Dinebra retroflexa Herbal Phytotherapy: A Simulation Study Based on Bleomycin-Induced Pulmonary Fibrosis Retraction Potential in Swiss Albino Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Plant Material
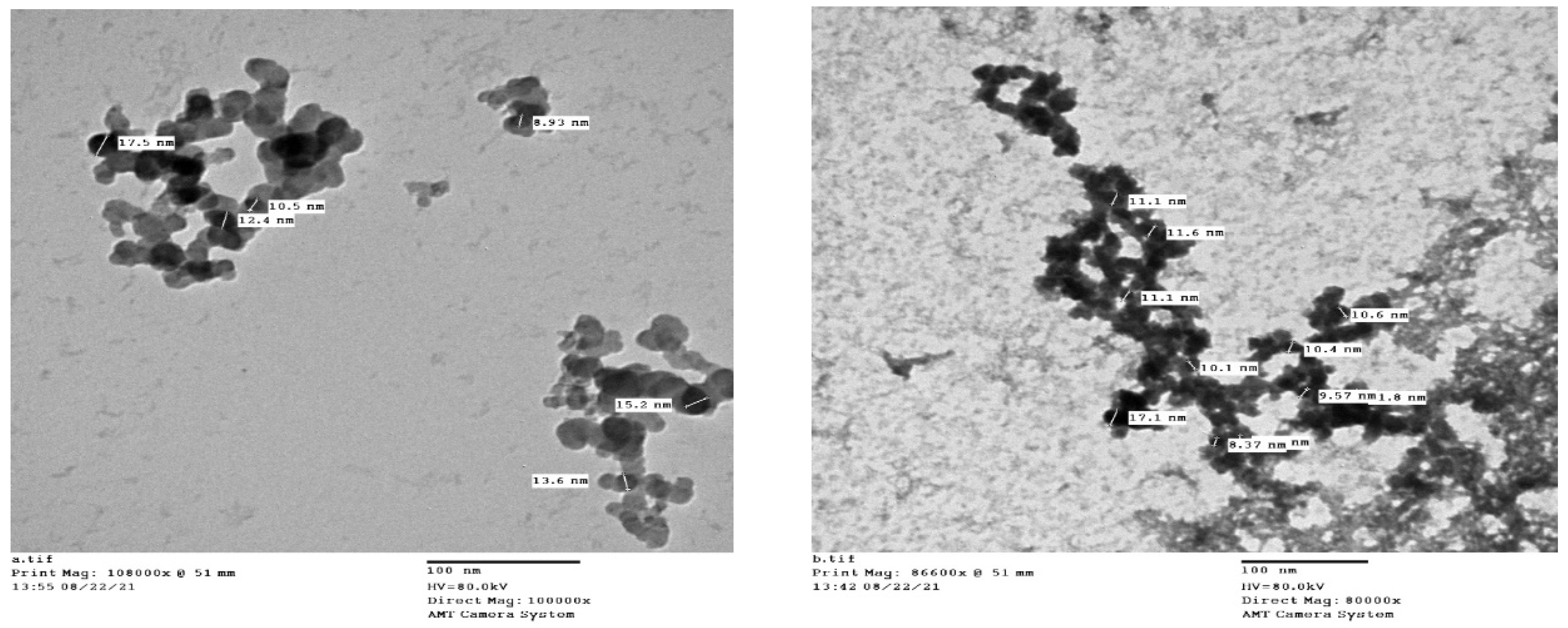
2.2. Synthesis of Ag-NPs
2.3. Lung Fibrosis Modality
2.3.1. Animals
2.3.2. Induction of Pulmonary Fibrosis
2.3.3. Experimental Design
- Control group: 10 Rats were injected with the drug vehicle (saline) in a dosing volume of (5 mL/kg, IT).
- Drug control group: 10 Rats were injected with a single dose of DRE (35 mg/100 mL/kg-DMSO, IT).
- BLM group: 10 Rats were injected with a single dose of BLM (5 mg/5 mL/kg-Saline, IT).
- DRE group: 10 Rats were injected with a single dose of DRE (35 mg/100 mL/kg-DMSO, IT) after one hour of BLM instillation.
- DRN group: 10 Rats were injected with a single dose of DRN (35 mg/100 mL/kg-DMSO, IT) after one hour of BLM instillation.
- Ag-NPs group: 10 Rats were injected with a single dose of Ag-NPs prepared by citrate reduction (35 mg/100 mL/kg-DMSO, IT) after one hour of BLM instillation.
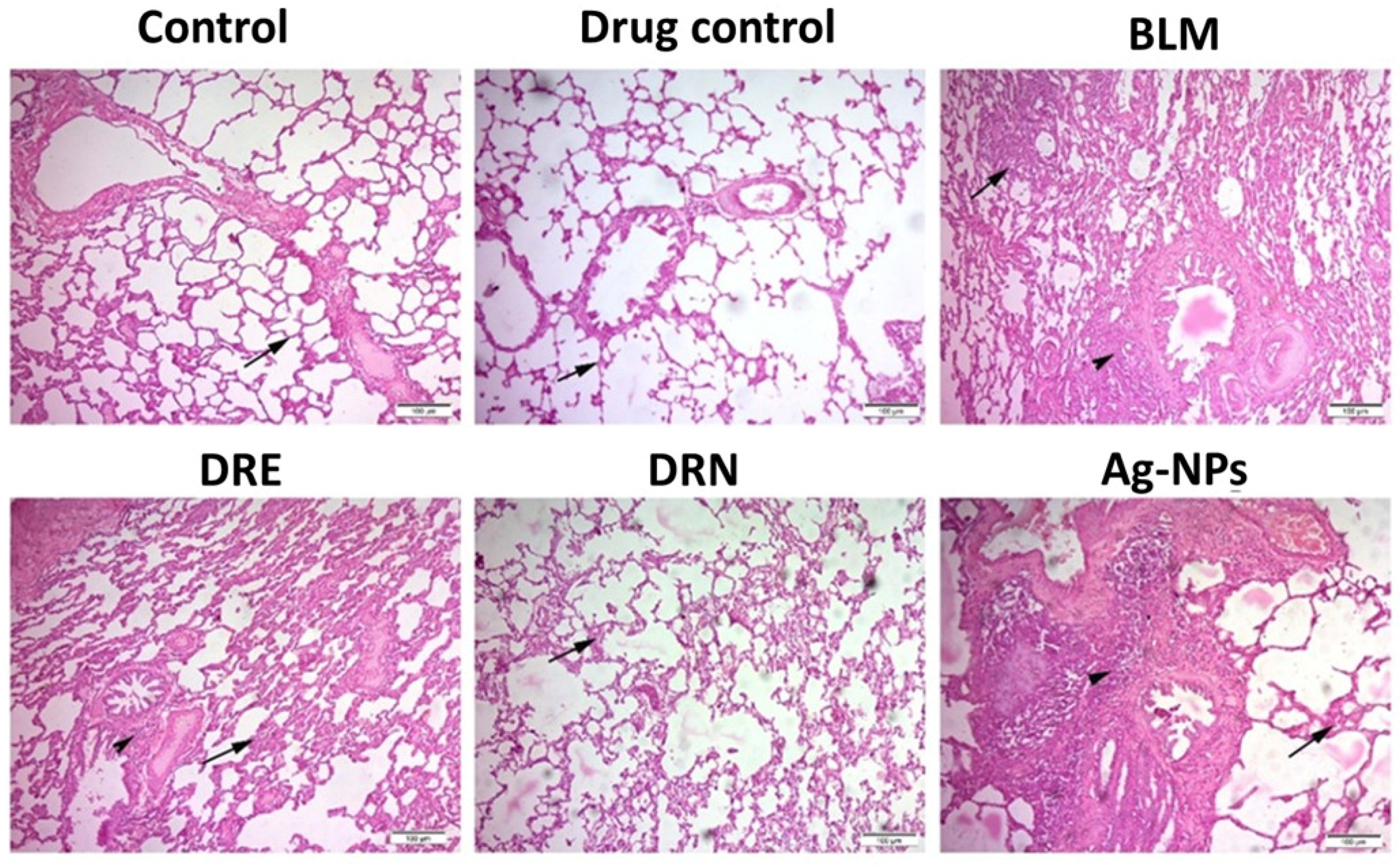
2.3.4. Lung Histopathology
2.3.5. Determination of the Intensity of Lung Fibrosis Using the Modified Ashcroft Score Technique
- Grade 0: Normal lung tissue.
- Grade 1: Minimal fibrous thickening of alveolar or bronchiolar walls.
- Grade 2: Mild fibrous thickening of alveolar or bronchiolar walls.
- Grade 3: Moderate thickening of walls without obvious damage to lung architecture.
- Grade 4: Increased fibrosis with focal damage to lung structure and formation of fibrous bands or single fibrous nodule.
- Grade 5: Increased fibrosis with definite damage to lung structure and formation of fibrous bands or small fibrous nodules.
- Grade 6: Wide fibrosis with wide damage to lung structure and formation of fibrous bands or fibrous masses.
- Grade 7: Severe distortion of structure and large fibrous areas.
- Grade 8: Total fibrous obliteration of the fields.
2.3.6. Statistical Analysis
2.4. UHPLC/Q-TOF-MS-MS Metabolic Profiling
2.5. Quantitative Determination of Polyphenolics
3. Results
3.1. Synthesis of Ag-NPs
3.2. Effect of DRE and DRN on Lung Histopathology and Aschroft Fibrosis Score in BLM Male Swiss Albino Rats
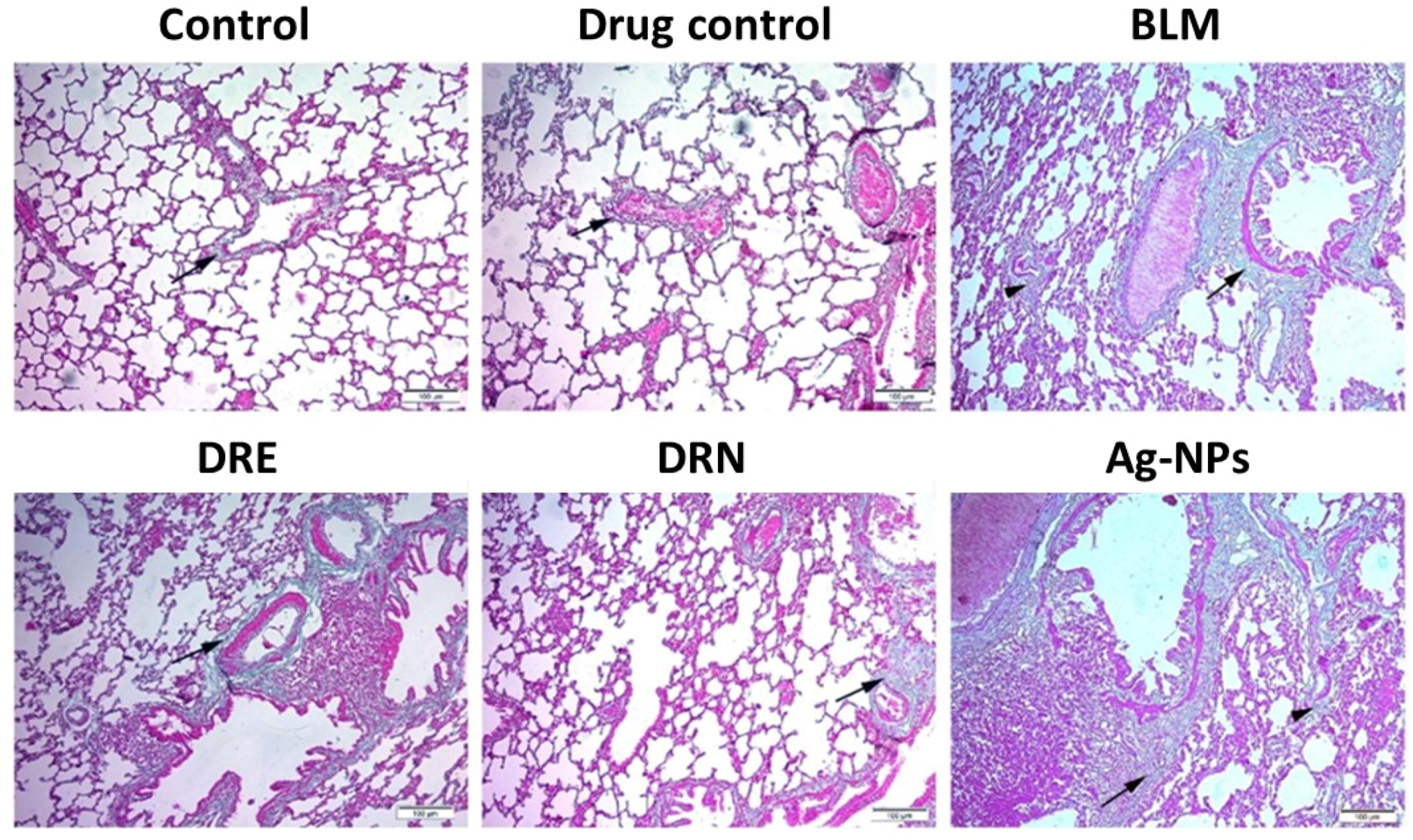
3.3. Effect of DRE and DRN on Collagen Disposition in the Lungs of BLM Male Swiss Albino Rats
3.4. UHPLC/Q-TOF-MS-MS Metabolic Profiling
3.5. Quantitative Determination of Polyphenolics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noble, P.W.; Barkauskas, C.E.; Jiang, D. Pulmonary fibrosis: Patterns and perpetrators. J. Clin. Investig. 2012, 122, 2756–2762. [Google Scholar] [CrossRef]
- Sharaf, Y.A.; El Deeb, S.; Ibrahim, A.E.; Al-Harrasi, A.; Sayed, R.A. Two Green Micellar HPLC and Mathematically Assisted UV Spectroscopic Methods for the Simultaneous Determination of Molnupiravir and Favipiravir as a Novel Combined COVID-19 Antiviral Regimen. Molecules 2022, 27, 2330. [Google Scholar] [CrossRef]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- White, E.S.; Thomas, M.; Stowasser, S.; Tetzlaff, K. Challenges for Clinical Drug Development in Pulmonary Fibrosis. Front. Pharmacol. 2022, 13, 823085. [Google Scholar] [CrossRef] [PubMed]
- Dudala, S.S.; Venkateswarulu, T.; Kancharla, S.C.; Kodali, V.P.; Babu, D.J. A review on importance of bioactive compounds of medicinal plants in treating idiopathic pulmonary fibrosis (special emphasis on isoquinoline alkaloids). Futur. J. Pharm. Sci. 2021, 7, 156. [Google Scholar] [CrossRef]
- Hosseini, S.; Imenshahidi, M.; Hosseinzadeh, H.; Karimi, G. Effects of plant extracts and bioactive compounds on attenuation of bleomycin-induced pulmonary fibrosis. Biomed. Pharmacother. 2018, 107, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, H.S.; Muhammad, F.S.; Muhammad, S.; Niaz, A.Q.; Safia, M. The importance of cereals (Poaceae: Gramineae) nutrition in human health: A review. J. Cereals Oilseeds 2013, 4, 32–35. [Google Scholar] [CrossRef]
- Marwat, S.K.; Rehman, F.; Usman, K.; Rashid, A.; Ghulam, S. Biodiversity of grassy weeds and their ethnobotanical importance in Dera Ismail Khan District (DI Khan), KPK, Pakistan. Pak. J. Bot 2012, 44, 733–738. [Google Scholar]
- Burkill, H. The Useful Plants of West Tropical Africa. Volume 2: Families E–I, 2nd ed.; Royal Botanic Gardens: London, UK, 1994. [Google Scholar]
- Ahirrao, Y.A.; Patil, D.A. 5. Ethnobotanical Probe in Fodder Resources of Buldhana District (Maharashtra, India) by Ya Ahirrao1 and Da Patil. Life Sci. Leafl. 2012, 25, 23–35. [Google Scholar]
- Bandeira, S.; Massingue Manjate, A.; Filipe, O. An Ecological Assessment of the Health of the Chibuto Wetland in the Dry Season Mozambique: Emphasis on Resources Assessment Utilization and Sustainability Analysis. 2006. Available online: https://cgspace.cgiar.org/bitstream/handle/10568/21604/21604.pdf (accessed on 12 October 2022).
- Abousabee, G.M. Ecology of Echinochloa colona (L.) Link (Poaceae), a Weed Flora in Summer Crops (Oryza) of El Sharkyia Province, Egypt; Lashin, G.M.A., Ziada, M.A., Tohamy, E.Y., Nourhan, O.A., Eds.; The Scientific Research and Studies Center, Faculty of Science, Zagazig University: Zagazig, Egypt, 2015; pp. 47–61. [Google Scholar]
- Patidar, J.; Kewat, M.; Jha, A. Present status of weed flora in soybean crop in Jabalpur district of Kymore plateau & Satpura Hills Zone of Madhya Pradesh. Pharma Innov. J. 2019, 8, 717–720. [Google Scholar]
- Iraz, M.; Erdoğan, H.; Kotuk, M.; Yagmurca, M.; Kılıc, T.; Ermis, H.; Fadillioglu, E.; Yildirim, Z. Ginkgo biloba inhibits bleomycin-induced lung fibrosis in rats. Pharmacol. Res. 2006, 53, 310–316. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, F.; Kang, L.; Wang, Z.; Wang, Y. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm. Med. 2017, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Youssif, K.A.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.; Afifi, W.M.; Salem, M.A.; Albohy, A.; Abdelmohsen, U.R.; Haggag, E.G. Cytotoxic Potential of Green Synthesized Silver Nanoparticles of Lampranthus coccineus Extracts, Metabolic Profiling and Molecular Docking Study. ChemistrySelect 2020, 5, 12278–12286. [Google Scholar] [CrossRef]
- Gazdhar, A.; Susuri, N.; Hostettler, K.; Gugger, M.; Knudsen, L.; Roth, M.; Ochs, M.; Geiser, T. HGF Expressing Stem Cells in Usual Interstitial Pneumonia Originate from the Bone Marrow and Are Antifibrotic. PLoS ONE 2013, 8, e65453. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, T.; Wang, X.; Sun, B.-B.; Li, J.-Q.; Liu, D.-S.; Zhang, S.-F.; Liu, L.; Xu, D.; Chen, Y.-J.; et al. Blockade of advanced glycation end product formation attenuates bleomycin-induced pulmonary fibrosis in rats. Respir. Res. 2009, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Hartz, P.H. Simultaneous Histologic Fixation and Gross Demonstration of Calcification. Am. J. Clin. Pathol. 1947, 17, 750. [Google Scholar] [CrossRef]
- Psaty, B.M.; Prentice, R.L. Minimizing bias in randomized trials: The importance of blinding. JAMA 2010, 304, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Kushwah, L.; Gohel, D.; Patel, M.; Marvania, T.; Balakrishnan, S. Evaluating the Ameliorative Potential of Quercetin against the Bleomycin-Induced Pulmonary Fibrosis in Wistar Rats. Pulm. Med. 2013, 2013, 921724. [Google Scholar] [CrossRef] [PubMed]
- Hübner, R.-H.; Gitter, W.; El Mokhtari, N.E.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wolf, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. BioTechniques 2008, 44, 514–517. [Google Scholar] [CrossRef]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef]
- Abbass, H.S.; El-Hela, A.A.; Hegazy, M.M.; Abu Bakr, M.S. Profiling of antiviral and antioxidant phytochemicals of Pterocephalus frutescens hochist. using high-resolution ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometer. Pharmacogn. Mag. 2020, 16, 592. [Google Scholar] [CrossRef]
- Mohamed, S. Phytochemical and Biological Study of (Senecio glaucus subsp. coronopifolius) (Maire) C. Alexander Growing in Egypt. Al-Azhar J. Pharm. Sci. 2015, 52, 283–298. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Awwad, A.M.; Salem, N.M. Green Synthesis of Silver Nanoparticles byMulberry LeavesExtract. Nanosci. Nanotechnol. 2012, 2, 125–128. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Foglia, P.; Pastorini, E.; Samperi, R.; Laganà, A. Identification and mass spectrometric characterization of glycosylated flavonoids inTriticum durum plants by high-performance liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 3143–3158. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of Flavonoid Subgroups and Hydroxy Substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.C.; Liu, L.T.; Bian, J.J.; Yan, C.Q.; Ye, L.; Zhao, M.X.; Huang, Q.S.; Wang, W.; Liang, K.; Shi, Z.F.; et al. Identification of multiple constituents in shuganjieyu capsule and rat plasma after oral administration by ultra-performance liquid chromatography coupled with electrospray ionization and ion trap mass spectrometry. Acta Chromatogr. 2018, 30, 95–102. [Google Scholar] [CrossRef]
- Farag, M.A.; El Fishawy, A.M.; El-Toumy, S.A.; Amer, K.F.; Mansour, A.M.; Taha, H.E. Antihepatotoxic effect and metabolite profiling of Panicum turgidum extract via UPLC-qTOF-MS. Pharmacogn. Mag. 2016, 12 (Suppl. 4), S446. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Gadimli, A.I.; Isaev, J.I.; Kashchenko, N.I.; Prokopyev, A.S.; Kataeva, T.N.; Chirikova, N.K.; Vennos, C. Caucasian Gentiana Species: Untargeted LC-MS Metabolic Profiling, Antioxidant and Digestive Enzyme Inhibiting Activity of Six Plants. Metabolites 2019, 9, 271. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Z.; Liao, X.; Zhou, C.; Xie, Z.; Zhu, S.; Wei, G.; Huang, Y. Identification of C-glycosyl flavones by high performance liquid chromatography electrospray ionization mass spectrometry and quantification of five main C-glycosyl flavones in Flickingeria fimbriata. BMC Chem. 2019, 13, 94. [Google Scholar] [CrossRef]
- Jang, G.H.; Kim, H.W.; Lee, M.K.; Jeong, S.Y.; Bak, A.R.; Lee, D.J.; Kim, J.B. Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J. Biol. Sci. 2018, 25, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; Chaves, D.S.D.A.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L.; et al. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Rev. Bras. Farm. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Wang, X.; Fan, R.; Li, J.; Li, C.; Zhang, Y. Molecular cloning and functional characterization of a novel (iso) flavone 4′,7-O-diglucoside glucosyltransferase from Pueraria lobata. Front. Plant Sci. 2016, 7, 387. [Google Scholar] [CrossRef]
- Wang, S.-F.; Leng, J.; Xu, Y.-M.; Feng, M.-L. Identification and determination of major constituents in a traditional Chinese medicine compound recipe Xiongdankaiming tablet using HPLC-PDA/ESI-MS n and HPLC-UV/ELSD. J. Zhejiang Univ. Sci. B 2013, 14, 604–614. [Google Scholar] [CrossRef]
- Bonaccorsi, P.; Caristi, C.; Gargiulli, C.; Leuzzi, U. Flavonol Glucoside Profile of Southern Italian Red Onion (Allium cepa L.). J. Agric. Food Chem. 2005, 53, 2733–2740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, J.; Bi, W.; Ferruzzi, M.; Yemul, S.; Freire, D.; Mazzola, P.; Ho, L.; Dubner, L.; Pasinetti, G.M. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 2015, 89, 191–197. [Google Scholar] [CrossRef]
- Ju, W.-T.; Kwon, O.-C.; Kim, H.-B.; Sung, G.-B.; Kim, H.-W.; Kim, Y.-S. Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J. Food Sci. Technol. 2018, 55, 1789–1796. [Google Scholar] [CrossRef]
- Flamini, R.; Traldi, P. Mass Spectrometry in Grape and Wine Chemistry; John Wiley & Sons: New York, NY, USA, 2009; Volume 42. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Mukhopadhyay, S.; Robbins, R.J.; Harnly, J.M. Identification and quantification of flavonoids of Mexican oregano (Lippia graveolens) by LC-DAD-ESI/MS analysis. J. Food Compos. Anal. 2007, 20, 361–369. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS Profiles of Phenolic Compounds and Antioxidant Activity of Fruits from Three Citrus Species Consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T.; Smirnov, S.V.; Damdinsuren, O.; Kondo, K. Flavonoids from Reaumuria soongarica (Tamaricaceae) in Mongolia. Bull. Natl. Mus. Nat. Sci. Ser. B 2012, 38, 189–195. [Google Scholar]
- Madeira, P.J.A.; Borges, C.M.; Florêncio, M.H. Electrospray ionization Fourier transform ion cyclotron resonance mass spectrometric and semi-empirical calculations study of five isoflavone aglycones. Rapid Commun. Mass Spectrom. 2010, 24, 3432–3440. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Almeida, J.M.; Negri, G.; Salatino, A.; de Carvalho, J.E.; Lajolo, F.M. Antiproliferative and antioxidant activities of a tricin acylated glycoside from sugarcane (Saccharum officinarum) juice. Phytochemistry 2007, 68, 1165–1171. [Google Scholar] [CrossRef]
- Han, L.; Piao, X.C.; Jiang, J.; Jiang, X.L.; Yin, C.R.; Lian, M.L. A high production of flavonoids and anthraquinones via adventitious root culture of Oplopanax elatus and evaluating antioxidant activity. Plant Cell Tissue Organ. Cult. (PCTOC) 2019, 137, 173–179. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, M.; Cheng, X.; Liang, C.; Diao, X.; Zhang, L. Ultrahigh-performance liquid chromatography coupled with triple quadrupole and time-of-flight mass spectrometry for the screening and identification of the main flavonoids and their metabolites in rats after oral administration of Cirsium japonicum DC. extract. Rapid Commun. Mass Spectrom. 2018, 32, 1451–1461. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.Y.; Luo, X.M.; Wang, X.B.; Wu, T. Analysis of the chemical components of Qixianqingming granules and their metabolites in rats by UPLC-ESI-Q-TOF-MS. J. Mass Spectrom. 2020, 55, e4484. [Google Scholar] [CrossRef]
- Niessen, W.M. Liquid Chromatography-Mass Spectrometry; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Srithi, K.; Balslev, H.; Tanming, W.; Trisonthi, C. Weed Diversity and Uses: A Case Study from Tea Plantations in Northern Thailand. Econ. Bot. 2017, 71, 147–159. [Google Scholar] [CrossRef]
- Umezawa, H.; Maeda, K.; Takeuchi, T.; Okami, Y. New antibiotics, bleomycin A and B. J. Antibiot. 1966, 19, 200–209. [Google Scholar]
- Reinert, K.R.S.; Po’E, E.K.; Barkin, S.L. The Relationship between Executive Function and Obesity in Children and Adolescents: A Systematic Literature Review. J. Obes. 2013, 2013, 820956. [Google Scholar] [CrossRef]
- Ojo, A.S.; Balogun, S.A.; Williams, O.T.; Ojo, O.S. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm. Med. 2020, 2020, 6175964. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.B. Possible mechanisms of bleomycin-induced fibrosis. Clin. Chest Med. 1990, 11, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Ali, R.B.; Abidi, A.; Jameleddine, S. The efficacy of plant extract and bioactive compounds approaches in the treatment of pulmonary fibrosis: A systematic review. Biomed. Pharmacother. 2017, 93, 666–673. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Zahedipour, F.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Pulmonary fibrosis: Therapeutic and mechanistic insights into the role of phytochemicals. BioFactors 2021, 47, 250–269. [Google Scholar] [CrossRef]
- Bahri, S.; Ben Ali, R.; Gasmi, K.; Mlika, M.; Fazaa, S.; Ksouri, R.; Serairi, R.; Jameleddine, S.; Shlyonsky, V. Prophylactic and curative effect of rosemary leaves extract in a bleomycin model of pulmonary fibrosis. Pharm. Biol. 2017, 55, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Veith, C.; Albrecht, C.; Bartholome, R.; Drittij, M.-J.; Claessen, S.M.; Bast, A.; Rosenbruch, M.; Jonkers, L.; van Schooten, F.-J.; et al. The dietary antioxidant quercetin reduces hallmarks of bleomycin-induced lung fibrogenesis in mice. BMC Pulm. Med. 2020, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Park, S.-H.; Choi, Y.-J.; Kim, Y.-H.; Antika, L.D.; Habibah, N.U.; Kang, M.-K.; Kang, Y.-H. Dietary Compound Kaempferol Inhibits Airway Thickening Induced by Allergic Reaction in a Bovine Serum Albumin-Induced Model of Asthma. Int. J. Mol. Sci. 2015, 16, 29980–29995. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, W. Apigenin protects against bleomycin-induced lung fibrosis in rats. Exp. Ther. Med. 2015, 11, 230–234. [Google Scholar] [CrossRef]
- Bai, L.; Li, A.; Gong, C.; Ning, X.; Wang, Z. Protective effect of rutin against bleomycin induced lung fibrosis: Involvement of TGF-β1/α-SMA/Col I and III pathway. Biofactors 2020, 46, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Peng, W.-H.; Wu, L.-C.; Wu, C.-C.; Hsu, S.-L. Luteolin Ameliorates Experimental Lung Fibrosis Both in Vivo and in Vitro: Implications for Therapy of Lung Fibrosis. J. Agric. Food Chem. 2010, 58, 11653–11661. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-X.; Zeng, S.; Wan, B.-B.; Wang, Y.-Y.; Sun, H.-X.; Liu, G.; Gao, Z.-Q.; Chen, D.; Chen, Y.-Q.; Lu, M.-D.; et al. Sophoricoside attenuates lipopolysaccharide-induced acute lung injury by activating the AMPK/Nrf2 signaling axis. Int. Immunopharmacol. 2020, 90, 107187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Dong, J.; Nie, J.; Zhu, J.-X.; Wang, H.; Chen, Q.; Chen, J.-Y.; Xia, J.-M.; Shuai, W. Amelioration of bleomycin-induced pulmonary fibrosis by chlorogenic acid through endoplasmic reticulum stress inhibition. Apoptosis 2017, 22, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Ge, A.; Liu, Y.; Zeng, X.; Kong, H.; Ma, Y.; Zhang, J.; Bai, F.; Huang, M. Effect of diosmetin on airway remodeling in a murine model of chronic asthma. Acta Biochim. Biophys. Sin. 2015, 47, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Liu, Y.; Shi, L.; Li, B.; Liu, L.; Bai, B.; Meng, X.; Hou, M.; Liu, X.; Sheng, L.; et al. Blueberry anthocyanins-enriched extracts attenuate the cyclophosphamide-induced lung toxicity. Chem. Interact. 2014, 222, 106–111. [Google Scholar] [CrossRef]
- Lee, S.-S.; Baek, Y.-S.; Eun, C.-S.; Yu, M.-H.; Baek, N.-I.; Chung, D.-K.; Bang, M.-H.; Yang, S.-A. Tricin derivatives as anti-inflammatory and anti-allergic constituents from the aerial part of Zizania latifolia. Biosci. Biotechnol. Biochem. 2015, 79, 700–706. [Google Scholar] [CrossRef]
- Cai, C.; Xiang, Y.; Wu, Y.; Zhu, N.; Zhao, H.; Xu, J.; Lin, W.; Zeng, C. Formononetin attenuates monocrotaline-induced pulmonary arterial hypertension via inhibiting pulmonary vascular remodeling in rats. Mol. Med. Rep. 2019, 20, 4984–4992. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Zhang, X.; Ji, X.; Xie, M.; Liu, H. Calycosin attenuates pulmonary fibrosis by the epithelial-mesenchymal transition repression upon inhibiting the AKT/GSK3β/β-catenin signaling pathway. Acta Histochem. 2021, 123, 151746. [Google Scholar] [CrossRef]
- Andugulapati, S.B.; Gourishetti, K.; Tirunavalli, S.K.; Shaikh, T.B.; Sistla, R. Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-β mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems. Phytomedicine 2020, 78, 153298. [Google Scholar] [CrossRef]
| Groups | Parameter | |
|---|---|---|
| H&E (Ashcroft Score) | Masson Trichrome (OD) | |
| Control group | 0 ± 0.18 | 82.29 ± 2.75 |
| Drug control group | 0 ± 0.00 | 93.81± 3.32 |
| BLM group | 6 ± 0.22 ab | 161.66 ± 2.39 ab |
| DRE group | 3 ± 0.18 abc | 106.95 ± 5.63 abc |
| DRN group | 1 ± 0.22 cd | 87.15 ± 3.44 cd |
| Ag-NPs group | 6 ± 0.22 abde | 158.26 ± 3.09 abde |
| Rt (min) | Precursor/Adduct Ion | Error (ppm) | Characteristic Fragmentation | Proposed Compound | Identification References | |
|---|---|---|---|---|---|---|
| 1 | 1.23 | 355.1029 [M+H]+ | 0 | 355[M+H]+ 193[M+H-caffeoyl moiety]+ 163[M+H-Quinic acid]+ | Chlorogenic acid | [32] |
| 2 | 1.65 | 611.1608 [M+H]+ | −0.7 | 611[M+H]+ 431[M+H-glucose-H2O]+ 413[M+H-glucose-2H2O]+ 395[M+H-glucose-3H2O]+ | Luteolin 8 C-hexosyl-O-hexoside | [33] |
| 3 | 1.94 | 595.1660 [M+H]+ | −0.5 | 595[M+H]+ 577[M+H-H2O]+ 433[M+H-glucose]+ 415[M+H-glucose- H2O]+ 379[M+H-glucose-3H2O]+ 271[M+H-2glucose]+ | Isovitexin-7-O-glucoside (Saponarin) | [34] |
| 4 | 2.85 | 565.1555 [M+H]+ | −3.5 | 565[M+H]+ 547[M+H-H2O]+ 529[M+H-2H2O]+ 511[M+H-3H2O]+ 469[M+H-60-2H2O]+ 457[M+H-90-H2O]+ 445[M+H-120]+ 427[M+H-120-H2O]+ 409[M+H-120-2H2O]+ | Apigenin 6-C-glucoside 8-C-xyloside | [35] |
| 5 | 3.27 | 565.1562 [M+H]+ | 0.9 | 565[M+H]+ 313[M+H-pentose-120]+ 283 [M+H-pentose-150]+ | Apigenin O-pentosyl-C-hexoside | [30] |
| 6 | 3.49 | 611.1603 [M+H]+ | −1.5 | 611[M+H]+ 303 [M+H-rutinoe]+ 465 [M+H-rhamnose]+ 285[M+H-rhamnose-H2O]+ 153 1,3A+ | Rutin | [31,36] |
| 7 | 3.51 | 433.1131 [M+H]+ | −0.9 | 433[M+H]+ 415[M+H-H2O]+ 397[M+H-2H2O]+ 313[M+H-120]+ 283[M+H-150]+ | Apigenin 6-C glucoside | [37] |
| 8 | 3.53 | 433.1134 [M+H]+ | −0.2 | 433[M+H]+ 271[M+H-glucose]+ 197 [M+H-glucose-2CO-H2O]+ 159 [M+H-glucose-2CH2CO-CO]+ | Genistein-4’-O-glucoside(Sophoricoside) | [38] |
| 9 | 3.54 | 433.1135 [M+H]+ | 0 | 271[M+H-glucose]+ 433[M+H]+ 153 1,3A+ | Apigenin-7-O-glucoside (Cosmosiin) | [39] |
| 10 | 3.63 | 465.1031 [M+H]+ | −0.4 | 465[M+H]+ 303[M+H-glucose]+ 229[M+H-glucose-H2O-2CO]+ 153 1,3A+ 137 0,2B+ | Quercetin 4’-O-β-D-glucopyranoside (Spiraeoside) | [31,40] |
| 11 | 3.71 | 479.0828 [M+H]+ | 0.4 | 479[M+H]+ 303[M+H-glucuronic acid]+ 229 [M+H-glucuronic acid-H2O-2CO]+ 153 1,3A+ 137 0,2B+ | Quercetin-3-O-Glucuronide | [31,41] |
| 12 | 3.84 | 449.1080 [M+H]+ | −0.9 | 449[M+H]+ 287[M+H-glucose]+ | Kaempferol 3-O-glucoside (Astragalin) | [42] |
| 13 | 3.88 | 479.1193 [M+H]+ | 0.6 | 302[M+H-glucose-CH3]+ 317[M+H-glucose]+ 479[M+H]+ | Isorhamnetin-3-O-glucoside | [43] |
| 14 | 3.92 | 433.1154 [M+H]+ | 4.4 | 433[M+H]+ 287[M+H-rhamnose]+ | Kaempferol-3-O-α-L-rhamnoside (afzelin) | [36] |
| 15 | 4.02 | 449.1083 [M+H]+ | −0.2 | 449[M+H]+ 287[M+H-glucose]+ | Luteolin-7-O-glucoside | [44] |
| 16 | 4.23 | 625.1757 [M+H]+ | −1.9 | 317[M+H-Rutinose]+ 479[M+H-Rhamnose]+ 625[M+H]+ | Isorhamnetin-3-O-rutinoside (Narcissin) | [44] |
| 17 | 4.26 | 287.0554 [M+H]+ | −0.7 | 287[M+H]+ 241[M+H-H2O-CO]+ 213[M+H-H2O-2CO]+ 153 1,3A+ 121 0,2B+ | Kaempferol | [31] |
| 18 | 4.27 | 303.0517 [M+H]+ | 4 | 137 0,2B+ 153 1,3 A+ 229[M+H-H2O-2CO]+ 257[M+H-H2O-CO]+ 303[M+H]+ | Quercetin | [31] |
| 19 | 4.31 | 449.1081 [M+H]+ | −0.7 | 449[M+H]+ 303[M+H-rhamnose]+ 285[M+H-rhamnose-H2O]+ 257[M+H-rhamnose-H2O-CO]+ 165 0,2A+ 153 1,3A+ | Quercetin-3-O-rhamnoside (Quercitrin) | [31,36] |
| 20 | 4.47 | 609.1804 [M+H]+ | −2.5 | 286[M+H-Neohesperidose-CH3]+ 301[M+H-Rhamnose-glucose]+ 463[M+H-Rhamnose]+ 609[M+H]+ | Diosmetin 7-O-neohesperidoside (Neodiosmin) | [45] |
| 21 | 4.71 | 449.1077 [M+H]+ | −1.6 | 449[M+H]+ 303[M+H-rhamnose]+ 257[M+H-rhamnose-H2O-CO]+ 153 1,3A+ | Quercetin-7-O-rhamnoside (Vincetoxicoside B) | [31,46] |
| 22 | 5.01 | 493.1335 [M]+ | −2.2 | 331[M-glucose]+ 493[M]+ | Malvidin-3-O-glucoside | [47] |
| 23 | 6.87 | 331.0809 [M+H]+ | −2.7 | 273[M+H-2CH3-CO]+ 285[M+H-H2O-CO]+ 301[M+H-2CH3]+ 313[M+H-H2O]+ 316[M+H-CH3]+ 331 [M+H]+ | Tricin | [31,48] |
| 24 | 6.93 | 301.0717 [M+H]+ | 1.6 | 286[M+H-CH3]+ 301[M+H]+ | Kaempferide(4’-O-methyl kaempferol) | [49] |
| 25 | 7.89 | 269.0813 [M+H]+ | −0.4 | 137 1,3A+ 213[M+H-2CO]+ 237[M+H-CH3OH]+ 253[M+H-CH3-H]+ 254[M+H-CH3]+ 269[M+H]+ | Formononetin Isoflavonoid | [47] |
| 26 | 9.38 | 285.0761 [M+H]+ | −0.7 | 285[M+H]+ 270[M+H-CH3]+ 242 [M+H-CH3-CO]+ 153 1,3A+ | Acacetin | [50] |
| 27 | 9.39 | 285.0761 [M+H]+ | −0.7 | 285[M+H]+ 270[M+H-CH3]+ 242 [M+H-CH3-CO]+ | Calycosin | [51] |
| 28 | 9.57 | 285.0757 [M+H]+ | −2.1 | 133 1,3 B+ 153 1,3A+ 270[M+H-CH3]+ 285[M+H]+ | Biochanin-A | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hela, A.A.; Hegazy, M.M.; Abbass, H.S.; Ahmed, A.H.; Bakr, M.S.A.; Elkousy, R.H.; Ibrahim, A.E.; El Deeb, S.; Sayed, O.M.; Gad, E.S. Dinebra retroflexa Herbal Phytotherapy: A Simulation Study Based on Bleomycin-Induced Pulmonary Fibrosis Retraction Potential in Swiss Albino Rats. Medicina 2022, 58, 1719. https://doi.org/10.3390/medicina58121719
El-Hela AA, Hegazy MM, Abbass HS, Ahmed AH, Bakr MSA, Elkousy RH, Ibrahim AE, El Deeb S, Sayed OM, Gad ES. Dinebra retroflexa Herbal Phytotherapy: A Simulation Study Based on Bleomycin-Induced Pulmonary Fibrosis Retraction Potential in Swiss Albino Rats. Medicina. 2022; 58(12):1719. https://doi.org/10.3390/medicina58121719
Chicago/Turabian StyleEl-Hela, Atef A., Mostafa M. Hegazy, Hatem S. Abbass, Amal H. Ahmed, Marwa S. Abu Bakr, Rawah H. Elkousy, Adel Ehab Ibrahim, Sami El Deeb, Ossama M. Sayed, and Enas S. Gad. 2022. "Dinebra retroflexa Herbal Phytotherapy: A Simulation Study Based on Bleomycin-Induced Pulmonary Fibrosis Retraction Potential in Swiss Albino Rats" Medicina 58, no. 12: 1719. https://doi.org/10.3390/medicina58121719
APA StyleEl-Hela, A. A., Hegazy, M. M., Abbass, H. S., Ahmed, A. H., Bakr, M. S. A., Elkousy, R. H., Ibrahim, A. E., El Deeb, S., Sayed, O. M., & Gad, E. S. (2022). Dinebra retroflexa Herbal Phytotherapy: A Simulation Study Based on Bleomycin-Induced Pulmonary Fibrosis Retraction Potential in Swiss Albino Rats. Medicina, 58(12), 1719. https://doi.org/10.3390/medicina58121719







