Development and Survival of Human Ovarian Cells in Chitosan Hydrogel Micro-Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chitosan Characterisation
2.2. Chitosan Bioreactor Preparation
2.3. Ovarian Tissue Culture
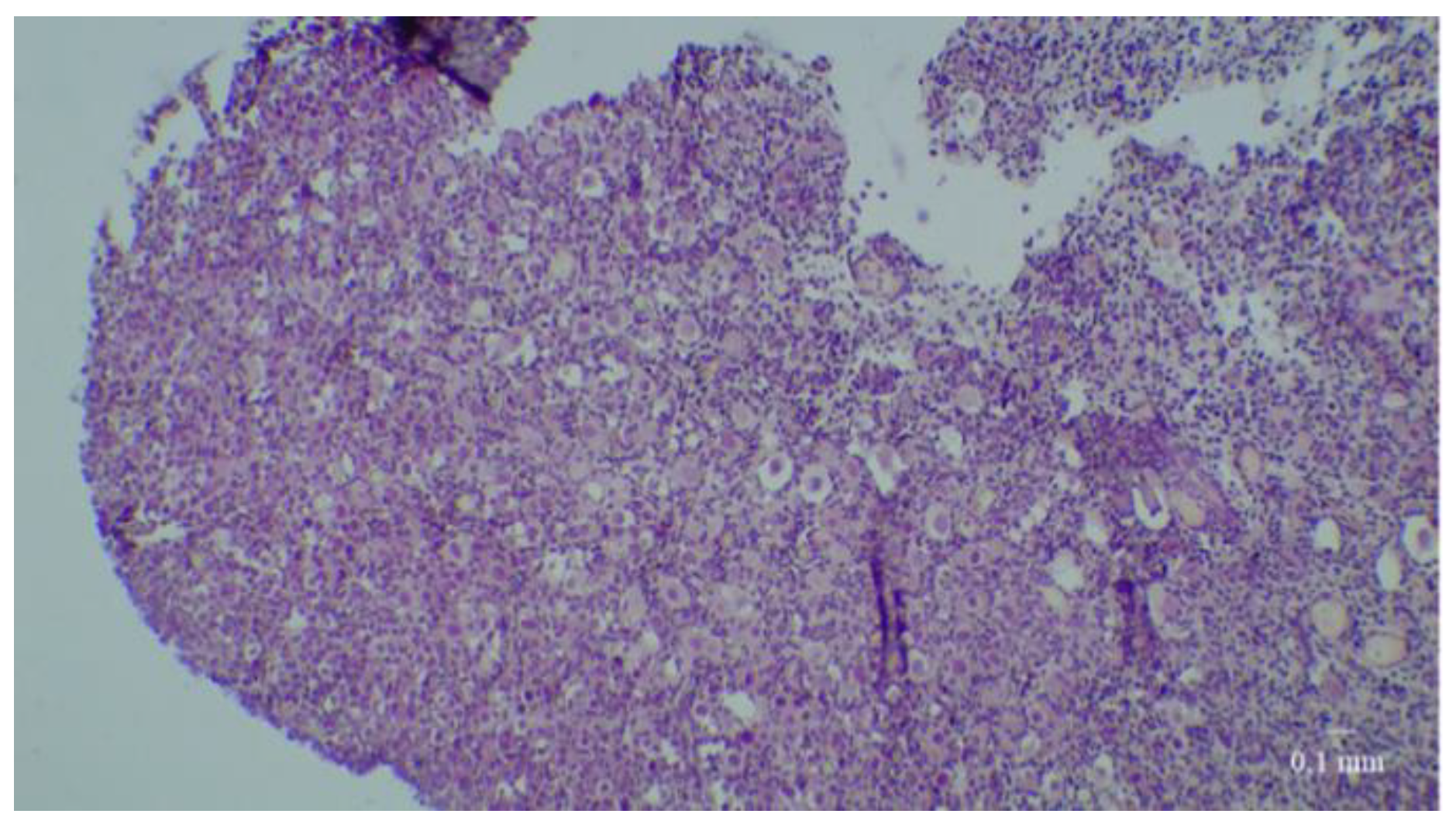
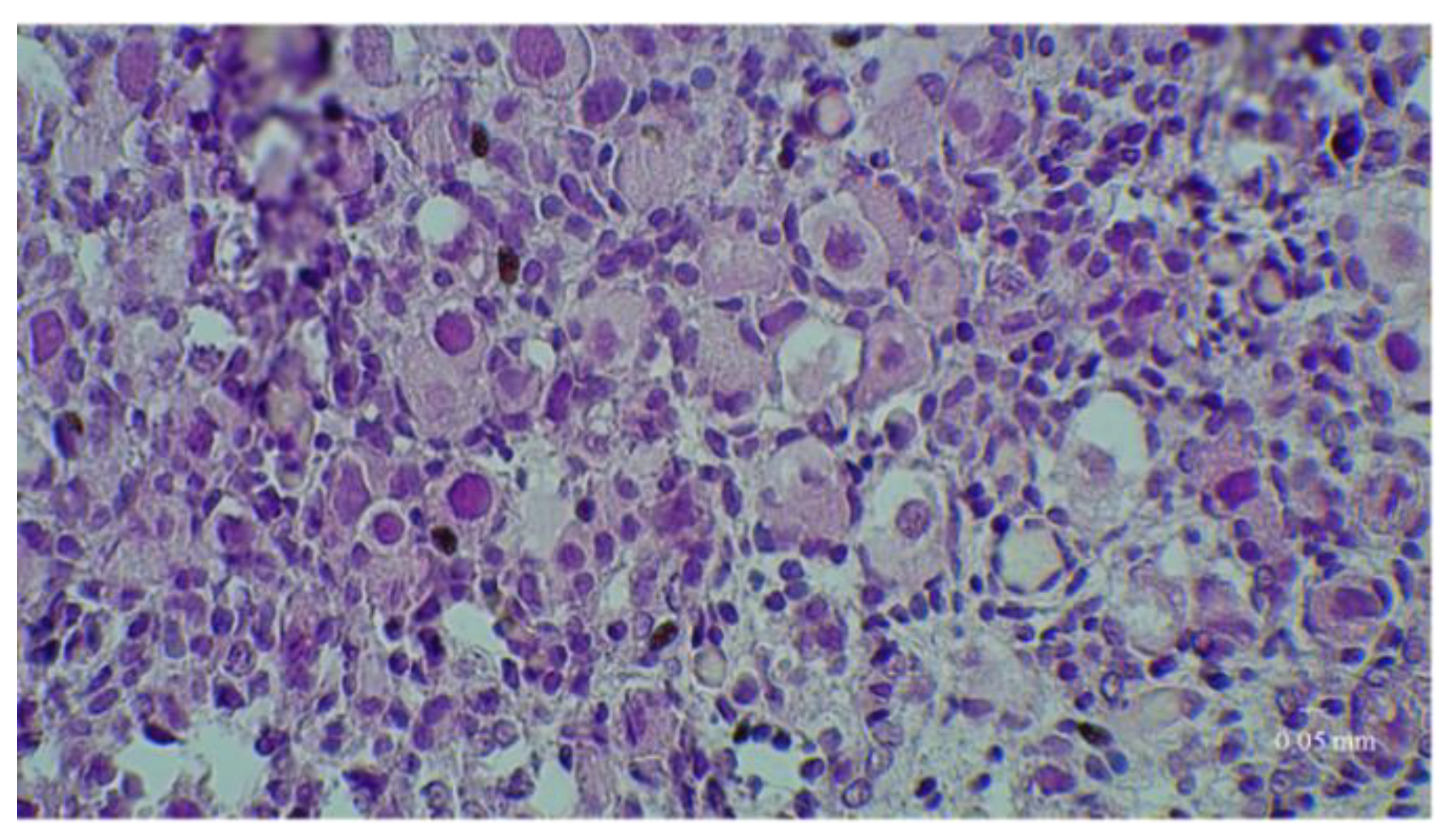
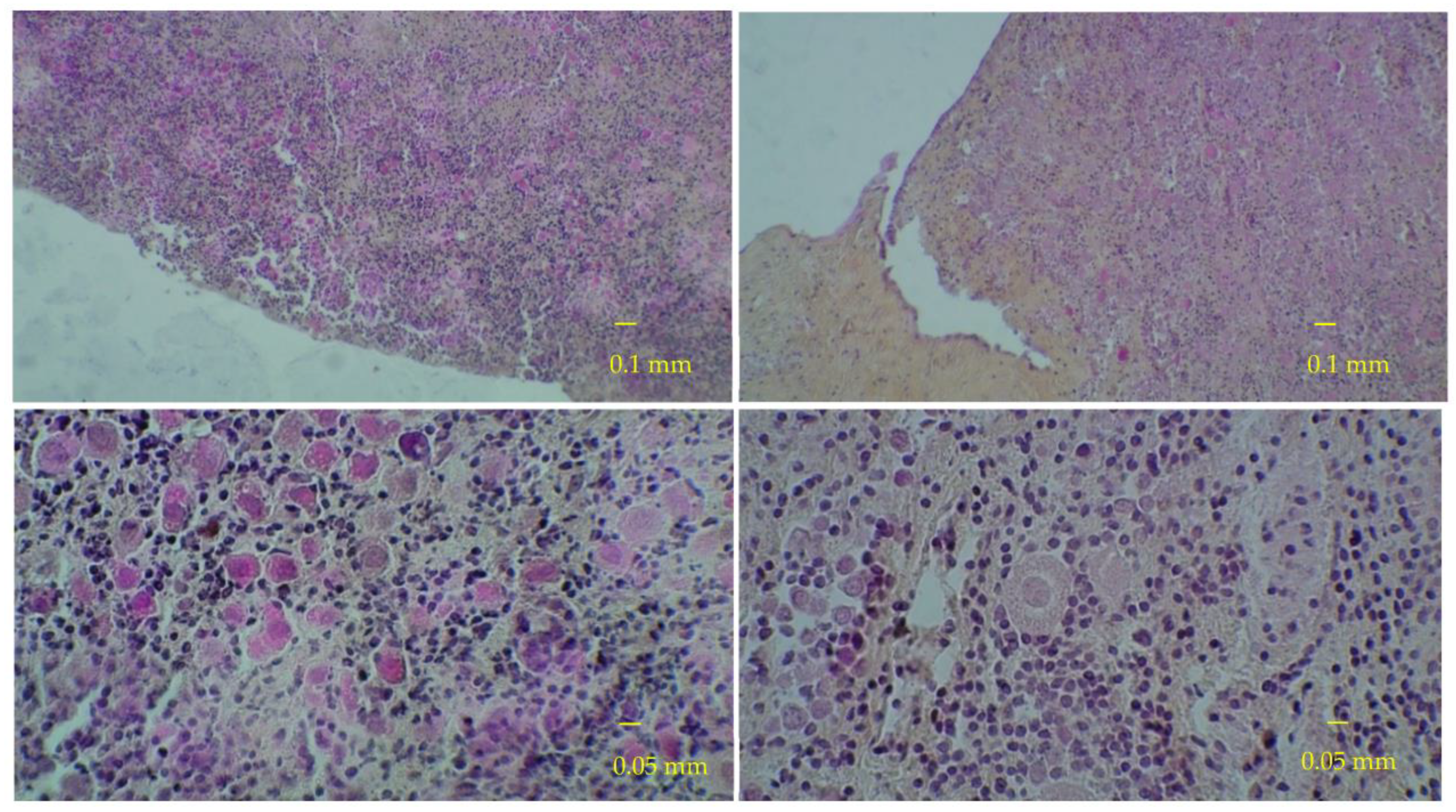
2.4. Histological Analyses
2.5. Immunostaining of Ovarian Tissue Slices
2.6. Statistical Analyses
2.7. Legal and Ethical Considerations
3. Results
3.1. Results of the First Set of Analyses with Intact Follicles as Denominator
3.2. Results of the Second Set of Analyses with All Present Follicles as Denominator
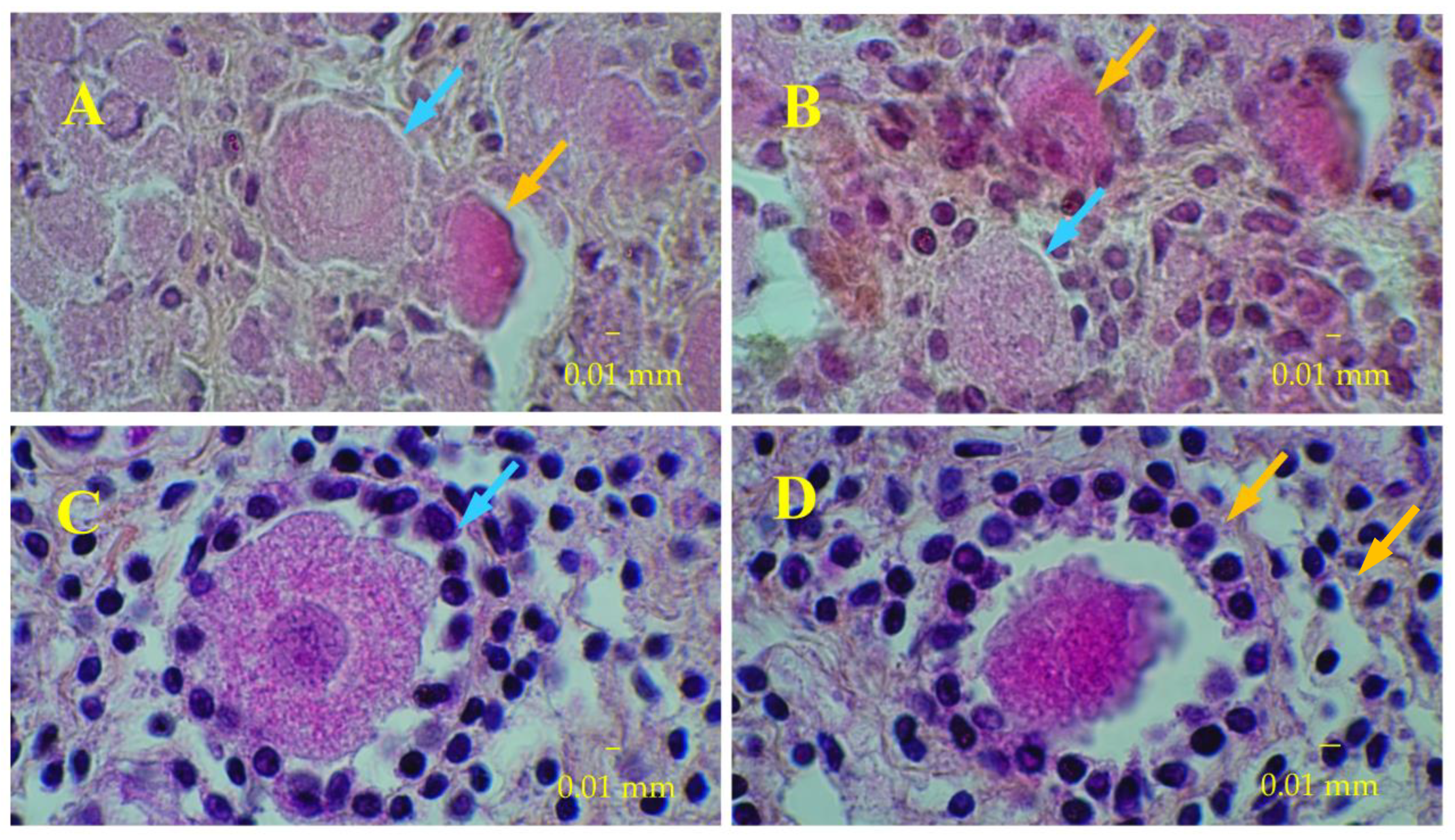
3.3. Immunostaining for Cell Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takae, S.; Suzuki, N. Current state and future possibilities of ovarian tissue transplantation. Reprod. Med. Biol. 2019, 18, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnez, J.; Dolmans, M.-M. Fertility preservation in women. Nat. Rev. Endocrinol. 2013, 9, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Falcone, T.; Patrizio, P. Importance of patient selection to analyze in vitro fertilization outcome with transplanted cryopreserved ovarian tissue. Fertil. Steril. 2020, 114, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J.; Martinez-Madrid, B.; Van Langendonckt, A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004, 364, 1405–1410. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Catt, S.; Pangestu, M.; Temple-Smith, P. Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: Oocyte maturation, fertilization, and live births. Reproduction 2011, 141, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.H.; Sankai, T.; Kada, H. Live Offspring from Cryopreserved Embryos Following In Vitro Growth, Maturation and Fertilization of Oocytes Derived from Preantral Follicles in Mice. J. Reprod. Dev. 2011, 57, 715–722. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, M.; Albertini, D.F.; Wallace, W.H.B.; Anderson, R.A.; Telfer, E.E. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol. Hum. Reprod. 2018, 24, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Lawson, M.S.; Bean, Y.; Ting, A.Y.; Pejovic, T.; De Geest, K.; Moffitt, M.; Mitalipov, S.M.; Xu, J. Matrix-free 3D culture supports human follicular development from the unilaminar to the antral stage in vitro yielding morphologically normal metaphase II oocytes. Hum. Reprod. 2021, 36, 1326–1338. [Google Scholar] [CrossRef]
- Laronda, M.; Duncan, F.E.; Hornick, J.E.; Xu, M.; Pahnke, J.E.; Whelan, K.A.; Shea, L.D.; Woodruff, T.K. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J. Assist. Reprod. Genet. 2014, 31, 1013–1028. [Google Scholar] [CrossRef]
- Wang, T.; Yan, L.-Y.; Yan, J.; Lu, C.-L.; Xia, X.; Yin, T.-L.; Zhu, X.-H.; Gao, J.-M.; Ding, T.; Hu, W.-H.; et al. Basic fibroblast growth factor promotes the development of human ovarian early follicles during growth in vitro. Hum. Reprod. 2014, 29, 568–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, L.; Shirazi, A.; Naderi, M.M.; Shams-Esfandabadi, N.; Boroujeni, S.B.; Sarvari, A.; Sadeghnia, S.; Behzadi, B.; Akhondi, M.M. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod. Biomed. Online 2017, 35, 343–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassani, F.; Ebrahimi, B.; Moini, A.; Ghiaseddin, A.; Bazrafkan, M.; Hassanzadeh, G.; Valojerdi, M.R. Chitosan Hydrogel Supports Integrity of Ovarian Follicles during In Vitro Culture: A Preliminary of A Novel Biomaterial for Three Dimensional Culture of Ovarian Follicles. Cell J. 2020, 21, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Pors, S.E.; Ramløse, M.; Nikiforov, D.; Lundsgaard, K.; Cheng, J.; Andersen, C.Y.; Kristensen, S.G. Initial steps in reconstruction of the human ovary: Survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum. Reprod. 2019, 34, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, A.; Talaei-Khozani, T.; Kargar-Abarghouei, E.; Razban, V.; Vojdani, Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res. Ther. 2018, 9, 252. [Google Scholar] [CrossRef] [Green Version]
- Bourdon, G.; Cadoret, V.; Charpigny, G.; Couturier-Tarrade, A.; Dalbies-Tran, R.; Flores, M.-J.; Froment, P.; Raliou, M.; Reynaud, K.; Saint-Dizier, M.; et al. Progress and challenges in developing organoids in farm animal species for the study of reproduction and their applications to reproductive biotechnologies. Veter Res. 2021, 52, 42. [Google Scholar] [CrossRef]
- Jones, A.S.K.; Shikanov, A. Follicle development as an orchestrated signaling network in a 3D organoid. J. Biol. Eng. 2019, 13, 2. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Domard, A.; David, L.; Riva-Araiza, R.N. Hollow, Notably Multi-Membrane Fibers, Method for Preparation Thereof by Spinning and Device for Applying Said Method. Patent Application Publication US2012040463A1, 5 September 2008. [Google Scholar]
- Araiza, R.N.R.; Rochas, C.; David, L.; Domard, A. Interrupted Wet-Spinning Process for Chitosan Hollow Fiber Elaboration. Macromol. Symp. 2008, 266, 1–5. [Google Scholar] [CrossRef]
- Montembault, A.; Viton, C.; Domard, A. Physico-chemical studies of the gelation of chitosan in a hydroalcoholic medium. Biomaterials 2005, 26, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, A. Dynamics of follicular growth in the human: A model from preliminary results. Hum. Reprod. 1986, 1, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Labrune, E.; Jaeger, P.; Santamaria, M.C.; Fournier, M.C.; Benchaib, M.; Rabilloud, M.; Salle, B.; Lornage, J. Cellular and Molecular Impact of Vitrification Versus Slow Freezing on Ovarian Tissue. Tissue Eng. Part C: Methods 2020, 26, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Hovatta, O.; Silye, R.; Abir, R.; Krausz, T.; Winston, R.M. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum. Reprod. 1997, 12, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, J.; Xu, K.P.; Liu, B.; DiMattina, M. Extracorporeal development and ultrarapid freezing of human fetal ova. J. Assist. Reprod. Genet. 1995, 12, 361–368. [Google Scholar] [CrossRef]
- Wright, C.; Hovatta, O.; Margara, R.; Trew, G.; Winston, R.; Franks, S.; Hardy, K. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum. Reprod. 1999, 14, 1555–1562. [Google Scholar] [CrossRef]
- Hovatta, O.; Wright, C.; Krausz, T.; Hardy, K.; Winston, R.M. Human primordial, primary and secondary ovarian follicles in long-term culture: Effect of partial isolation. Hum. Reprod. 1999, 14, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- Hreinsson, J.G.; Scott, J.E.; Rasmussen, C.; Swahn, M.L.; Hsueh, A.J.W.; Hovatta, O. Growth Differentiation Factor-9 Promotes the Growth, Development, and Survival of Human Ovarian Follicles in Organ Culture. J. Clin. Endocrinol. Metab. 2002, 87, 316–321. [Google Scholar] [CrossRef]
- Sadeu, J.; Smitz, J. Growth differentiation factor-9 and anti-Müllerian hormone expression in cultured human follicles from frozen–thawed ovarian tissue. Reprod. Biomed. Online 2008, 17, 537–548. [Google Scholar] [CrossRef]
- Telfer, E.E.; McLaughlin, M.; Ding, C.; Thong, K.J. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum. Reprod. 2008, 23, 1151–1158. [Google Scholar] [CrossRef] [Green Version]
- Lerer-Serfaty, G.; Samara, N.; Fisch, B.; Shachar, M.; Kossover, O.; Seliktar, D.; Ben-Haroush, A.; Abir, R. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. J. Assist. Reprod. Genet. 2013, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, F.; Reid, R.L.; Moini, A.; Abolhassani, F.; Valojerdi, M.R.; Kan, F.W.K. In vitro development of human primordial follicles to preantral stage after vitrification. J. Assist. Reprod. Genet. 2013, 30, 1397–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, M.; Kinnell, H.L.; Anderson, R.A.; Telfer, E.E. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol. Hum. Reprod. 2014, 20, 736–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, E.; Najafi, A.; Moeini, A.; Pirjani, R.; Hassanzadeh, G.; Mikaeili, S.; Salehi, E.; Adutwum, E.; Soleimani, M.; Khosravi, F.; et al. Ovarian tissue culture in the presence of VEGF and fetuin stimulates follicle growth and steroidogenesis. J. Endocrinol. 2017, 232, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Grosbois, J.; Demeestere, I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum. Reprod. 2018, 33, 1705–1714. [Google Scholar] [CrossRef]
- Salama, M.; Woodruff, T.K. From bench to bedside: Current developments and future possibilities of artificial human ovary to restore fertility. Acta Obstet. Gynecol. Scand. 2019, 98, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.S.; Sabouni, R.; Vaught, K.C.C.; Owen, C.M.; Albertini, D.F.; Segars, J.H. Biomechanics and mechanical signaling in the ovary: A systematic review. J. Assist. Reprod. Genet. 2018, 35, 1135–1148. [Google Scholar] [CrossRef]
- Zhou, H.; Shikanov, A. Three-Dimensional Hydrogel-Based Culture to Study the Effects of Toxicants on Ovarian Follicles. In Biomaterials for Tissue Engineering; Humana Press: New York, NY, USA, 2018; Volume 1758, pp. 55–72. [Google Scholar] [CrossRef]


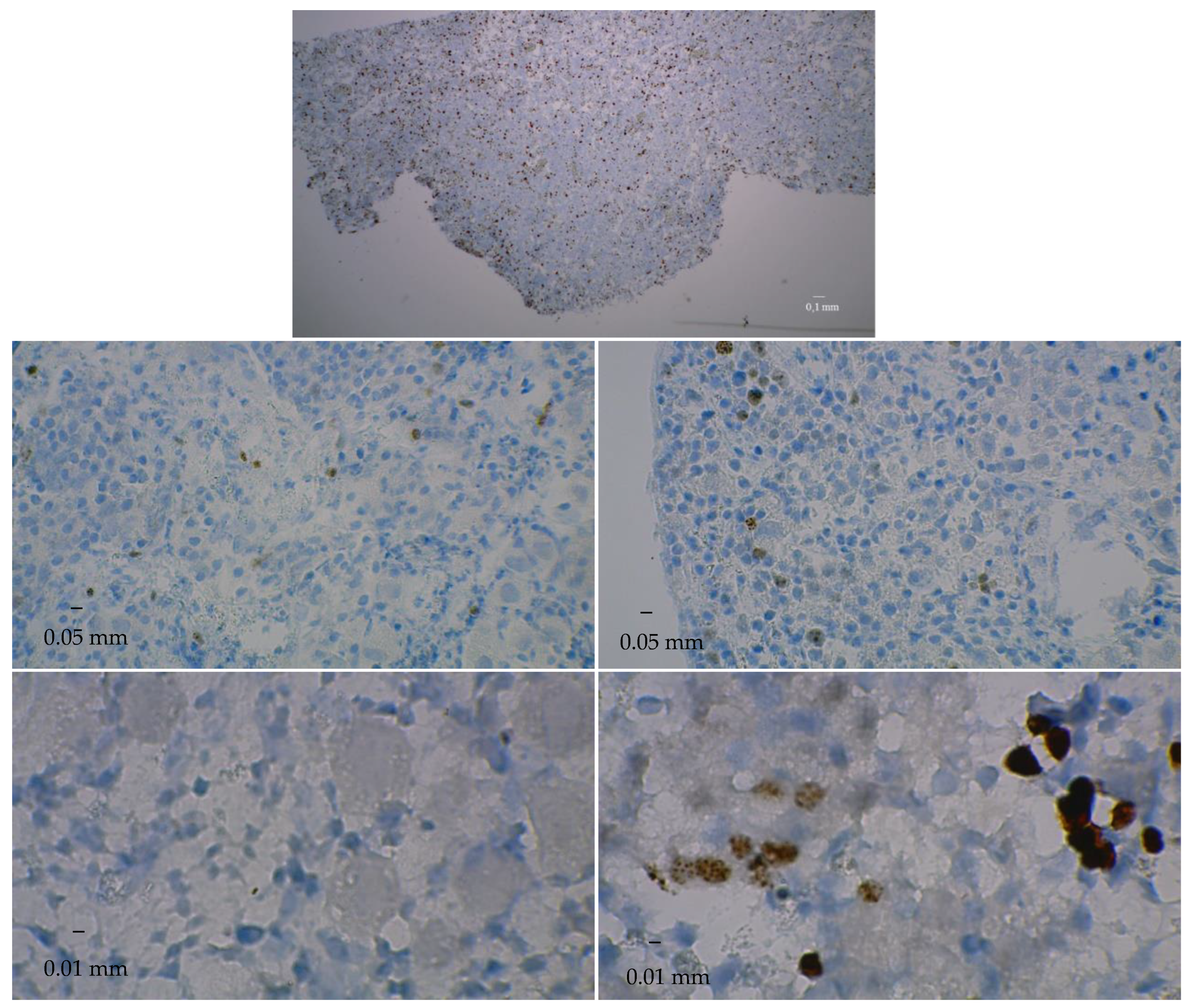
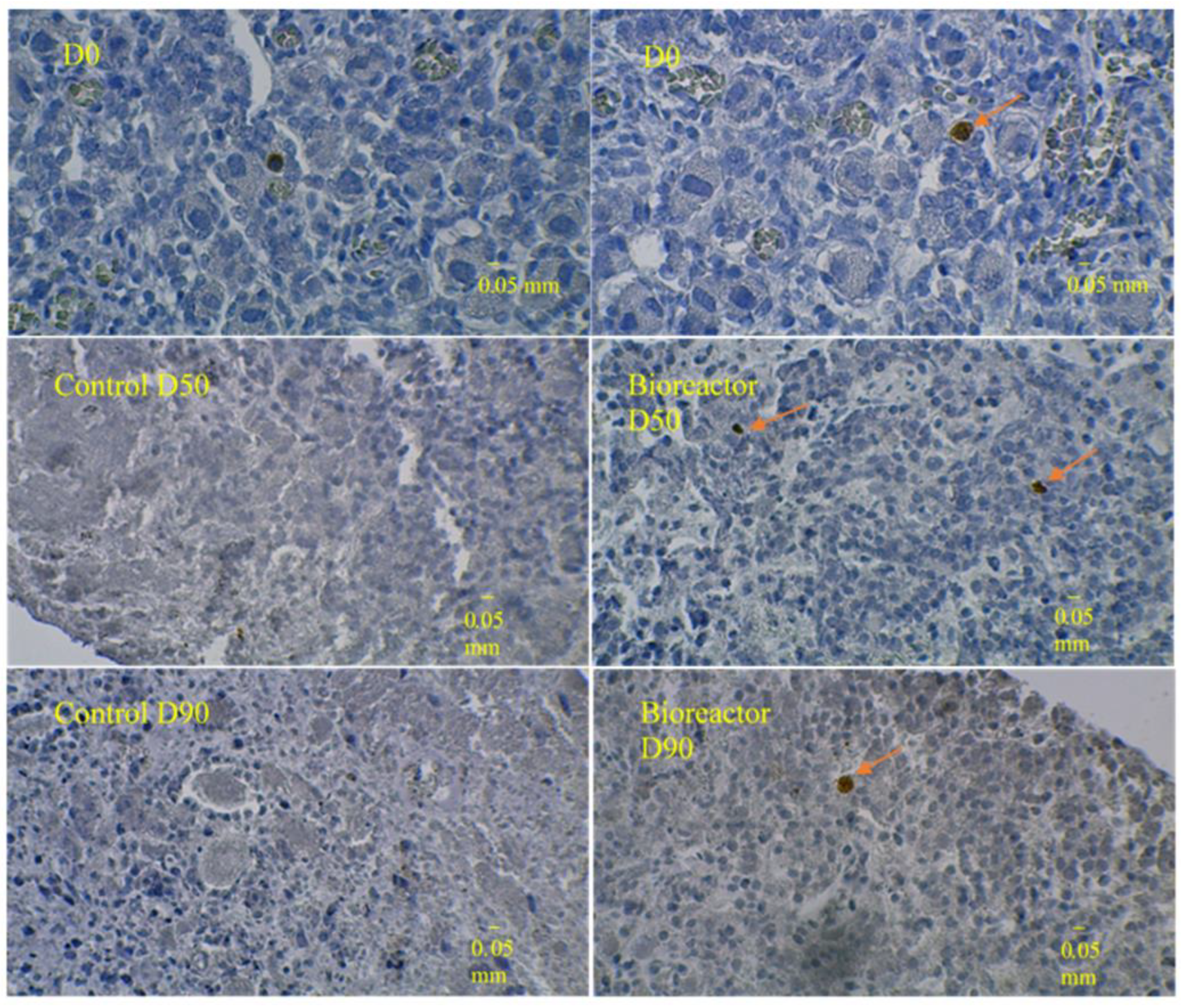
| Ovary | pGF at D0 | pGF at D50 in Bioreactor | pGF at D50 in Control Culture | pGF at D90 in Bioreactor | pGF at D90 in Control Culture |
|---|---|---|---|---|---|
| 1 | 31.7 | 60 | 16.7 | 51 | 82.4 |
| 2 | 9.7 | 24.3 | 11.4 | 39.7 | 48.5 |
| 3 | 2.5 | 1.2 | 1.1 | 1.8 | 1.3 |
| 4 | 0 | 12.5 | – | 35.3 | 0 |
| 5 | 0 | 0 | – | 25 | – |
| Ovary | pGF at D0 | pGF at D50 in Bioreactor | pGF at D50 in Control Culture | pGF at D90 in Bioreactor | pGF at D90 in Control Culture |
|---|---|---|---|---|---|
| 1 | 29.3 | 35.7 | 6.1 | 30.9 | 18.2 |
| 2 | 8.2 | 18.4 | 7.8 | 27.8 | 20.9 |
| 3 | 1.3 | 0.2 | 0.4 | 1 | 0.4 |
| 4 | 0 | 4.4 | 0 | 27.3 | 0 |
| 5 | 0 | 0 | 0 | 8.1 | 0 |
| Day 50 | Day 90 | |
|---|---|---|
| Proliferating cells | ||
| Bioreactor cultures | 14.1 ± 7.5 a | 10.5 ± 6.4 |
| Control cultures | 3.7 ± 3.6 | 0.0 ± 0.0 |
| Apoptotic cells | ||
| Bioreactor cultures | 6.8 ± 2.2 b | 2.2 ± 1.1 |
| Control cultures | 0.0 ± 0.0 | 0.0 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labrune, E.; Fournier, C.; Riche, B.; David, L.; Montembault, A.; Collardeau-Frachon, S.; Benchaib, M.; Lornage, J.; Iwaz, J.; Salle, B. Development and Survival of Human Ovarian Cells in Chitosan Hydrogel Micro-Bioreactor. Medicina 2022, 58, 1565. https://doi.org/10.3390/medicina58111565
Labrune E, Fournier C, Riche B, David L, Montembault A, Collardeau-Frachon S, Benchaib M, Lornage J, Iwaz J, Salle B. Development and Survival of Human Ovarian Cells in Chitosan Hydrogel Micro-Bioreactor. Medicina. 2022; 58(11):1565. https://doi.org/10.3390/medicina58111565
Chicago/Turabian StyleLabrune, Elsa, Cyrielle Fournier, Benjamin Riche, Laurent David, Alexandra Montembault, Sophie Collardeau-Frachon, Mehdi Benchaib, Jacqueline Lornage, Jean Iwaz, and Bruno Salle. 2022. "Development and Survival of Human Ovarian Cells in Chitosan Hydrogel Micro-Bioreactor" Medicina 58, no. 11: 1565. https://doi.org/10.3390/medicina58111565
APA StyleLabrune, E., Fournier, C., Riche, B., David, L., Montembault, A., Collardeau-Frachon, S., Benchaib, M., Lornage, J., Iwaz, J., & Salle, B. (2022). Development and Survival of Human Ovarian Cells in Chitosan Hydrogel Micro-Bioreactor. Medicina, 58(11), 1565. https://doi.org/10.3390/medicina58111565






