 |
| Laboratory data | ||||||
| WBC, ×109/L | 6.4 (5.0–8.2) | 4.9 (4.2–6.1) | 6.3 (5.7–8.9) | 6.8 (5.1–7.6) | 9.1 (6.7–13.1) | 0.007 |
| Neutrophils, ×109/L | 4.8 (3.2–6.7) | 3.1 (2.5–3.8) | 4.7 (4.3–5.9) | 5.0 (3.6–6.4) | 7.9 (5.5–11.5) | 0.002 |
| Lymphocytes, ×109/L | 1.0 (0.7–1.5) | 1.4 (0.8–1.6) | 1.0 (0.7–1.5) | 1.0 (0.6–1.5) | 0.8 (0.6–1.1) | 0.268 |
| Hb, g/L | 117.0 (109.5–130.5) | 117.0 (110.0–132.5) | 114.5 (102.5–132.0) | 116.0 (101.5–129.5) | 122.5 (115.0–130.0) | 0.915 |
| PLT, ×109/L | 213.0 (156.5–301.5) | 234.5 (141.5–322.5) | 235.5 (184.0–293.0) | 193.5 (156.5–309.5) | 193.0 (144.0–269.5) | 0.901 |
| AST, IU/L | 31.5 (24.5–47.0) | 28.0 (23.5–44.5) | 28.0 (22.0–32.0) | 43.5 (26.5–63.5) | 39.5 (30.0–54.0) | 0.085 |
| ALT, IU/L | 26.0 (15.3–44.0) | 25.0 (15.0–46.0) | 25.0 (14.0–32.0) | 33.0 (17.0–55.0) | 37.0 (17.3–46.3) | 0.537 |
| ALP, IU/L | 72.0 (62.5–89.5) | 67.5 (60.0–76.0) | 80.5 (72.5–93.5) | 69.0 (57.0–124.0) | 71.0 (63.0–63.5) | 0.399 |
| Total bilirubin, umol/L | 12.7 (9.0–17.5) | 9.5 (6.2–11.5) | 12.3 (8.8–16.8) | 12.5 (8.7–13.9) | 23.2 (15.6–36.3) | 0.002 |
| LDH, IU/L | 513.5 (420.5–665.5) | 419.5 (338.0–548.0) | 457.0 (390.8–514.8) | 545.0 (474.5–638.5) | 876.0 (697.8–1061.3) | <0.001 |
| BUN, mmol/L | 5.7 (3.6–8.0) | 3.8 (2.8–5.7) | 5.9 (5.4–9.4) | 4.8 (3.2–6.8) | 7.1 (5.4–9.9) | 0.064 |
| Cr, umol/L | 68.0 (53.0–91.9) | 61.9 (49.5–76.0) | 74.3 (57.4–98.6) | 65.9 (54.8–83.9) | 80.0 (49.1–133.1) | 0.372 |
| CRP, mg/L | 45.0 (9.0–122.0) | 10.0 (2.0–47.0) | 22.0 (13.0–116.0) | 57.0 (12.0–112.0) | 99.0 (63.0–156.0) | 0.039 |
| Initial KL-6, U/mL | 234.5 (160.0–449.0) | 114.0 (98.0–128.0) | 198.5 (175.0–206.0) | 290.0 (251.5–356.0) | 661.5 (530.5–812.0) | < 0.001 |
| Severity assessment | ||||||
| CCI | 4.0 (2.0–5.0) | 3.5 (2.0–5.0) | 4.0 (2.0–5.5) | 4.0 (2.5–5.0) | 4.0 (3.0–4.5) | 0.951 |
| SOFA | 3.0 (1.0–6.0) | 2.0 (0.5–3.5) | 3.5 (1.5–4.5) | 2.5 (1.0–3.5) | 9.0 (5.0–11.5) | 0.005 |
| WHO disease severity § | ||||||
| Moderate disease | 13 (27.1) | 5 (41.7) | 3 (25.0) | 4 (33.3) | 1 (7.7) | 0.297 |
| Critical disease ‖ | 35 (72.9) | 7 (58.3) | 9 (75.0) | 8 (66.7) | 11 (91.7) | |
| ARDS | 10 (20.8) | 0 (0.0) | 1 (8.3) | 2 (16.7) | 7 (58.3) | <0.001 |
| Sepsis | 29 (60.4) | 7 (58.3) | 9 (75.0) | 7 (58.3) | 6 (50.0) | 0.647 |
| Septic shock | 6 (12.5) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 5 (41.7) | 0.005 |
| Clinical outcome | ||||||
| ICU admission | 13 (27.1) | 1 (8.3) | 2 (16.7) | 1 (8.3) | 9 (75.0) | <0.001 |
| Ventilator use | 11 (22.9) | 0 (0.0) | 1 (8.3) | 1 (8.3) | 9 (75.0) | <0.001 |
| ECMO use ¶ | 6 (12.5) | 0 (0.0) | 0 (0.0) | 1 (8.3) | 5 (41.7) | 0.005 |
| 30-day mortality | 8 (16.7) | 2 (16.7) | 0 (0.0) | 2 (16.7) | 4 (33.3) | 0.187 |
| In-hospital mortality | 12 (25.0) | 2 (16.7) | 2 (16.7) | 3 (25.0) | 5 (41.7) | 0.446 |
| Hospital stay, days | 28.0 (21.0–41.0) | 24.0 (17.5–29.0) | 28.0 (22.0–42.0) | 40.0 (26.0–53.5) | 40.0 (26.0–53.5) | 0.196 |
| Variables * | ICU Admission | Ventilator Use | ECMO Use | 30-Day Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 13) | No (n = 35) | p | Yes (n = 11) | No (n = 37) | p | Yes (n = 6) | No (n = 42) | p | Non-Survival (n = 8) | Survival (n = 40) | p | |
| Age > 70 years | 6 (46.2) | 19 (54.3) | 0.620 | 5 (45.5) | 20 (54.1) | 0.619 | 2 (33.3) | 23 (54.8) | 0.407 | 7 (87.5) | 18 (45.0) | 0.049 |
| Initial KL-6 level | ||||||||||||
| >322 U/mL | 10 (76.9) | 5 (14.3) | <0.001 | 10 (90.9) | 5 (14.3) | <0.001 | 6 (100) | 9 (21.4) | <0.001 | 5 (62.5) | 10 (25.0) | 0.087 |
| >412 U/mL † | 10 (76.9) | 3 (8.6) | <0.001 | 10 (90.9) | 3 (8.1) | <0.001 | 6 (100) | 7 (16.7) | <0.001 | 4 (50.0) | 9 (22.5) | 0.187 |
| CCI > 3 | 6 (46.2) | 21 (60.0) | 0.395 | 5 (45.5) | 22 (59.5) | 0.416 | 2 (33.3) | 25 (59.5) | 0.383 | 7 (87.5) | 20 (50.0) | 0.064 |
| Critical disease | 12 (92.3) | 23 (65.7) | 0.081 | 11 (100.0) | 24 (64.9) | 0.023 | 6 (100.0) | 29 (69.0) | 0.171 | 7 (87.5) | 28 (70.0) | 0.418 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.; Hur, M.; Kim, H.; Lee, C.H.; Lee, J.H.; Nam, M. Usefulness of KL-6 for Predicting Clinical Outcomes in Hospitalized COVID-19 Patients. Medicina 2022, 58, 1317. https://doi.org/10.3390/medicina58101317
Park M, Hur M, Kim H, Lee CH, Lee JH, Nam M. Usefulness of KL-6 for Predicting Clinical Outcomes in Hospitalized COVID-19 Patients. Medicina. 2022; 58(10):1317. https://doi.org/10.3390/medicina58101317
Chicago/Turabian StylePark, Mikyoung, Mina Hur, Hanah Kim, Chae Hoon Lee, Jong Ho Lee, and Minjeong Nam. 2022. "Usefulness of KL-6 for Predicting Clinical Outcomes in Hospitalized COVID-19 Patients" Medicina 58, no. 10: 1317. https://doi.org/10.3390/medicina58101317
APA StylePark, M., Hur, M., Kim, H., Lee, C. H., Lee, J. H., & Nam, M. (2022). Usefulness of KL-6 for Predicting Clinical Outcomes in Hospitalized COVID-19 Patients. Medicina, 58(10), 1317. https://doi.org/10.3390/medicina58101317








