Anti-CXCL8 Autoantibody: A Potential Diagnostic Biomarker for Esophageal Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Differential Expression Gene Analysis
2.2. Functional Annotation and Hub Genes Screening
2.3. CXCL8 mRNA Expression Validation
2.4. Study Population
2.5. Enzyme-Linked Immunosorbent Assay
2.6. Statistical Analysis
3. Results
3.1. Identification of DEGs
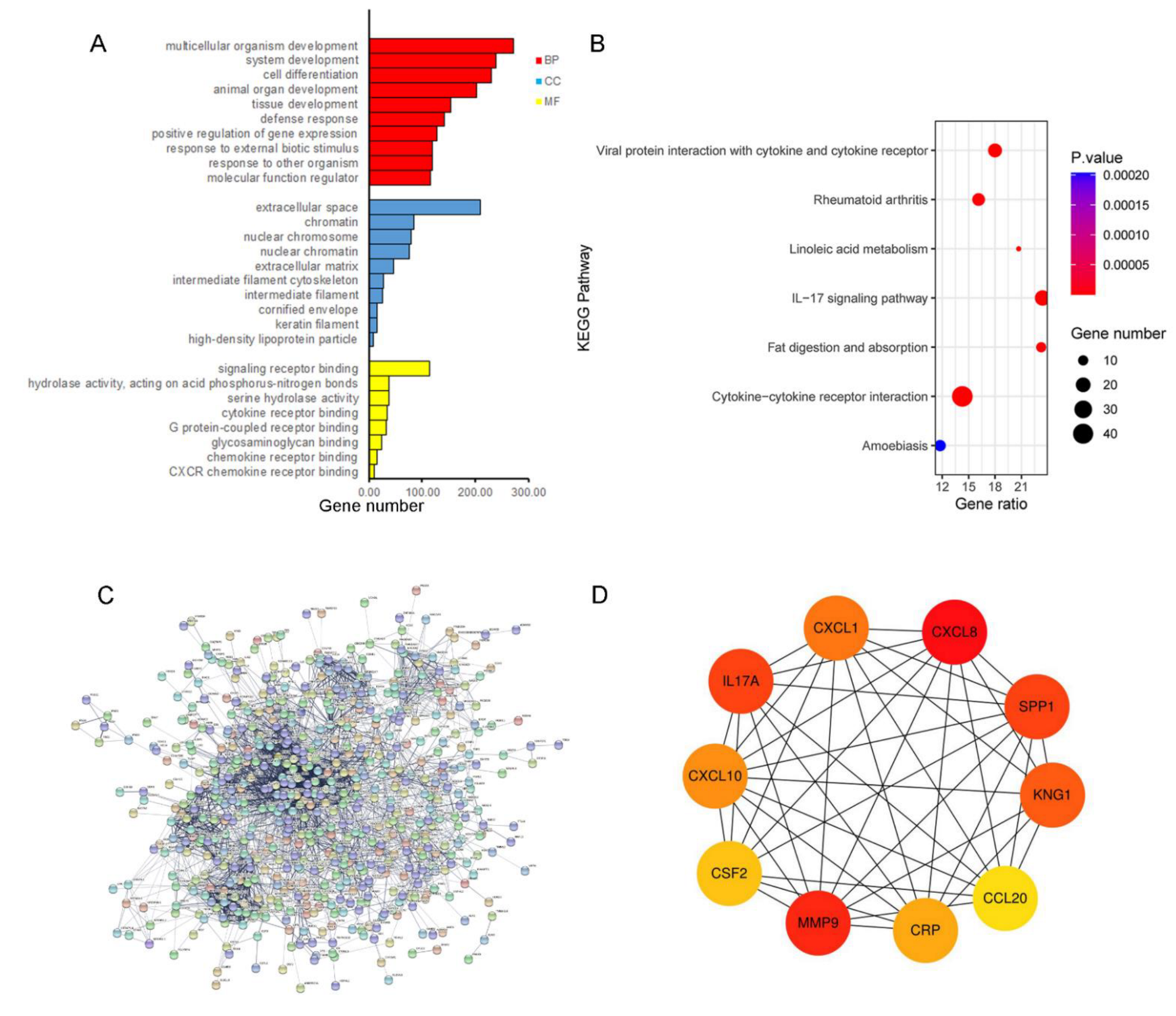
3.2. Functional Annotation and PPI Analysis for the Up-Regulated Genes
3.3. Validation mRNA Expression of CXCL8
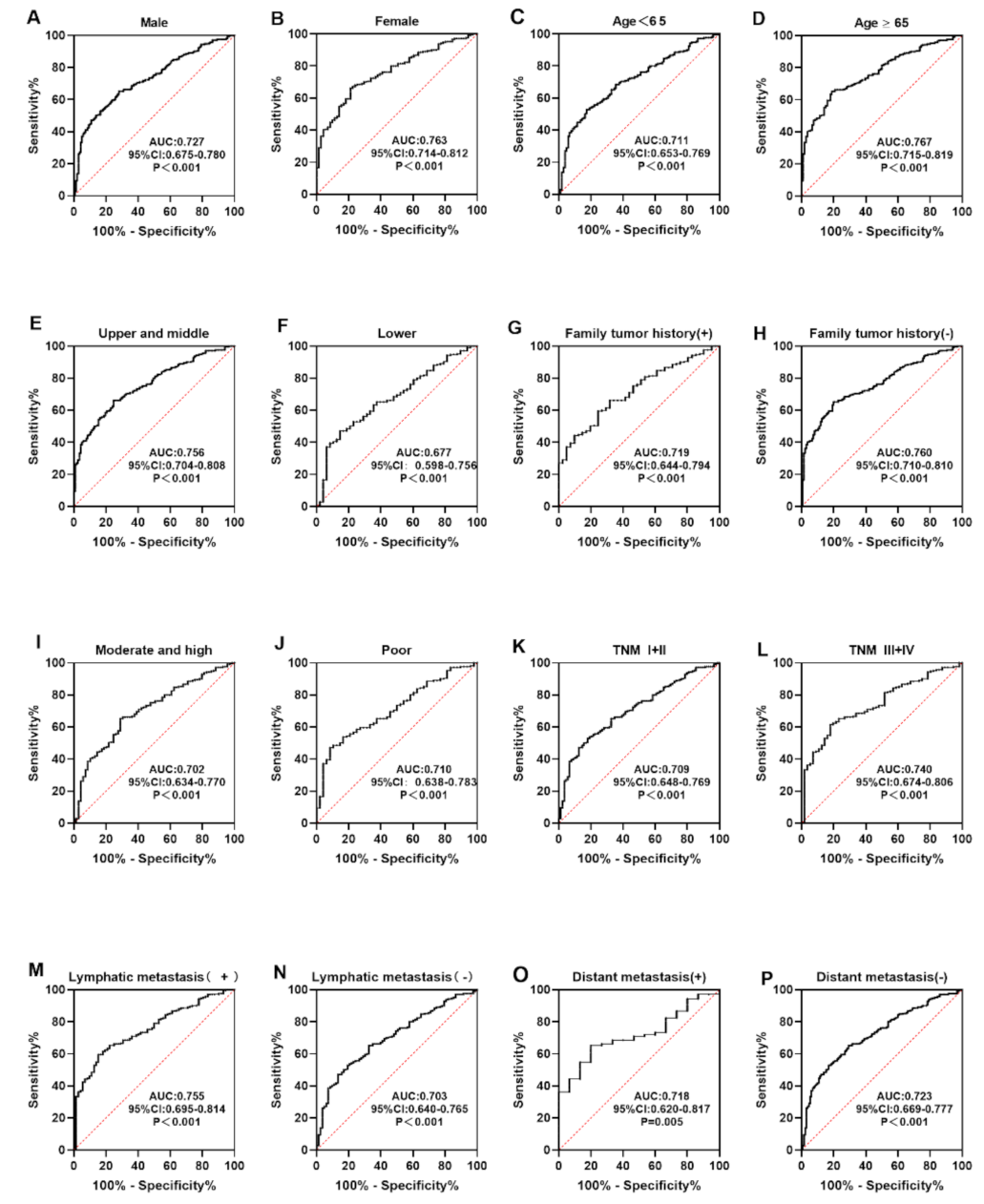
3.4. Level and Diagnostic Value of Anti-CXCL8 Autoantibody
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.Q.; Jiao, G.G.; Chang, F.B.; Fang, W.H.; Song, J.X.; Lu, N.; Lin, D.M.; Xie, Y.Q.; Yang, L. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann. Thorac. Surg. 2004, 77, 1740–1744. [Google Scholar] [CrossRef]
- Bird-Lieberman, E.L.; Fitzgerald, R.C. Early diagnosis of oesophageal cancer. Br. J. Cancer 2009, 101, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lao-Sirieix, P.; Fitzgerald, R.C. Screening for oesophageal cancer. Nat. Rev. Clin. Oncol. 2012, 9, 278–287. [Google Scholar] [CrossRef]
- Liang, H.; Fan, J.H.; Qiao, Y.L. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol. Med. 2017, 14, 33–41. [Google Scholar]
- Codipilly, D.C.; Qin, Y.; Dawsey, S.M.; Kisiel, J.; Topazian, M.; Ahlquist, D.; Iyer, P.G. Screening for esophageal squamous cell carcinoma: Recent advances. Gastrointest. Endosc. 2018, 88, 413–426. [Google Scholar] [CrossRef]
- Tan, E.M.; Zhang, J. Autoantibodies to tumor-associated antigens: Reporters from the immune system. Immunol. Rev. 2008, 222, 328–340. [Google Scholar] [CrossRef] [Green Version]
- Heo, C.K.; Bahk, Y.Y.; Cho, E.W. Tumor-associated autoantibodies as diagnostic and prognostic biomarkers. BMB Rep. 2012, 45, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Liu, F.; Pan, Y.; Xu, R.; Li, F.; Liu, A.; Yang, H.; Duan, L.; Shen, L.; Wu, Q.; et al. Tumor-associated autoantibodies in ESCC screening: Detecting prevalent early-stage malignancy or predicting future cancer risk? EBioMedicine 2021, 73, 103674. [Google Scholar] [CrossRef]
- Xu, Y.W.; Peng, Y.H.; Chen, B.; Wu, Z.Y.; Wu, J.Y.; Shen, J.H.; Zheng, C.P.; Wang, S.H.; Guo, H.P.; Li, E.M.; et al. Autoantibodies as potential biomarkers for the early detection of esophageal squamous cell carcinoma. Am. J. Gastroenterol. 2014, 109, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Zhang, W.W.; Xiang, J.L.; Wang, X.H.; Li, J.; Wang, J.L. Identification of microRNAs as novel biomarkers for esophageal squamous cell carcinoma: A study based on The Cancer Genome Atlas (TCGA) and bioinformatics. Chin. Med. J. 2019, 132, 2213–2222. [Google Scholar] [CrossRef]
- Bhushan, A.; Singh, A.; Kapur, S.; Borthakar, B.B.; Sharma, J.; Rai, A.K.; Kataki, A.C.; Saxena, S. Identification and Validation of Fibroblast Growth Factor 12 Gene as a Novel Potential Biomarker in Esophageal Cancer Using Cancer Genomic Datasets. OMICS 2017, 21, 616–631. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, Z.; Zhang, Z.; Zhao, D.; Xu, L.; Zhang, L. RNA-Associated Co-expression Network Identifies Novel Biomarkers for Digestive System Cancer. Front. Genet. 2021, 12, 659788. [Google Scholar] [CrossRef]
- Zhang, H.F.; Qin, J.J.; Ren, P.F.; Shi, J.X.; Xia, J.F.; Ye, H.; Wang, P.; Song, C.H.; Wang, K.J.; Zhang, J.Y. A panel of autoantibodies against multiple tumor-associated antigens in the immunodiagnosis of esophageal squamous cell cancer. Cancer Immunol. Immunother. 2016, 65, 1233–1242. [Google Scholar] [CrossRef]
- Ohashi, S.; Miyamoto, S.; Kikuchi, O.; Goto, T.; Amanuma, Y.; Muto, M. Recent Advances From Basic and Clinical Studies of Esophageal Squamous Cell Carcinoma. Gastroenterology 2015, 149, 1700–1715. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.N.; Chen, L.Q.; Shang, Q.X.; Yuan, Y.; Yang, Y.S. A meta-analysis on surgery with or without postoperative radiotherapy to treat squamous cell esophageal carcinoma. Int. J. Surg. 2020, 80, 184–191. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, L.; Tu, M.; Yin, X.; Cai, L.; Zhang, S.; Yu, L.; Pan, X.; Huang, Y. Development of a panel of autoantibody against NSG1 with CEA, CYFRA21-1, and SCC-Ag for the diagnosis of esophageal squamous cell carcinoma. Clin. Chim. Acta 2021, 520, 126–132. [Google Scholar] [CrossRef]
- Wang, X.B.; Jiang, X.R.; Yu, X.Y.; Wang, L.; He, S.; Feng, F.Y.; Guo, L.P.; Jiang, W.; Lu, S.H. Macrophage inhibitory factor 1 acts as a potential biomarker in patients with esophageal squamous cell carcinoma and is a target for antibody-based therapy. Cancer Sci. 2014, 105, 176–185. [Google Scholar] [CrossRef]
- Trigos, A.S.; Pearson, R.B.; Papenfuss, A.T.; Goode, D.L. How the evolution of multicellularity set the stage for cancer. Br. J. Cancer 2018, 118, 145–152. [Google Scholar] [CrossRef]
- Trigos, A.S.; Pearson, R.B.; Papenfuss, A.T.; Goode, D.L. Altered interactions between unicellular and multicellular genes drive hallmarks of transformation in a diverse range of solid tumors. Proc. Natl. Acad. Sci. USA 2017, 114, 6406–6411. [Google Scholar] [CrossRef] [Green Version]
- Zamecnik, J. The extracellular space and matrix of gliomas. Acta Neuropathol. 2005, 110, 435–442. [Google Scholar] [CrossRef]
- O’Hayre, M.; Vazquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef] [Green Version]
- Diakowska, D. Cytokines association with clinical and pathological changes in esophageal squamous cell carcinoma. Dis. Markers 2013, 35, 883–893. [Google Scholar] [CrossRef]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef]
- Kumar, S.; O’Malley, J.; Chaudhary, A.K.; Inigo, J.R.; Yadav, N.; Kumar, R.; Chandra, D. Hsp60 and IL-8 axis promotes apoptosis resistance in cancer. Br. J. Cancer 2019, 121, 934–943. [Google Scholar]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Cherukumilli, M.; Mahmoudpour, S.H.; Brand, K.; Bandapalli, O.R. ShRNA-mediated knock-down of CXCL8 inhibits tumor growth in colorectal liver metastasis. Biochem. Biophys. Res. Commun. 2018, 500, 731–737. [Google Scholar] [CrossRef]
- Long, X.; Ye, Y.; Zhang, L.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review). Int. J. Oncol. 2016, 48, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; He, H.; Liu, H.; Li, R.; Chen, Y.; Qi, Y.; Jiang, Q.; Chen, L.; Zhang, P.; Zhang, H.; et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut 2019, 68, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, J.; Li, Y.; Jiang, Y.; Ma, J.; Li, Q.; Pang, T. CXCL8 is associated with the recurrence of patients with acute myeloid leukemia and cell proliferation in leukemia cell lines. Biochem. Biophys. Res. Commun. 2018, 499, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, X.; Xu, C.; Sun, J.; Fang, Z.; Pan, H.; Han, W. Comprehensive analysis of the expression and prognostic value of CXC chemokines in colorectal cancer. Int. Immunopharmacol. 2020, 89, 107077. [Google Scholar] [CrossRef]
- Sun, F.; Wang, J.; Sun, Q.; Li, F.; Gao, H.; Xu, L.; Zhang, J.; Sun, X.; Tian, Y.; Zhao, Q.; et al. Interleukin-8 promotes integrin beta3 upregulation and cell invasion through PI3K/Akt pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 449. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Wang, X.; Miao, W.; Wang, B.; Qiu, Y. CXCL8 promotes the invasion of human osteosarcoma cells by regulation of PI3K/Akt signaling pathway. APMIS 2017, 125, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Hosono, M.; Koma, Y.I.; Takase, N.; Urakawa, N.; Higashino, N.; Suemune, K.; Kodaira, H.; Nishio, M.; Shigeoka, M.; Kakeji, Y.; et al. CXCL8 derived from tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression by promoting migration and invasion of cancer cells. Oncotarget 2017, 8, 106071–106088. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Gao, F.X.; Wang, C.; Qin, M.; Han, F.; Xu, T.; Hu, Z.; Long, Y.; He, X.M.; Deng, X.; et al. IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 321. [Google Scholar] [CrossRef]
- Pawluczuk, E.; Lukaszewicz-Zajac, M.; Gryko, M.; Kulczynska-Przybik, A.; Mroczko, B. Serum CXCL8 and Its Specific Receptor (CXCR2) in Gastric Cancer. Cancers 2021, 13, 5186. [Google Scholar] [CrossRef]
- Litman-Zawadzka, A.; Łukaszewicz-Zając, M.; Gryko, M.; Kulczyńska-Przybik, A.; Mroczko, B. Serum chemokine CXCL-8 as a better biomarker for diagnosis and prediction of pancreatic cancer than its specific receptor CXCR-2, CRP and classical tumor markers (CA 19-9 and CEA). Pol. Arch. Intern. Med. 2018, 128, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Paczek, S.; Lukaszewicz-Zajac, M.; Gryko, M.; Mroczko, P.; Kulczynska-Przybik, A.; Mroczko, B. CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression. Int. J. Mol. Sci. 2020, 21, 2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, D.; Xu, Y.; Ding, R.; Qiu, K.; Zhang, R.; Wang, H.; Huang, L.; Xie, X.; Yan, H.; Deng, Y.; et al. Extensive serum biomarker analysis in patients with non-small-cell lung carcinoma. Cytokine 2020, 126, 154868. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.S.; LaBaer, J. The sentinel within: Exploiting the immune system for cancer biomarkers. J. Proteome Res. 2005, 4, 1123–1133. [Google Scholar] [CrossRef] [PubMed]



| Variables | Verification Phase | Validation Phase | ||
|---|---|---|---|---|
| (n = 140) | (n = 280) | |||
| ESCC (n = 70) | NC (n = 70) | ESCC (n = 140) | NC (n = 140) | |
| Gender | ||||
| Male, n (%) | 40 (57.1) | 40 (57.1) | 99 (70.7) | 98 (70.0) |
| Female, n (%) Age, years | 30 (42.9) | 30 (42.9) | 41 (29.3) | 42 (30.0) |
| Mean age ± SD | 64.28 ± 8.23 | 64.74 ± 8.26 | 63.64 ± 8.64 | 64.31 ± 8.75 |
| Age range | 45–88 | 45–84 | 41–87 | 41–88 |
| Tumor site, n (%) | ||||
| Upper thorax | 1 (1.4) | 25 (17.9) | ||
| Middle thorax | 19 (27.2) | 72 (51.4) | ||
| Lower thorax | 8 (11.4) | 40 (28.6) | ||
| Unknown | 42 (60.0) | 3 (2.1) | ||
| Family tumor history, n (%) | ||||
| Yes | 12 (17.1) | 29 (20.7) | ||
| No | 56 (80.0) | 79 (56.4) | ||
| Unknown | 2 (2.9) | 32 (22.9) | ||
| Histological grade, n (%) | ||||
| High | 3 (4.3) | 3 (2.1) | ||
| Medium | 16 (22.8) | 46 (32.9) | ||
| Low | 8 (11.4) | 41 (29.3) | ||
| Unknown | 43 (61.5) | 50 (35.7) | ||
| TNM stage, n (%) | ||||
| I | 8 (11.4) | 45 (32.2) | ||
| II | 5 (7.1) | 31 (22.1) | ||
| III | 12 (17.1) | 30 (21.4) | ||
| IV | 6 (8.6) | 8 (5.7) | ||
| Unknown | 39 (55.7) | 26 (18.6) | ||
| Lymph node metastasis, n (%) | ||||
| Positive | 18 (25.8) | 54 (38.6) | ||
| Negative | 12 (17.1) | 71 (50.7) | ||
| Unknown | 40 (57.1) | 15 (10.7) | ||
| Distant metastasis, n (%) | ||||
| Yes | 6 (8.6) | 9 (6.4) | ||
| No | 25 (35.7) | 105 (75.0) | ||
| Unknown | 39 (55.7) | 26 (18.6) | ||
| Gene Symbol | Full Name | Degree | Function |
|---|---|---|---|
| CXCL8 | C-X-C motif chemokine ligand 8 | 86 | CXCL8 is a chemotactic factor and participates with inflammatory responses and neovascularization, and regulates immune response. |
| MMP9 | matrix metallopeptidase 9 | 82 | MMP9 is involved in the breakdown of extracellular matrix in normal physiological processes. |
| IL17A | interleukin 17A | 57 | IL17A mediated downstream pathways induce the production of inflammatory molecules, chemokines, and antimicrobial peptides. |
| SPP1 | secreted phosphoprotein 1 | 57 | The protein encoded by this gene is involved in the attachment of osteoclasts to the mineralized bone matrix. The encoded protein is secreted and binds hydroxyapatite with high affinity. |
| KNG1 | kininogen 1 | 55 | KNG1 is involved in signaling receptor binding and cysteine-type endopeptidase inhibitor activity. |
| CXCL1 | C-X-C motif chemokine ligand 1 | 53 | CXCL1 is associated with the growth and progression of certain tumors. |
| CXCL10 | C-X-C motif chemokine ligand 10 | 51 | Pro-inflammatory cytokine is involved in a wide variety of processes, such as chemotaxis and differentiation. |
| CRP | C-reactive protein | 50 | This gene is involved in complement activation and amplification via communication with complement initiation pattern recognition molecules. |
| CSF2 | colony stimulating factor 2 | 48 | CSF2 controls the production, differentiation, and function of granulocytes and macrophages. |
| CCL20 | C-C motif chemokine ligand 20 | 47 | CCL20 is involved in immunoregulatory and inflammatory processes. |
| Cohorts | AUC | 95%CI | Se (%) | Sp (%) | YI | +LR | −LR | PPV (%) | NPV (%) | Accuracy |
|---|---|---|---|---|---|---|---|---|---|---|
| Verification | 0.713 | 0.624–0.801 | 35.7 | 82.9 | 0.186 | 2.088 | 0.776 | 67.6 | 56.3 | 0.593 |
| Validation | 0.751 | 0.696–0.808 | 47.1 | 77.9 | 0.250 | 2.131 | 0.679 | 68.0 | 59.6 | 0.621 |
| Total | 0.739 | 0.692–0.787 | 44.3 | 81.4 | 0.257 | 2.420 | 0.684 | 70.5 | 59.4 | 0.628 |
| Variables | Number | Frequency (%) | p |
|---|---|---|---|
| Gender | |||
| Male | 139 | 41.7 | 0.296 |
| Female | 71 | 49.3 | |
| Age range (years) | |||
| <65 | 104 | 38.5 | 0.092 |
| ≥65 | 106 | 50.0 | |
| Family tumor history | |||
| Yes | 41 | 43.9 | 0.885 |
| No | 135 | 45.2 | |
| Tumor site | |||
| Upper and middle | 117 | 48.7 | 0.119 |
| Lower | 48 | 35.4 | |
| Differentiation | |||
| Moderate and high | 69 | 39.1 | 0.961 |
| Poor | 48 | 39.6 | |
| TNM stage | |||
| I–II | 89 | 38.2 | 0.234 |
| III–IV | 56 | 48.2 | |
| Lymphatic metastasis | |||
| Positive | 66 | 51.5 | 0.276 |
| Negative | 89 | 42.7 | |
| Distant metastasis | |||
| Yes | 15 | 33.3 | 0.436 |
| No | 130 | 43.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Sun, G.; Han, Z.; Wang, H.; Li, J.; Ye, H.; Song, C.; Zhang, J.; Wang, P. Anti-CXCL8 Autoantibody: A Potential Diagnostic Biomarker for Esophageal Squamous Cell Carcinoma. Medicina 2022, 58, 1480. https://doi.org/10.3390/medicina58101480
Chen H, Sun G, Han Z, Wang H, Li J, Ye H, Song C, Zhang J, Wang P. Anti-CXCL8 Autoantibody: A Potential Diagnostic Biomarker for Esophageal Squamous Cell Carcinoma. Medicina. 2022; 58(10):1480. https://doi.org/10.3390/medicina58101480
Chicago/Turabian StyleChen, Huili, Guiying Sun, Zhuo Han, Huimin Wang, Jiaxin Li, Hua Ye, Chunhua Song, Jianying Zhang, and Peng Wang. 2022. "Anti-CXCL8 Autoantibody: A Potential Diagnostic Biomarker for Esophageal Squamous Cell Carcinoma" Medicina 58, no. 10: 1480. https://doi.org/10.3390/medicina58101480







