Dostarlimab an Inhibitor of PD-1/PD-L1: A New Paradigm for the Treatment of Cancer
Abstract
:1. Introduction
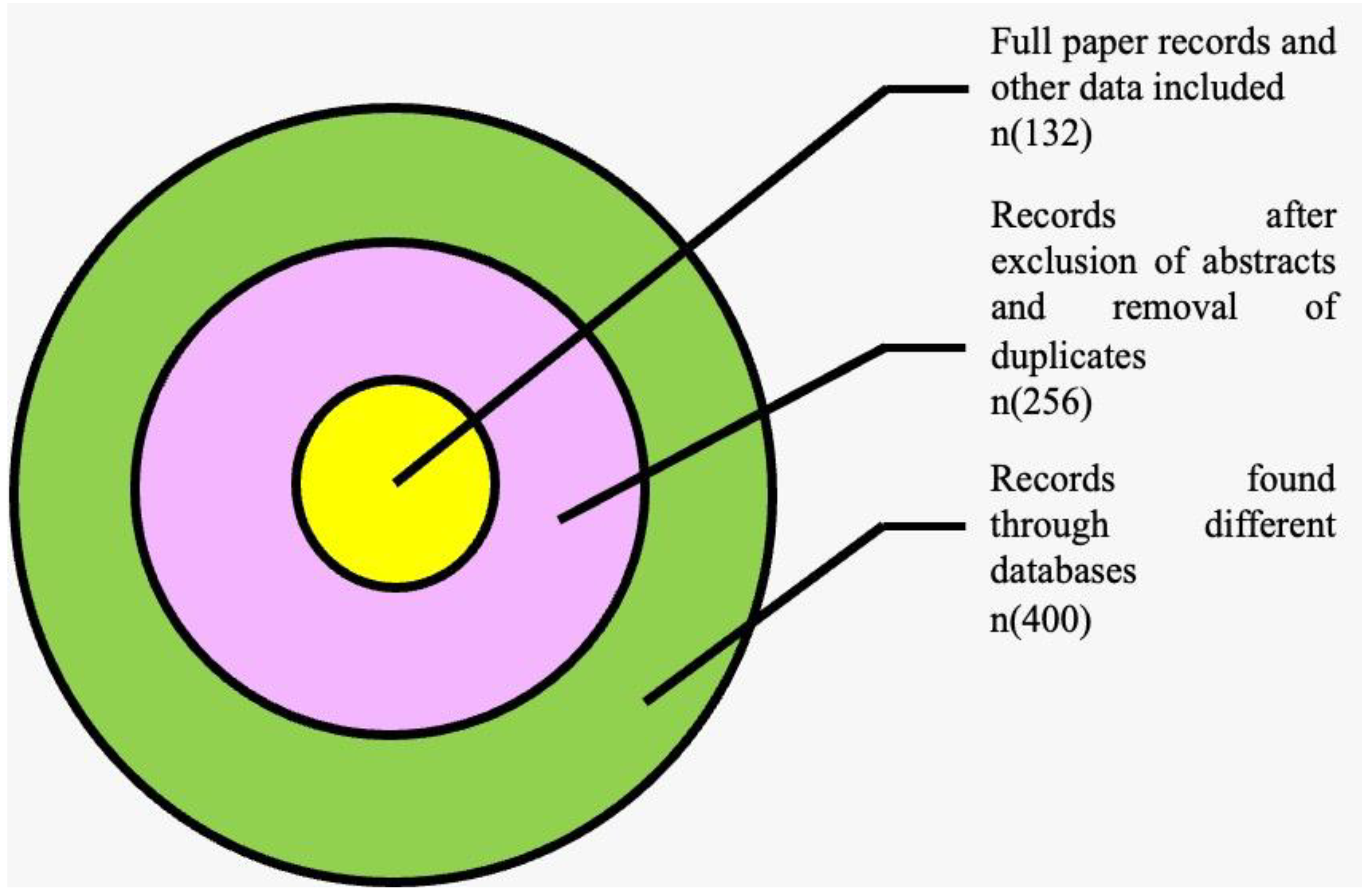
2. Methodology
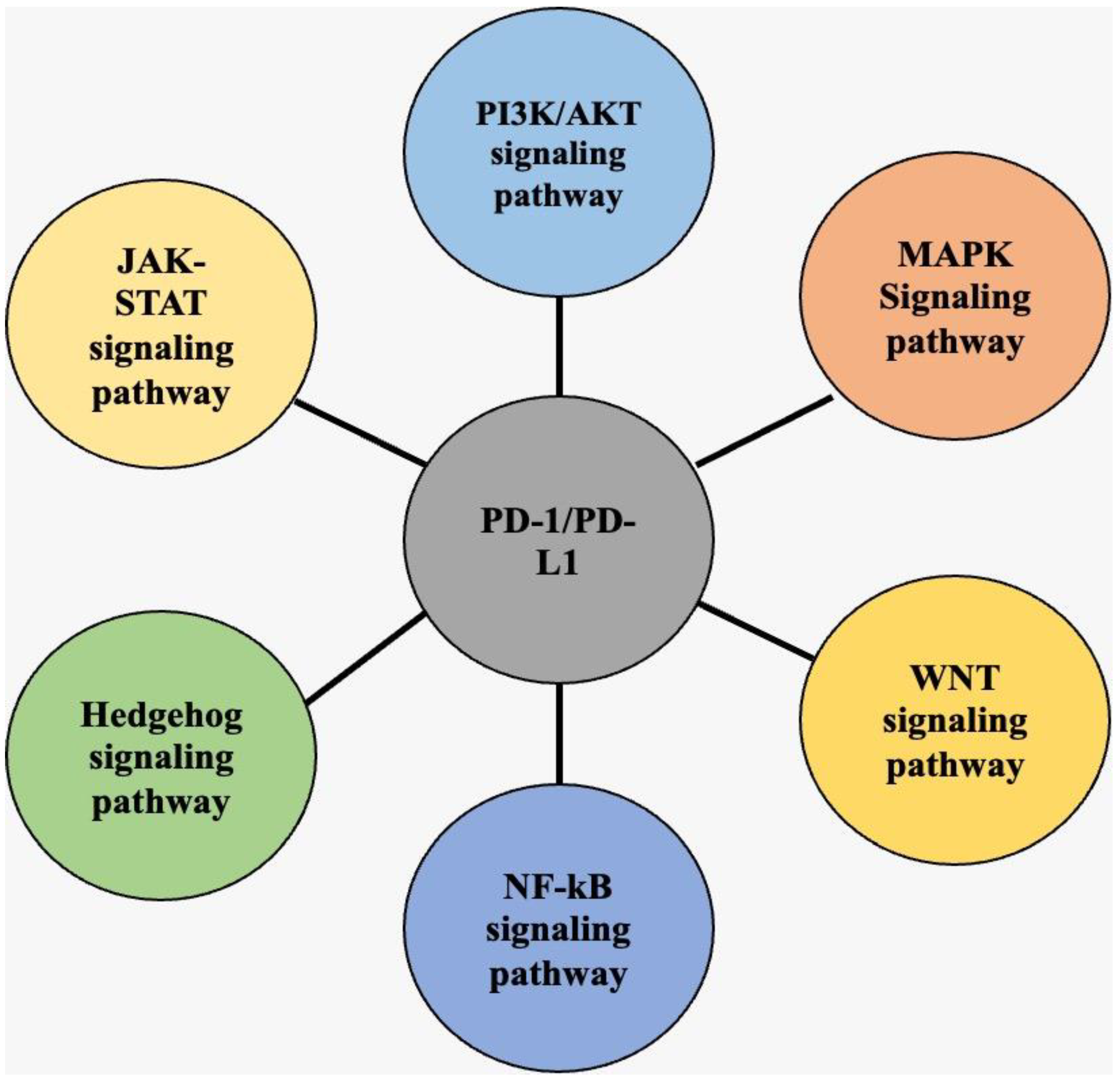
3. K3. Overview of PD-1/PD-L1 Signaling Pathways in Cancer
3.1. Programmed Death 1 (PD-1)
3.2. PD-L1
3.3. PI3K/AKT Signaling Pathway
3.4. MAPK Signaling Pathway
3.5. JAK-STAT Signaling Pathway
3.6. WNT Signaling Pathway
3.7. NF-κB Signaling Pathway
3.8. Hedgehog Signaling Pathway
4. Overview of the Role of PD-1 and PD-L1 in Cancer
4.1. PD-1/PD-L1 Inhibition and Their Inhibitors
4.2. Resistance Mechanisms
5. Jemperli™, Dostarlimab-Gxly (Dostarlimab)
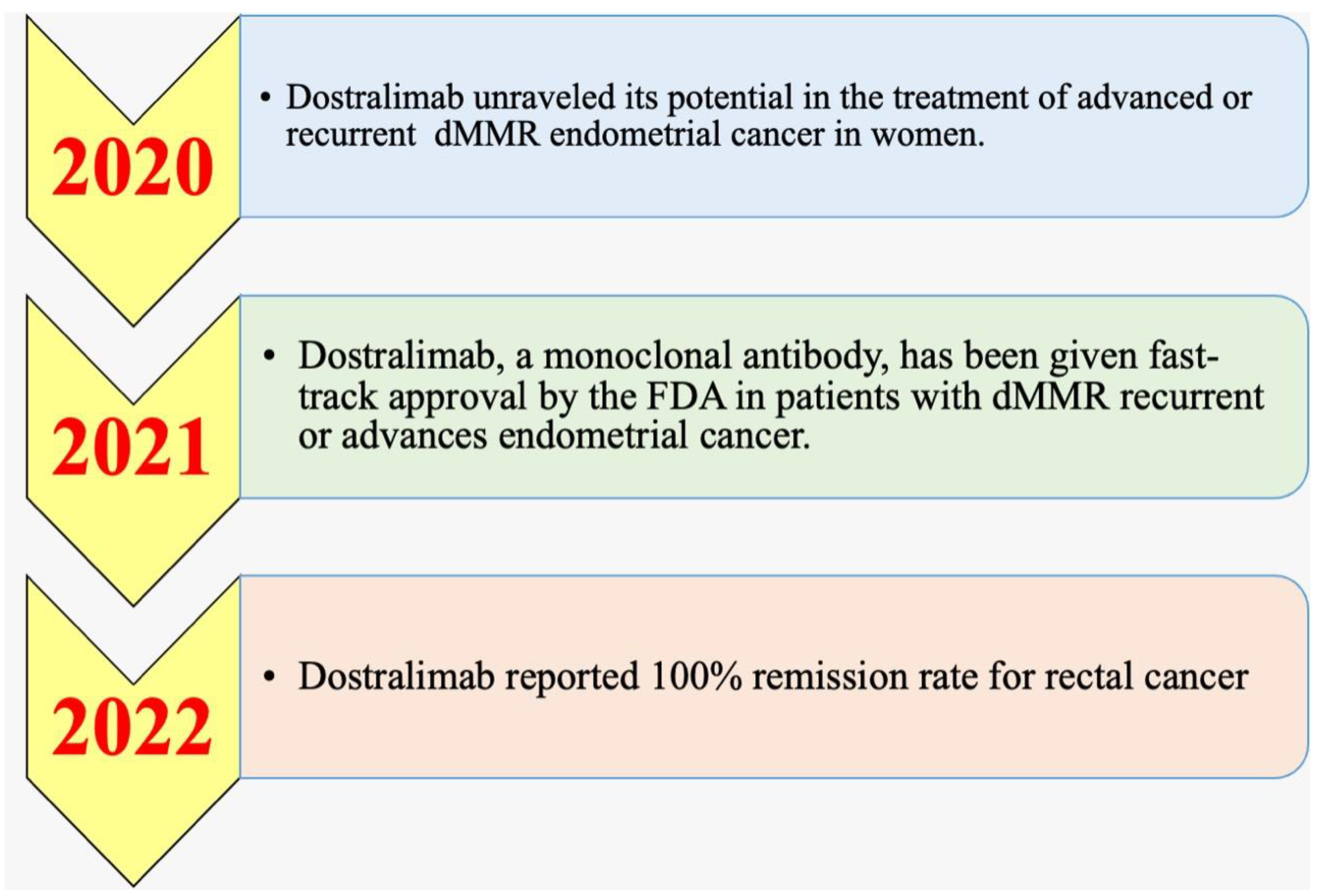
5.1. History and Timeline
5.2. Pharmacodynamics and Pharmacokinetics
5.3. Action Mechanism
5.4. Immunogenicity
6. GARNET Trial Overview
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | Anti-drug antibodies |
| ALK | Anaplastic lymphoma kinase |
| APC | Antigen-presenting cell |
| ATG | Antithymocyte globulin |
| BCG | Bacille Calmette–Guerin |
| CD | Cluster of differentiation |
| CRC | Colorectal cancer |
| DCs | Dendritic cells |
| DLBCL | Diffuse large B-cell lymphoma |
| dMMR | Deficient mismatch repair |
| DLs | Dose levels |
| EGFR | Epidermal growth-factor receptor |
| EMT | Epithelial-to-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| ER | Estrogen receptor |
| FDA | Food and Drug Administration |
| FGFR | Fibroblast growth-factor receptor |
| FOX | Forkhead box protein |
| GIST | Gastrointestinal stromal tumors |
| GCB | Germinal center B cell |
| GSK | GlaxoSmithKline |
| GLI | Glioma-associated oncogene homolog |
| Hh | Hedgehog |
| HNSCC | Head and neck squamous-cell carcinoma |
| HL | Hodgkin’s lymphoma |
| ICIs | Immune-checkpoint inhibitors |
| IFN | Interferon gamma |
| IL | Interleukin |
| IRF | Interferon regulatory factor |
| JAK | Janus kinase |
| Kras | Kirsten rat sarcoma viral oncogene |
| LAG | Lymphocyte-activation gene |
| MRI | Magnetic resonance imaging |
| MTD | Maximum tolerated dose |
| MCF | Michigan Cancer Foundation-7 |
| MHC | Major histocompatibility complex |
| MET | Microenvironment |
| MSI-H | Microsatellite instability—high |
| MAPK | Mitogen-activated protein kinase |
| NSCLC | Non-small-cell lung cancer |
| NOTCH | Neurogenic locus notch homolog protein 1 |
| TNF | Tumour necrosis factor |
| NFAT | Nuclear factor of activated T cells |
| OS | Overall survival |
| PDAC | Pancreatic ductal adenocarcinoma |
| PBMC | Peripheral blood mononuclear cells |
| PTEN | Phosphatase and tensin homolog |
| PKC | Protein kinase C |
| PET | Positron emission tomography |
| PD | Programmed cell death |
| PD-L | Programmed cell-death ligand |
| RAS | Rat sarcoma |
| RCC | Renal-cell carcinoma |
| STAT | Signal transducer and activator of transcription |
| SMO | Smoothened |
| TCR | T-cell receptor |
| TIM-3 | T-cell immunoglobulin and mucin-domain-containing-3 |
| TLR | Toll-like receptor |
| TNBC | Triple-negative breast cancer |
| TILs | Tumour-infiltrating lymphocytes |
| WNT | Wingless-related integration site |
References
- Pardoll, D. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couzin-Frankel, J. Cancer Immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Y.; Zhang, Q.; Feng, S.; Li, C.; Wang, L.; Zhao, X.; Yang, Z.; Li, Z.; Luo, H.; Liu, R.; et al. Safety and Efficacy of PD-1/PD-L1 inhibitors combined with radiotherapy in patients with non-small-cell lung cancer: A systematic review and meta-analysis. Cancer Med. 2021, 10, 1222–1239. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; Mc Dermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; Mc Dermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Powles, T.; Eder, J.P.; Fine, G.D.; Braiteh, F.S.; Loriot, Y.; Cruz, C.; Bellmunt, J.; Burris, H.A.; Petrylak, D.P.; Teng, S.-L.; et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014, 515, 558–562. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Leach, D.; Krummel, M.; Allison, J. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Yang, J.C.; Hughes, M.; Kammula, U.; Royal, R.; Sherry, R.M.; Topalian, S.L.; Suri, K.B.; Levy, C.; Allen, T.; Mavroukakis, S.; et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 2007, 30, 825–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, W.; Zhou, X.; Zhai, M.; Li, W.; Ran, Y.; Sun, Y.; Gao, Y. Blocking of the PD-1/PD-L1 interaction by a novel cyclic peptide inhibitor for cancer immunotherapy. Sci. China Life Sci. 2020, 64, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; Mc Dermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.; Momtaz, P. Immunologic Checkpoints in Cancer Therapy: Focus on the Programmed Death-1 (PD-1) Receptor Pathway. Pharm. Pers. Med. 2014, 7, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, P.; Gereke, M.; Bruder, D. Therapeutic intervention in cancer and chronic viral infections: Antibody mediated manipulation of PD-1/PD-L1 interaction. Rev. Recent Clin. Trials 2012, 7, 10–23. [Google Scholar] [CrossRef]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27 (Suppl. S2), S87–S97. [Google Scholar] [CrossRef]
- Blank, C.; Mackensen, A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: An update on implications for chronic infections and tumor evasion. Cancer Immunol. Immunother. 2007, 56, 739–745. [Google Scholar] [CrossRef]
- Ott, P.A.; Hodi, F.S.; Robert, C. CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013, 19, 5300–5399. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.; Vétizou, M.; Daillère, R.; Roberti, M.; Yamazaki, T.; Routy, B.; Lepage, P.; Boneca, I.; Chamaillard, M.; Kroemer, G.; et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity 2016, 44, 1255–1269. [Google Scholar] [CrossRef]
- Slovin, S.; Higano, C.; Hamid, O.; Tejwani, S.; Harzstark, A.; Alumkal, J.; Scher, H.; Chin, K.; Gagnier, P.; McHenry, M.; et al. Ipilimumab Alone or in Combination with Radiotherapy in Metastatic Castration-Resistant Prostate Cancer: Results from an Open-Label, Multicenter Phase I/II Study. Ann. Oncol. 2013, 24, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Shepherd, F.; Antonia, S.; Brahmer, J.; Chow, L.; Juergens, R.; Borghaei, H.; Shen, Y.; Harbison, C.; Alaparthy, S.; et al. First-Line Nivolumab (Anti-PD-1; BMS-936558, ONO-4538) Monotherapy in Advanced NSCLC: Safety, Efficacy, and Correlation of Outcomes with PD-L1 Status. J. Clin. Oncol. 2014, 32 (Suppl. S15), 8024. [Google Scholar] [CrossRef]
- Topalian, S.; Taube, J.; Anders, R.; Pardoll, D. Mechanism-Driven Biomarkers to Guide Immune Checkpoint Blockade in Cancer Therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Garon, E.; Rizvi, N.; Hui, R.; Leighl, N.; Balmanoukian, A.; Eder, J.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.; Arance, A.; Grob, J.; Mortier, L.; Daud, A.; Carlino, M.; Mc Neil, C.; Lotem, M.; et al. Pembrolizumab Versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.; Baas, P.; Kim, D.; Felip, E.; Pérez-Gracia, J.; Han, J.; Molina, J.; Kim, J.; Arvis, C.; Ahn, M.; et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rizvi, N.; Chaft, J.; Balmanoukian, A.; Goldberg, S.; Sanborn, R.; Steele, K.; Rebelatto, M.; Gu, Y.; Karakunnel, J.; Antonia, S. Tumor Response from Durvalumab (MEDI4736) + Tremelimumab Treatment in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC) Is Observed Regardless of PD-L1 Status. J. ImmunoTher. Cancer 2015, 3 (Suppl. S2), P193. [Google Scholar] [CrossRef] [Green Version]
- Antonia, S.; Goldberg, S.; Balmanoukian, A.; Chaft, J.; Sanborn, R.; Gupta, A.; Narwal, R.; Steele, K.; Gu, Y.; Karakunnel, J.; et al. Safety and Antitumour Activity of Durvalumab Plus Tremelimumab in Non-Small Cell Lung Cancer: A Multicentre, Phase 1B Study. Lancet Oncol. 2016, 17, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab Versus Docetaxel for Patients with Previously Treated Non-Small-Cell Lung Cancer (POPLAR): A Multicentre, Open-Label, Phase 2 Randomised Controlled Trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Mc Dermott, D.; Sosman, J.; Sznol, M.; Massard, C.; Gordon, M.; Hamid, O.; Powderly, J.; Infante, J.; Fassò, M.; Wang, Y.; et al. Atezolizumab, an Anti–Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates from a Phase Ia Study. J. Clin. Oncol. 2016, 34, 833–842. [Google Scholar] [CrossRef]
- Rosenberg, J.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.; Balar, A.; Necchi, A.; Dawson, N.; O’Donnell, P.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in Patients with Locally Advanced and Metastatic Urothelial Carcinoma Who have Progressed Following Treatment with Platinum-Based Chemotherapy: A Single-Arm, Multicentre, Phase 2 Trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.T.; Lee, J.Y.; Lim, H.; Lee, S.H.; Moon, Y.J.; Pyo, H.J.; Ryu, S.E.; Shin, W.; Heo, Y.-S. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci. Rep. 2017, 7, 553223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raval, R.; Sharabi, A.; Walker, A.; Drake, C.; Sharma, P. Tumor Immunology and Cancer Immunotherapy: Summary of the 2013 SITC Primer. J. ImmunoTher. Cancer 2014, 2, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oaknin, A.; Ellard, S.; Leath, C., III; Moreno, V.; Kristeleit, R.; Guo, W.; Lu, S.; Jenkins, D.; Mc Eachern, K.; Yu Jen, K.; et al. Preliminary Safety, Efficacy, and PK/PD Characterization from GARNET, a Phase I Clinical Trial of the Anti–PD-1 Monoclonal Antibody, TSR-042, in Patients with Recurrent or Advanced MSI-H Endometrial Cancer. Ann. Oncol. 2018, 29, viii334. [Google Scholar] [CrossRef]
- Oaknin, A.; Gilbert, L.; Tinker, A.; Brown, J.; Mathews, C.; Press, J.; Sabatier, R.; O’Malley, D.; Samouelian, V.; Boni, V.; et al. Safety and Antitumor Activity of Dostarlimab in Patients with Advanced or Recurrent DNA Mismatch Repair Deficient/Microsatellite Instability-High (Dmmr/MSI-H) or Proficient/Stable (Mmrp/MSS) Endometrial Cancer: Interim Results from GARNET—A Phase I, Single-Arm Study. J. ImmunoTher. Cancer 2022, 10, e003777. [Google Scholar]
- Moreno, V.; Barretina-Ginesta, M.; Guo, W.; Lu, S.; Jenkins, D.; McEachern, K.; Reichert, V.; Dunlap, S.; Im, E.; Gilbert, L.; et al. Abstract CT053: Preliminary Safety, Efficacy, and PK/PD Characterization from GARNET, A Phase 1 Clinical Trial of the Anti-PD-1 Monoclonal Antibody, TSR-042, in Patients with Recurrent or Advanced NSCLC and MSI-H Endometrial Cancer. Cancer Res. 2018, 78 (Suppl. S13), CT053. [Google Scholar] [CrossRef]
- Patnaik, A.; Weiss, G.; Rasco, D.; Blaydorn, L.; Mirabella, A.; Beeram, M.; Guo, W.; Lu, S.; Danaee, H.; McEachern, K.; et al. Safety, Antitumor Activity, and Pharmacokinetics of Dostarlimab, an Anti-PD-1, in Patients with Advanced Solid Tumors: A Dose–Escalation Phase 1 Trial. Cancer Chemother. Pharmacol. 2021, 89, 93–103. [Google Scholar] [CrossRef]
- Kristeleit, R.; Mathews, C.; Redondo, A.; Boklage, S.; Hanlon, J.; Im, E.; Brown, J. Patient-Reported Outcomes in the GARNET Trial in Patients with Advanced or Recurrent Mismatch Repair-Deficient/Microsatellite Instability-High Endometrial Cancer Treated with Dostarlimab. Int. J. Gynecol. Cancer 2022, 32, 1250–1257. [Google Scholar] [CrossRef]
- Kumar, S.; Ghosh, S.; Sharma, G.; Wang, Z.; Kehry, M.; Marino, M.; Neben, T.; Lu, S.; Luo, S.; Roberts, S.; et al. Preclinical Characterization of Dostarlimab, a Therapeutic Anti-PD-1 Antibody with Potent Activity to Enhance Immune Function in in vitro Cellular Assays and in vivo Animal Models. mAbs 2021, 13, 1954136. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Shao, W.; Lin, Z.; Melhem, M.; Lu, S. A Competitive Ligand-Binding Assay for the Detection of Neutralizing Antibodies against Dostarlimab (TSR-042). AAPS Open 2021, 7, 1–14. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Jemperli (Dostarlimab) Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761174s000lbl.pdf (accessed on 10 June 2020).
- Raedler, L.A. Opdivo (Nivolumab): Second PD-1 Inhibitor Receives FDA Approval for Unresectable or Metastatic Melanoma. Am. Health Drug Benefits 2015, 8, 180–183. [Google Scholar] [PubMed]
- Opdivo Approval History. Available online: https://www.drugs.com/history/opdivo.html (accessed on 10 January 2019).
- Opdivo: EPAR—Product Information. Available online: https://www.ema.europa.eu/documents/productinformation/opdivo-epar-product-information_en.pdf (accessed on 10 January 2019).
- Raedler, L.A. Keytruda (pembrolizumab): First PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am. Health Drug Benefits 2015, 8, 96–100. [Google Scholar] [PubMed]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- Shaffer, A. Checkpoint Inhibitor Changes Take Hold: Approval Standards Stir Debate 2022. Available online: https://www.onclive.com//view/checkpoint-inhibitor-changes-take-hold-approval-standards-stir-debate (accessed on 21 October 2022).
- Al-Salama, Z.T. Durvalumab: A Review in Extensive-Stage SCLC. Target. Oncol. 2021, 16, 857–864. [Google Scholar] [CrossRef]
- Danehy, S. European Commission Approves BAVENCIO®(avelumab) for First-Line Maintenance Treatment of Locally Advanced or Metastatic Urothelial Carcinoma 2021. Available online: https://www.prnewswire.com/news-releases/european-commission-approves-bavencio-avelumab-for-first-line-maintenance-treatment-of-locally-advanced-or-metastatic-urothelial-carcinoma-301213683.html (accessed on 20 October 2022).
- Hossain, M.; Liu, G.; Dai, B.; Si, Y.; Yang, Q.; Wazir, J.; Birnbaumer, L.; Yang, Y. Reinvigorating Exhausted CD8+ Cytotoxic T Lymphocytes in the Tumor Microenvironment and Current Strategies in Cancer Immunotherapy. Med. Res. Rev. 2020, 41, 156–201. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, D.; Zhang, G.; Guo, X. The Role of PD-1/PD-L1 Axis in Treg Development and Function: Implications for Cancer Immunotherapy. OncoTargets Ther. 2019, 12, 8437–8445. [Google Scholar] [CrossRef] [Green Version]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Paschall, A.V.; Shi, H.; Savage, N.; Waller, J.L.; Sabbatini, M.E.; Oberlies, N.H.; Pearce, C.; Liu, K. The MLL1-H3K4me3 axis-mediated PD-L1 expression and pancreatic cancer immune evasion. JNCI J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [Green Version]
- Efremova, M.; Rieder, D.; Klepsch, V.; Charoentong, P.; Finotello, F.; Hackl, H.; Hermann-Kleiter, N.; Löwer, M.; Baier, G.; Krogsdam, A.; et al. Targeting Immune Checkpoints Potentiates Immunoediting and Changes the Dynamics of Tumor Evolution. Nat. Commun. 2018, 9, 32. [Google Scholar] [CrossRef]
- Wherry, E.; Kurachi, M. Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 Contributes to Immunosuppression and Is Associated with Anti-PD-1 Response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mahoney, K.; Giobbie-Hurder, A.; Zhao, F.; Lee, S.; Liao, X.; Rodig, S.; Li, J.; Wu, X.; Butterfield, L.; et al. Soluble PD-L1 as a Biomarker in Malignant Melanoma Treated with Checkpoint Blockade. Cancer Immunol. Res. 2017, 5, 480–492. [Google Scholar] [CrossRef] [Green Version]
- Theodoraki, M.; Yerneni, S.; Hoffmann, T.; Gooding, W.; Whiteside, T. Clinical Significance of PD-L1 + Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patsoukis, N.; Li, L.; Sari, D.; Petkova, V.; Boussiotis, V. PD-1 Increases PTEN Phosphatase Activity while Decreasing PTEN Protein Stability by Inhibiting Casein Kinase 2. Mol. Cell. Biol. 2013, 33, 3091–3098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wartewig, T.; Kurgyis, Z.; Keppler, S.; Pechloff, K.; Hameister, E.; Öllinger, R.; Maresch, R.; Buch, T.; Steiger, K.; Winter, C.; et al. PD-1 Is a Haploinsufficient Suppressor of T Cell Lymphomagenesis. Nature 2017, 552, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Abushukair, H.; Ababneh, O.; Zaitoun, S.; Saeed, A. Primary and Secondary Immune Checkpoint Inhibitors Resistance in Colorectal Cancer: Key Mechanisms and Ways to Overcome Resistance. Cancer Treat. Res. Commun. 2022, 100643. [Google Scholar] [CrossRef]
- Brom, V.; Burger, C.; Wirtz, D.; Schildberg, F. The Role of Immune Checkpoint Molecules on Macrophages in Cancer, Infection, and Autoimmune Pathologies. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Robainas, M.; Otano, R.; Bueno, S.; Ait-Oudhia, S. Understanding the Role of PD-L1/PD1 Pathway Blockade and Autophagy in Cancer Therapy. OncoTargets Ther. 2017, 10, 1803–1807. [Google Scholar] [CrossRef] [Green Version]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced Expression of PD-1, a Novel Member of the Immunoglobulin Gene Superfamily, upon Programmed Cell Death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Akinleye, A.; Rasool, Z. Immune Checkpoint Inhibitors Of PD-L1 as Cancer Therapeutics. J. Hematol. Oncol. 2019, 12, 92. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Filippone, A.; Lanza, M.; Mannino, D.; Raciti, G.; Colarossi, C.; Sciacca, D.; Cuzzocrea, S.; Paterniti, I. PD1/PD-L1 Immune Checkpoint as a Potential Target for Preventing Brain Tumor Progression. Cancer Immunol. Immunother. 2022, 71, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.; Collins, M. The B7 Family of Ligands and its Receptors: New Pathways for Costimulation and Inhibition of Immune Responses. Annu. Rev. Immunol. 2002, 20, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Neel, B.; Gu, H.; Pao, L. The ‘Shp’ing News: SH2 Domain-Containing Tyrosine Phosphatases in Cell Signaling. Trends Biochem. Sci. 2003, 28, 284–293. [Google Scholar] [CrossRef]
- Ohaegbulam, K.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human Cancer Immunotherapy with Antibodies to the PD-1 and PD-L1 Pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.; Liu, Y.; Li, Q.; Li, X.; Zhao, W.; Zhang, H.; Zhang, X.; Jiang, J.; Wu, C. PD-1/PD-L1 Pathway in Non-Small-Cell Lung Cancer and its Relation with EGFR Mutation. J. Transl. Med. 2015, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-Γ from Lymphocytes Induces PD-L1 Expression and Promotes Progression of Ovarian Cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef] [Green Version]
- Staron, M.; Gray, S.; Marshall, H.; Parish, I.; Chen, J.; Perry, C.; Cui, G.; Li, M.; Kaech, S. The Transcription Factor Foxo1 Sustains Expression of the Inhibitory Receptor PD-1 and Survival of Antiviral CD8+ T Cells during Chronic Infection. Immunity 2014, 41, 802–814. [Google Scholar] [CrossRef] [Green Version]
- Youngblood, B.; Oestreich, K.; Ha, S.; Duraiswamy, J.; Akondy, R.; West, E.; Wei, Z.; Lu, P.; Austin, J.; Riley, J.; et al. Chronic Virus Infection Enforces Demethylation of the Locus that Encodes PD-1 in Antigen-Specific CD8+ T Cells. Immunity 2011, 35, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Deng, A.; Liu, H.; Ge, G.; Liu, X. Activator Protein 1 Suppresses Antitumor T-Cell Function via the Induction of Programmed Death 1. Proc. Natl. Acad. Sci. USA 2012, 109, 15419–15424. [Google Scholar] [CrossRef] [Green Version]
- Salmaninejad, A.; Khoramshahi, V.; Azani, A.; Soltaninejad, E.; Aslani, S.; Zamani, M.; Zal, M.; Nesaei, A.; Hosseini, S. PD-1 and Cancer: Molecular Mechanisms and Polymorphisms. Immunogenetics 2017, 70, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, R.; Song, W.; Sun, J.; Liu, D.; Li, Z. PD-1, PD-L1 (B7-H1) and Tumor-Site Immune Modulation Therapy: The Historical Perspective. J. Hematol. Amp Oncol. 2017, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 365–1369. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.; Long, A.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.; Malenkovich, N.; Okazaki, T.; Byrne, M.; et al. Engagement of the PD-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.-X.; et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef]
- Bellucci, R.; Martin, A.; Bommarito, D.; Wang, K.; Hansen, S.; Freeman, G.; Ritz, J. Interferon-Γ-Induced Activation of JAK1 and JAK2 Suppresses Tumor Cell Susceptibility to NK Cells through Upregulation of PD-L1 Expression. OncoImmunology 2015, 4, e1008824. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.; Moreno, B.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.; Zaretsky, J.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [Green Version]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.; Watari, H. Tumor-Intrinsic PD-L1 Signaling in Cancer Initiation, Development and Treatment: Beyond Immune Evasion. Front. Oncol. 2018, 8, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes-Xavier, C.; Angulo, J.; Pulido, R.; López, J. A Critical Insight into the Clinical Translation of PD-1/PD-L1 Blockade Therapy in Clear Cell Renal Cell Carcinoma. Curr. Urol. Rep. 2019, 20, 1. [Google Scholar] [CrossRef]
- Gowrishankar, K.; Gunatilake, D.; Gallagher, S.; Tiffen, J.; Rizos, H.; Hersey, P. Inducible but not Constitutive Expression of PD-L1 in Human Melanoma Cells is Dependent on Activation of NF-Κb. PLoS ONE 2015, 10, e0123410. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jin, H.; Du, N.; Wang, J.; Lu, H.; Xiao, J.; Li, X.; Yi, J.; Gu, T.; Dan, X.; et al. A Novel Computational Framework for Predicting the Survival of Cancer Patients with PD-1/PD-L1 Checkpoint Blockade Therapy. Front. Oncol. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Song, Y.; Wang, Y.; Huang, Y.; Li, Z.; Cui, Y.; Yi, M.; Xia, L.; Zhuang, W.; Wu, X.; et al. PD-1/PD-L1 Blockade Rescue Exhausted CD8+ T Cells in Gastrointestinal Stromal Tumours via the PI3K/Akt/Mtor Signalling Pathway. Cell Prolif. 2019, 52, e12571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, F.; Zhang, T.; Deng, S.; Wei, J.; Yang, P.; Wang, Q.; Chen, Z.; Li, W.; Chen, H.; Hu, H.; et al. PD-L1 Promotes Colorectal Cancer Stem Cell Expansion by Activating HMGA1-Dependent Signaling Pathways. Cancer Lett. 2019, 450, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Wang, M.; Hu, C.; Tang, Y.; Li, Y.; Hao, S. Circular RNA Regulates the Onset and Progression of Cancer through the Mitogen-Activated Protein Kinase Signaling Pathway (Review). Oncol. Lett. 2021, 22, 817. [Google Scholar] [CrossRef]
- Pradhan, R.; Singhvi, G.; Dubey, S.; Gupta, G.; Dua, K. MAPK Pathway: A Potential Target for the Treatment of Non-Small-Cell Lung Carcinoma. Future Med. Chem. 2019, 11, 793–795. [Google Scholar] [CrossRef] [Green Version]
- Stutvoet, T.; Kol, A.; Vries, E.; Bruyn, M.; Fehrmann, R.; van Scheltinga, A.T.; Jong, S. MAPK Pathway Activity Plays a Key Role in PD-L1 Expression of Lung Adenocarcinoma Cells. J. Pathol. 2019, 249, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Jalali, S.; Price-Troska, T.; Bothun, C.; Villasboas, J.; Kim, H.; Yang, Z.; Novak, A.; Dong, H.; Ansell, S. Reverse Signaling via PD-L1 Supports Malignant Cell Growth and Survival in Classical Hodgkin Lymphoma. Blood Cancer J. 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D. JAK–STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef]
- Groner, B.; von Manstein, V. Jak Stat signaling and Cancer: Opportunities, Benefits and Side Effects of Targeted Inhibition. Mol. Cell. Endocrinol. 2017, 451, 1–14. [Google Scholar] [CrossRef]
- Doi, T.; Ishikawa, T.; Okayama, T.; Oka, K.; Mizushima, K.; Yasuda, T.; Sakamoto, N.; Katada, K.; Kamada, K.; Uchiyama, K.; et al. The JAK/STAT Pathway Is Involved in the Upregulation of PD-L1 Expression in Pancreatic Cancer Cell Lines. Oncol. Rep. 2017, 37, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Huang, T.; Zou, Q.; Liu, D.; Wang, Y.; Tan, X.; Wei, Y.; Qiu, H. FGFR2 Promotes Expression Of PD-L1 in Colorectal Cancer via the JAK/STAT3 Signaling Pathway. J. Immunol. 2019, 202, 3065–3075. [Google Scholar] [CrossRef] [PubMed]
- Harb, J.; Lin, P.; Hao, J. Recent Development of WNT Signaling Pathway Inhibitors for Cancer Therapeutics. Curr. Oncol. Rep. 2019, 21, 12. [Google Scholar] [CrossRef]
- Galluzzi, L.; Spranger, S.; Fuchs, E.; López-Soto, A. WNT Signaling in Cancer Immunosurveillance. Trends Cell Biol. 2019, 29, 44–65. [Google Scholar] [CrossRef]
- Taylor, A.; Rothstein, D.; Rudd, C. Small-Molecule Inhibition of PD-1 Transcription Is an Effective Alternative to Antibody Blockade in Cancer Therapy. Cancer Res. 2018, 78, 706–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castagnoli, L.; Cancila, V.; Cordoba-Romero, S.; Faraci, S.; Talarico, G.; Belmonte, B.; Iorio, M.; Milani, M.; Volpari, T.; Chiodoni, C.; et al. WNT Signaling Modulates PD-L1 Expression in the Stem Cell Compartment of Triple-Negative Breast Cancer. Oncogene 2019, 38, 4047–4060. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.; Jeong, M.; Bazer, F.; Song, G. Curcumin Suppresses Proliferation and Migration and Induces Apoptosis on Human Placental Choriocarcinoma Cells via ERK1/2 and SAPK/JNK MAPK Signaling Pathways. Biol. Reprod. 2016, 95, 83. [Google Scholar] [CrossRef]
- Bi, X.; Wang, H.; Zhang, W.; Wang, J.; Liu, W.; Xia, Z.; Huang, H.; Jiang, W.; Zhang, Y.; Wang, L. PD-L1 Is Upregulated by EBV-Driven LMP1 through NF-Κb Pathway and Correlates with Poor Prognosis in Natural Killer/T-Cell Lymphoma. J. Hematol. Oncol. 2016, 128, 4134. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.; et al. Chemotherapy Induces Programmed Cell Death-Ligand 1 Overexpression via the Nuclear Factor-Κb to Foster an Immunosuppressive Tumor Microenvironment in Ovarian Cancer. Cancer Res. 2015, 75, 5034–5045. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Shiraishi, R.; Kawauchi, D. Sonic Hedgehog Signaling in Cerebellar Development and Cancer. Front. Cell Dev. Biol. 2022, 10, 864035. [Google Scholar] [CrossRef]
- Martin, A.; Nirschl, C.; Polanczyk, M.; Bell, W.; Nirschl, T.; Harris-Bookman, S.; Phallen, J.; Hicks, J.; Martinez, D.; Ogurtsova, A.; et al. PD-L1 Expression in Medulloblastoma: An Evaluation by Subgroup. Oncotarget 2018, 9, 19177–19191. [Google Scholar] [CrossRef]
- Chakrabarti, J.; Holokai, L.; Syu, L.; Steele, N.; Chang, J.; Wang, J.; Ahmed, S.; Dlugosz, A.; Zavros, Y. Hedgehog Signaling Induces PD-L1 Expression and Tumor Cell Proliferation in Gastric Cancer. Oncotarget 2018, 9, 37439–37457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Chen, S.; Yuan, W.; Wang, H.; Chen, K.; Li, D.; Li, D. PD-1/PD-L1 Interaction Up-Regulates MDR1/P-Gp Expression in Breast Cancer Cells via PI3K/AKT and MAPK/ERK Pathways. Oncotarget 2017, 8, 99901–99912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajona, D.; Ortiz-Espinosa, S.; Moreno, H.; Lozano, T.; Pajares, M.; Agorreta, J.; Bértolo, C.; Lasarte, J.; Vicent, S.; Hoehlig, K.; et al. A Combined PD-1/C5a Blockade Synergistically Protects against Lung Cancer Growth and Metastasis. Cancer Discov. 2017, 7, 694–703. [Google Scholar] [CrossRef] [Green Version]
- Takada, K.; Toyokawa, G.; Okamoto, T.; Shimokawa, M.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Takamori, S.; Katsura, M.; et al. A Comprehensive Analysis of Programmed Cell Death Ligand-1 Expression with the Clone SP142 Antibody in Non–Small-Cell Lung Cancer Patients. Clin. Lung Cancer 2017, 18, 572–582.e1. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Li, C.; Liang, L.; Zhang, Y.; Chen, Y.; Fang, J.; Xu, J. Rise Of PD-L1 Expression During Metastasis of Colorectal Cancer: Implications for Immunotherapy. J. Dig. Dis. 2017, 18, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Li, Y.; Luo, Y.; Xu, J.; Liufu, H.; Tian, Z.; Huang, C.; Li, J.; Huang, C. A Feedback Loop Formed by ATG7/Autophagy, Foxo3a/Mir-145 and PD-L1 Regulates Stem-Like Properties and Invasion in Human Bladder Cancer. Cancers 2019, 11, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okudaira, K.; Hokari, R.; Tsuzuki, Y.; Okada, Y.; Komoto, S.; Watanabe, C.; Kurihara, C.; Kawaguchi, A.; Nagao, S.; Azuma, M.; et al. Blockade of B7-H1 or B7-DC Induces an Anti-Tumor Effect in a Mouse Pancreatic Cancer Model. Int. J. Oncol. 2009, 35, 741–749. [Google Scholar] [PubMed] [Green Version]
- Masugi, Y.; Abe, T.; Ueno, A.; Fujii-Nishimura, Y.; Ojima, H.; Endo, Y.; Fujita, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; et al. Characterization of Spatial Distribution of Tumor-Infiltrating CD8+ T Cells Refines Their Prognostic Utility for Pancreatic Cancer Survival. Mod. Pathol. 2019, 32, 1495–1507. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Liu, S.; Wang, A.R.; You, Z. PD-1, PD-L1 and PD-L2 expression in mouse prostate cancer. Am. J. Clin. Exp. Urol. 2016, 4, 1–8. [Google Scholar]
- Gevensleben, H.; Dietrich, D.; Golletz, C.; Steiner, S.; Jung, M.; Thiesler, T.; Majores, M.; Stein, J.; Uhl, B.; Müller, S.; et al. The Immune Checkpoint Regulator PD-L1 Is Highly Expressed in Aggressive Primary Prostate Cancer. Clin. Cancer Res. 2016, 22, 1969–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Dashnamoorthy, R.; Galera, P.; Makarenko, V.; Chang, H.; Ghosh, S.; Evens, A. The Immune Checkpoint Molecules PD-1, PD-L1, TIM-3 And LAG-3 in Diffuse Large B-Cell Lymphoma. Oncotarget 2019, 10, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Han, X. Melanoma Cancer Immunotherapy Using PD-L1 Sirna and Imatinib Promotes Cancer-Immunity Cycle. Pharm. Res. 2020, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function Of PD-1/PD-L Pathway beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lussier, D.; O’Neill, L.; Nieves, L.; McAfee, M.; Holechek, S.; Collins, A.; Dickman, P.; Jacobsen, J.; Hingorani, P.; Blattman, J. Enhanced T-Cell Immunity to Osteosarcoma through Antibody Blockade of PD-1/PD-L1 Interactions. J. Immunother. 2015, 38, 96–106. [Google Scholar] [CrossRef]
- Topalian, S.; Sznol, M.; McDermott, D.; Kluger, H.; Carvajal, R.; Sharfman, W.; Brahmer, J.; Lawrence, D.; Atkins, M.; Powderly, J.; et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients with Advanced Melanoma Receiving Nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef]
- Naidoo, J.; Page, D.; Li, B.; Connell, L.; Schindler, K.; Lacouture, M.; Postow, M.; Wolchok, J. Toxicities of the Anti-PD-1 and Anti-PD-L1 Immune Checkpoint Antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Chen, L.; Han, X. Anti–PD-1/PD-L1 Therapy of Human Cancer: Past, Present, and Future. J. Clin. Investig. 2015, 125, 3384–3391. [Google Scholar] [CrossRef] [Green Version]
- D’Incecco, A.; Andreozzi, M.; Ludovini, V.; Rossi, E.; Capodanno, A.; Landi, L.; Tibaldi, C.; Minuti, G.; Salvini, J.; Coppi, E.; et al. PD-1 And PD-L1 Expression in Molecularly Selected Non-Small-Cell Lung Cancer Patients. Br. J. Cancer 2014, 112, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, T.; Liu, J.; Yu, H.; Jiao, S.; Feng, B.; Zhou, F.; Fu, Y.; Yin, Q.; Zhang, P.; et al. Acid-Activatable Versatile Micelleplexes for PD-L1 Blockade-Enhanced Cancer Photodynamic Immunotherapy. Nano Lett. 2016, 16, 5503–5513. [Google Scholar] [CrossRef]
- Vaddepally, R.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbognin, L.; Pilotto, S.; Milella, M.; Vaccaro, V.; Brunelli, M.; Caliò, A.; Cuppone, F.; Sperduti, I.; Giannarelli, D.; Chilosi, M.; et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): Sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS ONE 2015, 10, e0130142. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, M. Searching in the Immune Checkpoint Black Box. Nat. Rev. Cancer 2017, 17, 511. [Google Scholar] [CrossRef]
- Nishino, M.; Ramaiya, N.; Hatabu, H.; Hodi, F. Monitoring Immune-Checkpoint Blockade: Response Evaluation and Biomarker Development. Nat. Rev. Clin. Oncol. 2017, 14, 655–668. [Google Scholar] [CrossRef]
- Bear, A.; Vonderheide, R.; O’Hara, M. Challenges and Opportunities for Pancreatic Cancer Immunotherapy. Cancer Cell 2020, 38, 788–802. [Google Scholar] [CrossRef]
- Karachaliou, N.; Gonzalez-Cao, M.; Crespo, G.; Drozdowskyj, A.; Aldeguer, E.; Gimenez-Capitan, A.; Teixido, C.; Molina-Vila, M.; Viteri, S.; De Gil, M.L.L.; et al. Interferon Gamma, an Important Marker of Response to Immune Checkpoint Blockade in Non-Small Cell Lung Cancer and Melanoma Patients. Ther. Adv. Med. Oncol. 2018, 10, 175883401774974. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Xu, H.; Liu, Q.; Zhao, W.; Han, X.; Wu, K. Biomarkers for Predicting Efficacy Of PD-1/PD-L1 Inhibitors. Mol. Cancer 2018, 17, 129. [Google Scholar] [CrossRef]
- Singh, V.; Sheikh, A.; Abourehab, M.; Kesharwani, P. Dostarlimab as a Miracle Drug: Rising Hope against Cancer Treatment. Biosensors 2022, 12, 617. [Google Scholar] [CrossRef]
- Kaplon, H.; Muralidharan, M.; Schneider, Z.; Reichert, J. Antibodies to Watch in 2020. mAbs 2019, 12, 1703531. [Google Scholar] [CrossRef] [Green Version]
- Temrikar, Z.; Suryawanshi, S.; Meibohm, B. Pharmacokinetics and Clinical Pharmacology of Monoclonal Antibodies in Pediatric Patients. Pediatr. Drugs 2020, 22, 199–216. [Google Scholar] [CrossRef] [Green Version]
- Green, A.; Feinberg, J.; Makker, V. A Review of Immune Checkpoint Blockade Therapy in Endometrial Cancer. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 238–244. [Google Scholar] [CrossRef]
- Deshpande, M.; Romanski, P.; Rosenwaks, Z.; Gerhardt, J. Gynecological Cancers Caused by Deficient Mismatch Repair and Microsatellite Instability. Cancers 2020, 12, 3319. [Google Scholar] [CrossRef]
- FDA Grants Accelerated Approval to Dostarlimab-Gxly for dMMR Advanced. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-advanced-solid-tumors (accessed on 3 October 2022).
- Clinical Trial Number NCT02715284 for “A Phase 1 Dose Escalation and Cohort Expansion Study of TSR-042, an Anti-PD-1 Monoclonal Antibody, in Patients with Advanced Solid Tumors (GARNET). Available online: ClinicalTrials.gov (accessed on 13 September 2022).
- Clinical Trial Number NCT03981796 for “A Study of Dostarlimab (TSR-042) Plus Carboplatin-Paclitaxel versus Placebo Plus Carboplatin-paclitaxel in Patients with Recurrent or Primary Advanced Endometrial Cancer (RUBY)”. Available online: ClinicalTrials.gov (accessed on 7 September 2022).
- Clinical trial Number NCT03602859 for “A Phase 3 Comparison of Platinum-Based Therapy with TSR-042 and Niraparib versus Standard of Care Platinum-Based Therapy as First-Line Treatment of Stage III or IV Nonmucinous Epithelial Ovarian Cancer (FIRST)”. Available online: ClinicalTrials.gov (accessed on 10 September 2022).
- Data from GARNET Study Indicates Robust Activity of Dostarlimab in Patients with Advanced or Recurrent Endometrial Cancer; GSK Press: Brentford, UK, 2019; Archived from the Original on 27 December 2019. Retrieved 1 January 2020; Available online: https://us.gsk.com/en-us/media/press-releases/data/from-garnet-study-indicates ro-bust-activity-of-dostarlimabin-patients-with advanced-or-recurrent-endometrial-cancer (accessed on 13 September 2022).
- Mulcahy, N. FDA Approves New Immunotherapy for Endometrial Cancer. Medscape; Archived from the Original on 22 April 2021. Retrieved 23 April 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-immunotherapy-endometrial-cancer-specific-biomarker (accessed on 10 September 2022).
- FDA Grants Accelerated Approval to Dostarlimab-Gxly for dMMR Endometrial Cancer; U.S. Food and Drug Administration (FDA): Silver Spring, MD, USA, 2021; Archived from the original on 22 April 2021. Retrieved 22 April 2021; Public Domain This article incorporates text from this source, which is in the public domain.
- André, T.; Banerjee, S.; Berton, D.; Ellard, L.; Jimenez, B.; Samouëlian, V.; Gilbert, L.; Boni, V.; Han, X.; Antony, G.; et al. Antitumor activity of dostarlimab by PD-L1 and tumor mutation burden (TMB) in patients (pts) with mismatch repair deficient and proficient (dMMR and MMRp) tumors in the GARNET trial. Cancer Res. 2022, 82, 5135. [Google Scholar] [CrossRef]
- Costa, B.; Vale, N. Dostarlimab: A Review. Biomolecules 2022, 12, 1031. [Google Scholar] [CrossRef]
- Commissioner, O. Of the Priority Review. Available online: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/priority-review (accessed on 16 October 2022).
- Park, U.; Jeong, T.; Gu, N.; Lee, H.; Heo, Y. Molecular Basis of PD-1 Blockade by Dostarlimab, the FDA-Approved Antibody for Cancer Immunotherapy. Biochem. Biophys. Res. Commun. 2022, 599, 31–37. [Google Scholar] [CrossRef]
- Markham, A. Dostarlimab: First Approval. Drugs 2021, 81, 1213–1219. [Google Scholar] [CrossRef]
- FDA; CDER. Highlights of Prescribing Information Tissue, Including the Following: Immune-Mediated Pneumonitis, (n.d.). Available online: www.fda.gov/medwatch (accessed on 16 June 2022).
- Home|GSK, (n.d.). Available online: https://www.gsk.com/en-gb/ (accessed on 16 June 2022).
- Committee for Medicinal Products Human Use (CHMP)—European Medicines Agency. Available online: https://www.ema.europa.eu/en/committees/committee-medicinal-products-human-use-chmp (accessed on 3 October 2022).
- Yap, T.; Bessudo, A.; Hamilton, E.; Sachdev, J.; Patel, M.; Rodon, J.; Evilevitch, L.; Duncan, M.; Guo, W.; Kumar, S.; et al. Iolite: Phase 1B Trial of Doublet/Triplet Combinations of Dostarlimab with Niraparib, Carboplatin–Paclitaxel, with or without Bevacizumab in Patients with Advanced Cancer. J. ImmunoTher. Cancer 2022, 10, e003924. [Google Scholar] [CrossRef]
- Melhem, M.; Hanze, E.; Lu, S.; Alskär, O.; Visser, S.; Gandhi, Y. Population Pharmacokinetics and Exposure–Response of Anti-Programmed Cell Death Protein-1 Monoclonal Antibody Dostarlimab in Advanced Solid Tumours. Br. J. Clin. Pharmacol. 2022, 88, 4142–4154. [Google Scholar] [CrossRef]
- Kasherman, L.; Ahrari, S.; Lheureux, S. Dostarlimab in the Treatment of Recurrent or Primary Advanced Endometrial Cancer. Future Oncol. 2021, 17, 877–892. [Google Scholar] [CrossRef]
- Jemperli|European Medicines Agency, (n.d.). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/jemperli (accessed on 16 June 2022).
- Lu, S.; Bowsher, R.; Clancy, A.; Rosen, A.; Zhang, M.; Yang, Y.; Koeck, K.; Gao, M.; Potocka, E.; Guo, W.; et al. An Integrated Analysis of Dostarlimab Immunogenicity. AAPS J. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Moreno, V.; Roda, D.; Pikiel, J.; Trigo, J.; Bosch-Barrera, J.; Drew, Y.; Kristeleit, R.; Hiret, S.; Bajor, D.L.; Cruz, P.; et al. Safety and Efficacy of Dostarlimab in Patients with Recurrent/Advanced Non-Small Cell Lung Cancer: Results from Cohort E of the Phase I GARNET Trial. Clin. Lung Cancer 2022, 23, e415–e427. [Google Scholar] [CrossRef]
- Laken, H.; Kehry, M.; McNeeley, P.; Neben, T.; Zhang, J.; Jenkins, D. Identification and characterization of TSR-042, a novel anti-PD-1 therapeutic antibody. Eur. J. Cancer 2016, 69 (Suppl. S1), S102. [Google Scholar] [CrossRef]

| S. No. | Drug Name | Trade Name | Year | Target | Developer | Refrences | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nivolumab | Opdivo | Melanoma (2014), NSCLC (2015), Hodgkin’s lymphoma (2016), head and neck squamous-cell cancer (2016), Hepatocellular carcer (2017), urothelial cancer (2017), CRC (2017), renal-cell cancer (2021), adenocarcinoma, esophageal cancer (2021) | PD-1 | Dako Bristol–Meyers Squibb | [42,43,44] | |||||
| 2 | Pembrolizumab | Keytruda | Melanoma (2014), NSCLC (2015), head and neck squamous-cell cancer (2016), bladder cancer (2017), gastroesophageal cancer (2017), Hodgkin’s lymphoma (2017), | PD-1 | Dako Merck | [45,46] | |||||
| 3 | Cemiplimab | Libtayo | Cutaneous squamous-cell cancer (2018), NSCLC (2021), basal-cell cancer (2021) | PD-1 | Regeneron | [46] | |||||
| 4 | Dostarlimab | Jemperli | Endometrial cancer and recurrent or advanced solid tumors (2021) | PD-1 | GlaxoSmithKline LLC | [41] | |||||
| 5 | Atezolizumab | Tecentriq | Urothelial cancer (2016), NSCLC (2016), hepatocellular melanoma (2020), carcinoma (2020), small-cell lung cancer (2021) | PD-LI | Ventana Genentech/Roche | [47] | |||||
| 6 | Durvalumab (MED14736) | Imfinzi | Urothelial carcinoma (2017), NSCLC (2018) | PD-LI | Ventana Medimmune/AstraZeneca | [48] | |||||
| 7 | Avelumab | Bavencio | Urothelial carcinoma (2017), Merkel-cell carcinoma (2017), renal-cell cancer (2019) | Merck KGaA, Darmstadt, Germany, and Pfizer | [49] |
| Condition or Disease | Intervention/Treatment Drug | Allocation | Phase | Estimated Enrollment | ClinicalTrials.gov Identifier: | Duration |
|---|---|---|---|---|---|---|
| Breast cancer | Dostarlimab, dostarlimab + combinations | N/A | Phase II | 4000 participants | NCT01042379 Recruiting | 1 March 2010 December 2031 |
| Neoplasms | Dostarlimab | Non-Randomized | Phase I | 740 participants | NCT02715284 Recruiting | 7 March 2016 30 July 2024 |
| Neoplasms | Dostarlimab, dostarlimab + combinations | Non-Randomized | Phase I | 369 participants | NCT02817633 Recruiting | 8 July 2016 3 October 2024 |
| Advanced solid tumors | Dostarlimab | Non-Randomized | Phase I | 111 participants | NCT03250832 Active, not recruiting | 8 August 2017 14 November 2022 |
| Neoplasms | Niraparib, pembrolizumab, TSR-042 (dostarlimab) | Non-Randomized | Phase II | 53 participants | NCT03308942 Completed | 29 September 2017 31 August 2021 |
| Advanced cancer, neoplasms, metastatic cancer, solid tumor, lung carcinoma | Niraparib, TSR-042, carboplatin–paclitaxil, bevacizumab, TSR-022, carboplatin–pemetrexed, carboplatin–nab-paclitaxe | Non-Randomized | Phase I | 58 participants | Active, not recruiting NCT03307785 | 12 October 2017 29 April 2022 |
| Endometrial cancer | Niraparib, dostarlimab | N/A | Phase II | 51 participants | NCT03016338 Active, not recruiting | 6 November 2017 December 2023 |
| Ovarian neoplasms, ovarian cancer | Niraparib, dostarlimab (TSR-042). | Randomized | Phase III | 1405 participants | NCT03602859 (First) Active, not recruiting | 11 October 2018 22 June 2026 |
| Ovarian neoplasms | Niraparib, TSR-042, bevacizumab, carboplatin, paclitaxel | Randomized | Phase I Phase II | 125 participants | NCT03574779 Recruiting | 15 November 2018 31 March 2026 |
| Neoplasms | Dostarlimab, carboplatin, paclitaxel, niraparib | N/A | Phase III | 740 participants | NCT03981796 (Ruby) Recruiting | 11 June 2019 29 December 2026 |
| Cervical cancer, advanced cancer | Dostarlimab | Randomized | Phase II | 132 participants | NCT03833479 Recruiting | 28 June 2019 December 2024 |
| Ovarian neoplasms | Dostarlimab, niraparib | N/A | Phase II | 41 participants | NCT03955471 (Moonstone) Terminated | 3 October 2019 12 January 2022 |
| Endometrial cancer | Dostarlimab Radiation: brachytherapy Procedure: endometrial, blood draw for immune-response biopsy | N/A | Phase I | 12 participants | NCT03955978 Recruiting | 15 October2019 31 October 2024 |
| Advanced mismatch repair-deficient solid tumors | Dostarlimab, capecitabine or 5-FU Radiation: intensity modulated radiation therapy | Non-randomized | Phase II | 30 participants | NCT04165772 Recruiting | 11 December 2019 30 November 2025 |
| Localized unresectable, adult primary liver cancer, adult primary liver cancer, advanced adult primary liver cancer | TSR-022 and dostarlimab | N/A | Phase II | 42 participants | NCT03680508 Recruiting | 19 December 2019 October 2023 |
| Melanoma stage III Melanoma stage IV | Dostarlimab (single), dostarlimab and TSR-022 (combination) | Randomized | Phase II | 56 participants | NCT04139902 Recruiting | 30 April 2020 October 2027 |
| Endometrial carcinoma, ovarian carcinoma | Niraparib (single), niraparib + ostarlimab drugs | Randomized | Phase II | Phase 3 196 participants | NCT03651206 Recruiting | 15 July 2020 June 2025 |
| Head and neck cancer | Dostarlimab, niraparib | N/A | Phase II | 23 participants | NCT04313504 Recruiting | 4 November 2020 1 June 2027 |
| Ovarian cancer | Dostarlimab, niraparib, pegylated liposomal doxorubicin, paclitaxel, bevacizumab, gemcitabine, topotecan | Randomized | Phase III | 427 participants | NCT04679064 Recruiting | 1 December 2020 1 January 2025 |
| Breast cancer | Dostarlimab, niraparib | Randomized | Phase II | 62 participants | NCT04584255 Recruiting | 18 December 2020 17 July 2029 |
| Neuroendocrine carcinomas | Dostarlimab, niraparib | N/A | Phase II | 48 participants | NCT04701307 Recruiting | 1 February 2021 30 May 2025 |
| Sarcoma, clear cell | Dostarlimab | N/A | Phase II | 16 participants | NCT04274023 Recruiting | 19 February 2021 1 May 2024 |
| Endometrial cancer | Dostarlimab, intensity-modulated radiation therapy | N/A | Phase II | 31 participants | NCT04774419 Recruiting | 2 April 2021 February 2023 |
| Breast cancer | Niraparib, dostarlimab Radiation: radiation therapy | N/A | Phase II | 32 participants | NCT04837209 Recruiting | 21 July 2021 1 December 2029 |
| HRD cholangiocarcinoma metastatic cancer | Niraparib, dostarlimab | N/A | Phase II | 47 participants | NCT04895046 Recruiting | 11 October 2021 September 2023 |
| RCA-mutated pancreas, breast, ovarian cancer | Dostarlimab, niraparib | N/A | Phase I | 18 participants | NCT04673448 Recruiting | 18 October 2021 10 March 2026 |
| Ovarian cancer | Niraparib, dostarlimab | Non-randomized | Phase II | 100 participants | NCT05126342 Not yet recruiting | 1 May 2022 1 November 2026 |
| Colon cancer, DMMR colorectal cancer | Dostarlimab | N/A | Phase II | 29 participants | NCT05239546 Not yet recruiting | June 2023 June 2029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkholifi, F.K.; Alsaffar, R.M. Dostarlimab an Inhibitor of PD-1/PD-L1: A New Paradigm for the Treatment of Cancer. Medicina 2022, 58, 1572. https://doi.org/10.3390/medicina58111572
Alkholifi FK, Alsaffar RM. Dostarlimab an Inhibitor of PD-1/PD-L1: A New Paradigm for the Treatment of Cancer. Medicina. 2022; 58(11):1572. https://doi.org/10.3390/medicina58111572
Chicago/Turabian StyleAlkholifi, Faisal K., and Rana M. Alsaffar. 2022. "Dostarlimab an Inhibitor of PD-1/PD-L1: A New Paradigm for the Treatment of Cancer" Medicina 58, no. 11: 1572. https://doi.org/10.3390/medicina58111572
APA StyleAlkholifi, F. K., & Alsaffar, R. M. (2022). Dostarlimab an Inhibitor of PD-1/PD-L1: A New Paradigm for the Treatment of Cancer. Medicina, 58(11), 1572. https://doi.org/10.3390/medicina58111572





