30 Days Mortality Prognostic Value of POCT Bio-Adrenomedullin and Proenkephalin in Patients with Sepsis in the Emergency Department
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
| Clinical Criteria | Risk Factor |
| fever or hypothermia (considered as body temperature > 38 °C or <35 °C) worsening of mental status presence of dyspnea or persistent cough nausea, diarrhea, vomiting or abdominal pain urinary tract symptoms, frequent diuresis, dysuria or strangury leukocytosis or leukopenia (WBC > 12,000 or <4000) | intravesical permanent catheter, endovascular or other implants frail patient nursing home resident recent hospitalization diabetes mellitusrecent surgical treatment or invasive maneuvers immunosuppression |
| Inclusion Criteria: 2 Clinical Criteria or 1 Clinical Criteria + 1 Risk Factor | |
2.2. Study Design
2.3. Biomarkers Analysis
2.4. Statistical Analysis
3. Results
3.1. Participants and Demographics
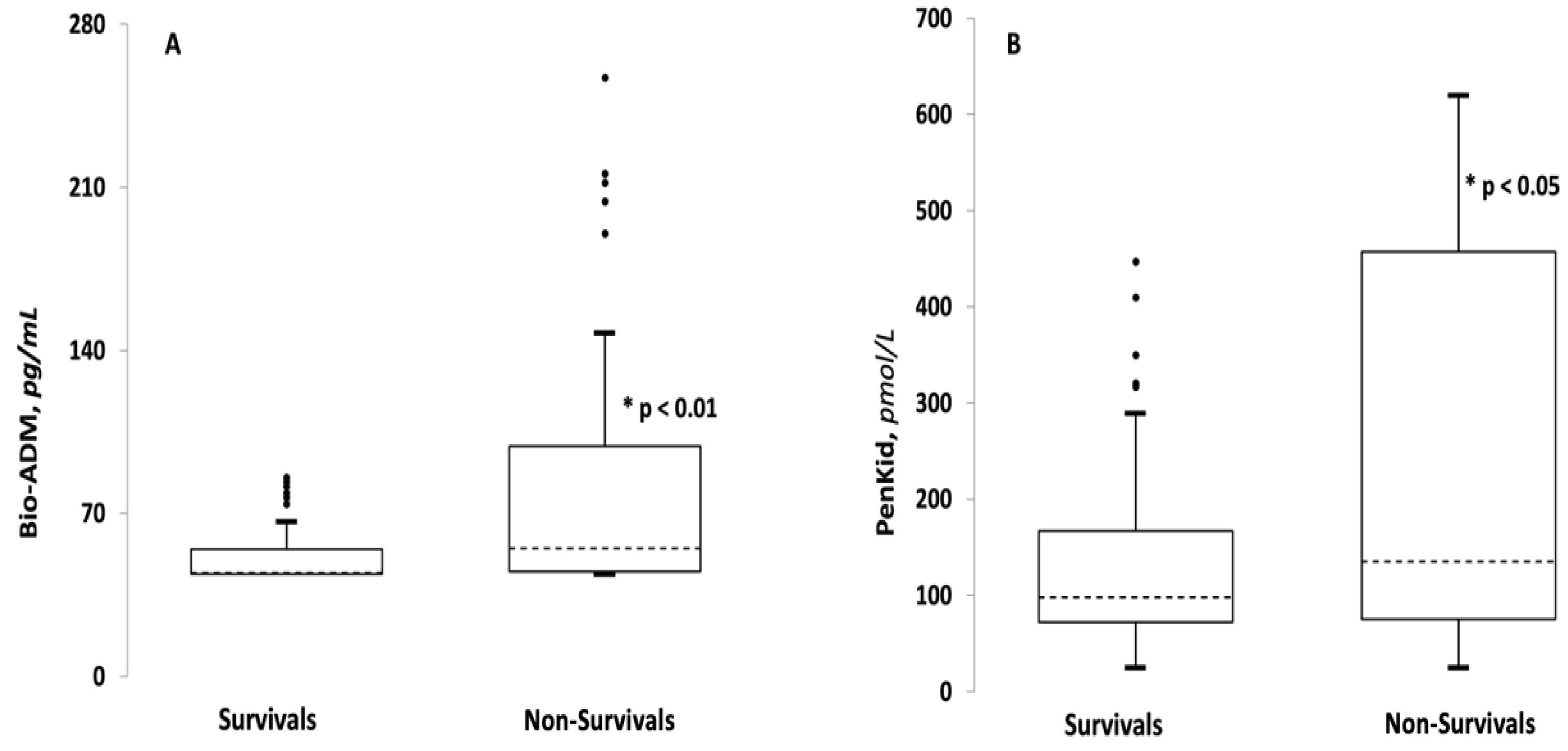
3.2. Comparison between Survivor and Non-Survivor Groups
3.3. Correlations between Demographic, Clinical and Laboratory Findings and POC Biomarkers
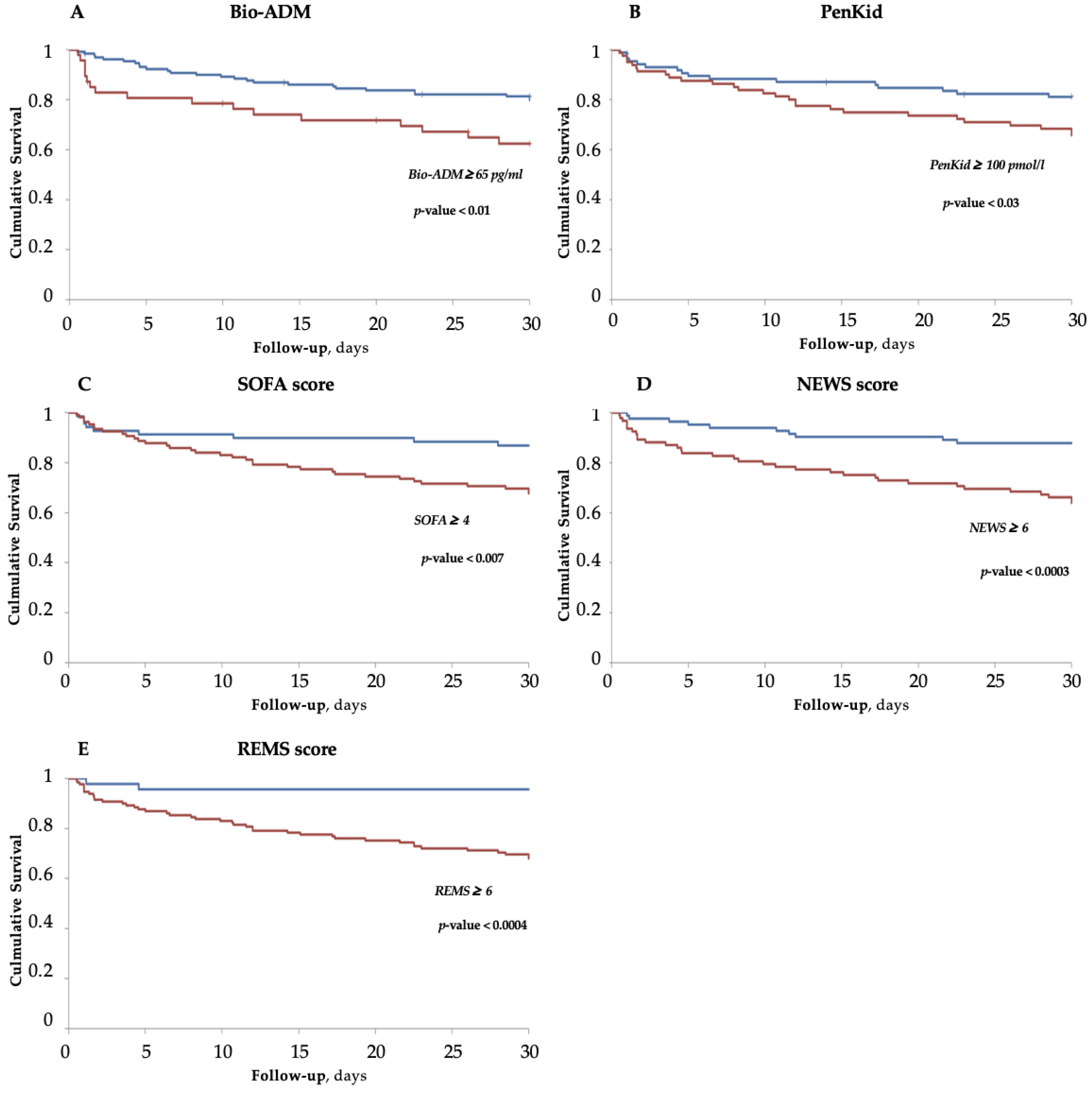
3.4. Survival Analysis for Bio-ADM, PenKid and Scores
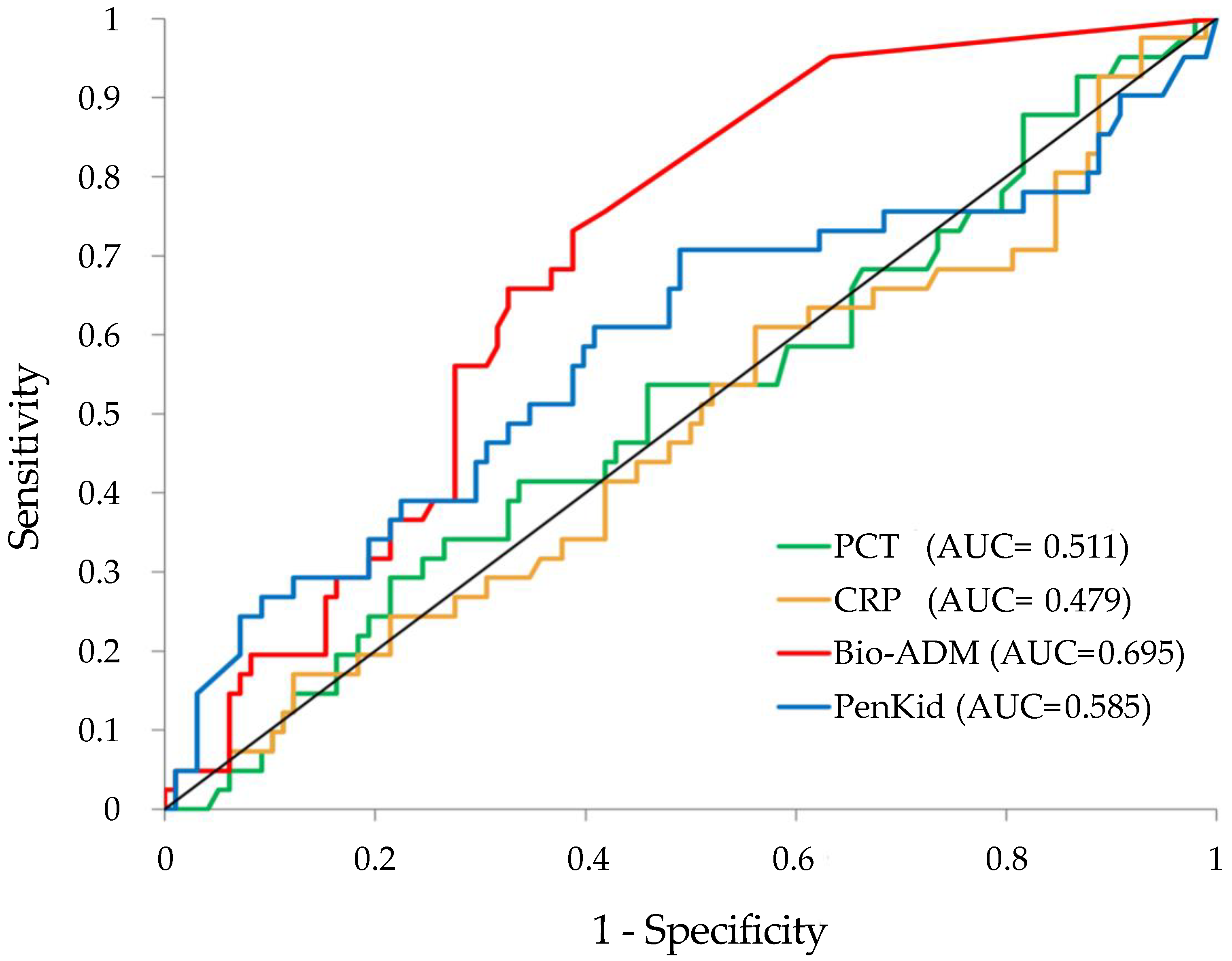
3.5. ROC Curves
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valli, G.; Galati, E.; De Marco, F.; Bucci, C.; Fratini, P.; Cennamo, E.; Ancona, C.; Volpe, N.; Ruggieri, M.P. In-Hospital Mortality in the Emergency Department: Clinical and Etiological Differences between Early and Late Deaths among Patients Awaiting Admission. Clin. Exp. Emerg. Med. 2021, 8, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Valli, G.; Fratini, P.; Volpe, N.; De Marco, F.; Pandolfi, C.; Ancona, C.; Ruggieri, M.P. Analysis of the Costs of Emergency Room Management of Critically Ill Patients. Ital. J. Emerg. Med. 2020, 9, 20–28. [Google Scholar] [CrossRef]
- Rhee, C.; Dantes, R.; Epstein, L.; Murphy, D.J.; Seymour, C.W.; Iwashyna, T.J.; Kadri, S.S.; Angus, D.C.; Danner, R.L.; Fiore, A.E.; et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs. Claims Data, 2009-2014. JAMA 2017, 318, 1241. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Wang, H.E.; Jones, A.R.; Donnelly, J.P. Revised National Estimates of Emergency Department Visits for Sepsis in the United States. Crit. Care Med. 2017, 45, 1443–1449. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef]
- Brink, A.; Alsma, J.; Verdonschot, R.J.C.G.; Rood, P.P.M.; Zietse, R.; Lingsma, H.F.; Schuit, S.C.E. Predicting Mortality in Patients with Suspected Sepsis at the Emergency Department; A Retrospective Cohort Study Comparing QSOFA, SIRS and National Early Warning Score. PLoS ONE 2019, 14, e0211133. [Google Scholar] [CrossRef]
- Loritz, M.; Busch, H.-J.; Helbing, T.; Fink, K. Prospective Evaluation of the QuickSOFA Score as a Screening for Sepsis in the Emergency Department. Intern. Emerg. Med. 2020, 15, 685–693. [Google Scholar] [CrossRef]
- Ruangsomboon, O.; Boonmee, P.; Limsuwat, C.; Chakorn, T.; Monsomboon, A. The Utility of the Rapid Emergency Medicine Score (REMS) Compared with SIRS, QSOFA and NEWS for Predicting in-Hospital Mortality among Patients with Suspicion of Sepsis in an Emergency Department. BMC Emerg. Med. 2021, 21, 2. [Google Scholar] [CrossRef]
- Litell, J.M.; Guirgis, F.; Driver, B.; Jones, A.E.; Puskarich, M.A. Most Emergency Department Patients Meeting Sepsis Criteria Are Not Diagnosed with Sepsis at Discharge. Acad. Emerg. Med. 2021, 28, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Struck, J.; Maisel, A.S.; Magrini, L.; Bergmann, A.; Di Somma, S. Plasma Adrenomedullin Is Associated with Short-Term Mortality and Vasopressor Requirement in Patients Admitted with Sepsis. Crit. Care Lond. Engl. 2014, 18, R34. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Struck, J.; Hartmann, O.; Maisel, A.S.; Rehfeldt, M.; Magrini, L.; Melander, O.; Bergmann, A.; Di Somma, S. Diagnostic and Short-Term Prognostic Utility of Plasma pro-Enkephalin (pro-ENK) for Acute Kidney Injury in Patients Admitted with Sepsis in the Emergency Department. J. Nephrol. 2015, 28, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, A.; Wittebole, X.; François, B.; Pickkers, P.; Antonelli, M.; Gayat, E.; Chousterman, B.G.; Lascarrou, J.-B.; Dugernier, T.; Di Somma, S.; et al. Proenkephalin A 119-159 (Penkid) Is an Early Biomarker of Septic Acute Kidney Injury: The Kidney in Sepsis and Septic Shock (Kid-SSS) Study. Kidney Int. Rep. 2018, 3, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- AdrenOSS-1 study investigators; Mebazaa, A.; Geven, C.; Hollinger, A.; Wittebole, X.; Chousterman, B.G.; Blet, A.; Gayat, E.; Hartmann, O.; Scigalla, P.; et al. Circulating Adrenomedullin Estimates Survival and Reversibility of Organ Failure in Sepsis: The Prospective Observational Multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) Study. Crit. Care 2018, 22, 354. [Google Scholar] [CrossRef]
- Lundberg, O.H.M.; Lengquist, M.; Spångfors, M.; Annborn, M.; Bergmann, D.; Schulte, J.; Levin, H.; Melander, O.; Frigyesi, A.; Friberg, H. Circulating Bioactive Adrenomedullin as a Marker of Sepsis, Septic Shock and Critical Illness. Crit. Care 2020, 24, 636. [Google Scholar] [CrossRef]
- Joshi, N.; Löffler, J.; Szczesna, K.; Do, T.; Vu, M.; Cobb, J.; Ngo, H.; Nguyen, J.; Carbone, V.; Bergmann, D.; et al. Analytical Performance Evaluation of Bioactive Adrenomedullin on Point-of-Care Platform. Clin. Chem. Lab. Med. CCLM 2022, 61, e13–e16. [Google Scholar] [CrossRef]
- Gruson, D.; Carbone, V.; Szczesna, K. Proenkephalin (PenKid) as a biomarker for acute kidney injury and its analytical performance on a point of care platform. Clin. Chem. Lab. Med. CCLM 2021, 59, S874. [Google Scholar] [CrossRef]
- Calandra, T.; Cohen, J. International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The International Sepsis Forum Consensus Conference on Definitions of Infection in the Intensive Care Unit. Crit. Care Med. 2005, 33, 1538–1548. [Google Scholar] [CrossRef]
- Esper, A.M.; Moss, M.; Lewis, C.A.; Nisbet, R.; Mannino, D.M.; Martin, G.S. The Role of Infection and Comorbidity: Factors That Influence Disparities in Sepsis. Crit. Care Med. 2006, 34, 2576–2582. [Google Scholar] [CrossRef]
- Page, D.B.; Donnelly, J.P.; Wang, H.E. Community-, Healthcare-, and Hospital-Acquired Severe Sepsis Hospitalizations in the University HealthSystem Consortium. Crit. Care Med. 2015, 43, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kweon, T.D.; Song, Y.; Lee, E.Y.; Kim, S.J.; Park, R. Analytical and Clinical Performance of a New Point of Care LABGEOIB D-Dimer Test for Diagnosis of Venous Thromboembolism. Ann. Clin. Lab. Sci. 2014, 44, 254–261. [Google Scholar] [PubMed]
- Lundberg, O.H.M.; Rosenqvist, M.; Bronton, K.; Schulte, J.; Friberg, H.; Melander, O. Bioactive Adrenomedullin in Sepsis Patients in the Emergency Department Is Associated with Mortality, Organ Failure and Admission to Intensive Care. PLoS ONE 2022, 17, e0267497. [Google Scholar] [CrossRef] [PubMed]
- Perner, A.; Gordon, A.C.; De Backer, D.; Dimopoulos, G.; Russell, J.A.; Lipman, J.; Jensen, J.-U.; Myburgh, J.; Singer, M.; Bellomo, R.; et al. Sepsis: Frontiers in Diagnosis, Resuscitation and Antibiotic Therapy. Intensiv. Care Med. 2016, 42, 1958–1969. [Google Scholar] [CrossRef]
- Hinson, J.P.; Kapas, S.; Smith, D.M. Adrenomedullin, a Multifunctional Regulatory Peptide. Endocr. Rev. 2000, 21, 138–167. [Google Scholar]
- Zudaire, E.; Portal-Núñez, S.; Cuttitta, F. The Central Role of Adrenomedullin in Host Defense. J. Leukoc. Biol. 2006, 80, 237–244. [Google Scholar] [CrossRef]
- Kato, J.; Kitamura, K. Bench-to-Bedside Pharmacology of Adrenomedullin. Eur. J. Pharmacol. 2015, 764, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Geven, C.; Bergmann, A.; Kox, M.; Pickkers, P. Vascular Effects of Adrenomedullin and the Anti-Adrenomedullin Antibody Adrecizumab in Sepsis. Shock Augusta Ga 2018, 50, 132–140. [Google Scholar] [CrossRef]
- Hippenstiel, S.; Witzenrath, M.; Schmeck, B.; Hocke, A.; Krisp, M.; Krüll, M.; Seybold, J.; Seeger, W.; Rascher, W.; Schütte, H.; et al. Adrenomedullin Reduces Endothelial Hyperpermeability. Circ. Res. 2002, 91, 618–625. [Google Scholar] [CrossRef]
- Geven, C.; van Lier, D.; Blet, A.; Peelen, R.; ten Elzen, B.; Mebazaa, A.; Kox, M.; Pickkers, P. Safety, Tolerability and Pharmacokinetics/Pharmacodynamics of the Adrenomedullin Antibody Adrecizumab in a First-in-Human Study and during Experimental Human Endotoxaemia in Healthy Subjects: First-in-Human Safety and Pharmacokinetics of Adrecizumab. Br. J. Clin. Pharmacol. 2018, 84, 2129–2141. [Google Scholar] [CrossRef]
- Laterre, P.-F.; Pickkers, P.; Marx, G.; Wittebole, X.; Meziani, F.; Dugernier, T.; Huberlant, V.; Schuerholz, T.; François, B.; Lascarrou, J.-B.; et al. Safety and Tolerability of Non-Neutralizing Adrenomedullin Antibody Adrecizumab (HAM8101) in Septic Shock Patients: The AdrenOSS-2 Phase 2a Biomarker-Guided Trial. Intensiv. Care Med. 2021, 47, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Beunders, R.; Struck, J.; Wu, A.H.B.; Zarbock, A.; Di Somma, S.; Mehta, R.L.; Koyner, J.L.; Nadim, M.K.; Maisel, A.S.; Murray, P.T.; et al. Proenkephalin (PENK) as a Novel Biomarker for Kidney Function. J. Appl. Lab. Med. 2017, 2, 400–412. [Google Scholar] [CrossRef]
- Khorashadi, M.; Beunders, R.; Pickkers, P.; Legrand, M. Proenkephalin: A New Biomarker for Glomerular Filtration Rate and Acute Kidney Injury. Nephron 2020, 144, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hur, M.; Struck, J.; Bergmann, A.; Di Somma, S. Proenkephalin Predicts Organ Failure, Renal Replacement Therapy, and Mortality in Patients with Sepsis. Ann. Lab. Med. 2020, 40, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.J.; Meeusen, J.W.; Lieske, J.C.; Bergmann, D.; Sparwaßer, A.; Jaffe, A.S. Analytical Performance of an Immunoassay to Measure Proenkephalin. Clin. Biochem. 2018, 58, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Lapinsky, S.; Dial, S.; Arabi, Y.; Dodek, P.; Wood, G.; Ellis, P.; Guzman, J.; Marshall, J.; Parrillo, J.E.; et al. Acute Kidney Injury in Septic Shock: Clinical Outcomes and Impact of Duration of Hypotension Prior to Initiation of Antimicrobial Therapy. Intensiv. Care Med. 2009, 35, 871–881. [Google Scholar] [CrossRef]
- Rosenqvist, M.; Bronton, K.; Hartmann, O.; Bergmann, A.; Struck, J.; Melander, O. Proenkephalin a 119-159 (PenKid)—A Novel Biomarker for Acute Kidney Injury in Sepsis: An Observational Study. BMC Emerg. Med. 2019, 19, 75. [Google Scholar] [CrossRef]
- Pierrakos, C.; Vincent, J.-L. Sepsis Biomarkers: A Review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef]
- Olsson, T.; Terent, A.; Lind, L. Rapid Emergency Medicine Score: A New Prognostic Tool for in-Hospital Mortality in Nonsurgical Emergency Department Patients. J. Intern. Med. 2004, 255, 579–587. [Google Scholar] [CrossRef]
| Patients | Characteristics | |
|---|---|---|
| n. | 177 | |
| Age, years ± SD | 73.1 ± 17.3 | |
| Female, n (%) | 98 (55.4%) | |
| SOFA, points (IQR) | 4 (2–6) | |
| NEWS, points (IQR) | 6 (3–9) | |
| REMS, points (IQR) | 6 (5–9) | |
| Deaths, n (%) | 43 (24,3%) | |
| Comorbidity, n (IQR) | 2 (1–3) | |
| Follow-up in Non-Survival, days (IQR) | 8 (2–17) | |
| Diuresis: | Active, n (%) | 123 (69.5%) |
| Oliguria, n (%) | 20 (12%) | |
| Anuria, n (%) | 10 (6%) | |
| Dialysis, n (%) | 4 (2%) | |
| Vasopressor, n (%) | 18 (10.2%) | |
| Site of infection: | Lung, n (%) | 78 (45%) |
| Urinary Tract, n (%) | 56 (32%) | |
| Abdominal, n (%) | 14 (8%) | |
| Others, n (%) | 24 (14%) | |
| O2 support: | NIV, n (%) | 4 (2.4%) |
| VMK, n (%) | 70 (42.7%) | |
| AA, n (%) | 90 (54.9%) | |
| Survivors | Non-Survivors | p-Value | |
|---|---|---|---|
| N | 134 | 43 | |
| Age, years (IQR) | 74 (59–83) | 85 (79–90) | <0.001 |
| Female, n (%) | 60 (45%) | 19 (44%) | n.s. |
| Comorbidity, n (IQR) | 2 (1–3) | 2 (1–3) | n.s. |
| Active Diuresis, n (%) | 113 (84%) | 29 (67%) | 0.01 |
| GCS, point (IQR) | 15 (14–15) | 14 (12–15) | <0.001 |
| RR, breath/min (IQR) | 20 (18–22) | 21 (18–27) | n.s. |
| HR, bpm (IQR) | 94 (82–105) | 90 (84–100) | n.s. |
| MAP, mmHg (IQR) | 88 (78–100) | 77 (69–99) | 0.03 |
| BT, °C (IQR) | 37 (36–38) | 36 (36–38) | n.s. |
| SpO2, % (IQR) | 96 (94–98) | 96 (92–97) | n.s. |
| P/F mmHg/% (IQR) | 350 (264–405) | 291 (214–396) | n.s. |
| pH, n (IQR) | 7.44 (7.40–7.48) | 7.42 (7.30–7.46) | 0.02 |
| La−, mmol/L (IQR) | 1.8 (1.4–2.6) | 2.2 (1.8–3.8) | 0.004 |
| Creatinine, mg/dL (IQR) | 1.0 (0.8–1.5) | 1.6 (1.1–2.5) | <0.001 |
| K+, mmol/L (IQR) | 3.9 (3.5–4.3) | 4.1 (3.5–5.0) | n.s. |
| Na+, mmol/L (IQR) | 136 (133–139) | 139 (135–144) | 0.003 |
| CRP, µg/dL (IQR) | 14 (5–25) | 14 (3–25) | n.s. |
| Glucose, mg/dL (IQR) | 122 (101–156) | 129 (101–179) | n.s. |
| BNP, pg/mL (IQR) | 162 (36–336) | 369 (254–483) | <0.001 |
| HsTnI, ng/L (IQR) | 15 (5–44) | 55 (26–138) | <0.001 |
| PCT, ng/mL (IQR) | 0.9 (0.2–7.0) | 1.3 (0.3–14.0) | n.s. |
| SOFA, point (IQR) | 4 (2–6) | 6 (4.5–7) | <0.001 |
| NEWS, point (IQR) | 5 (2–8) | 8 (6–10.5) | <0.001 |
| REMS, point (IQR) | 6 (5–8) | 8 (7–10) | <0.001 |
| Bio-ADM, pg/mL (IQR) | 44.5 (44–54.575) | 55 (45.05–98.75) | <0.001 |
| PenKid, pmol/L (IQR) | 97.85 (72.1–167) | 135.2 (75.3–457.4) | 0.05 |
| PenKid | Bio-ADM | |||
|---|---|---|---|---|
| R | p | R | p | |
| Age, years | 0.18 | 0.01 | 0.13 | n.s. |
| Comorbidity, n | 0.18 | 0.02 | 0.2 | 0.007 |
| MAP, mmHg | −0.13 | n.s. | −0.27 | <0.001 |
| Compromised diuresis | 0.35 | <0.001 | 0.32 | <0.001 |
| SOFA | 0.27 | <0.001 | 0.2 | 0.006 |
| pH, n | −0.27 | <0.001 | −0.31 | <0.001 |
| La−, mmol/L | 0.35 | <0.001 | 0.47 | 0.001 |
| K+, mmol/L | 0.25 | <0.001 | 0.21 | n.s. |
| Creatinine, mg/dL | 0.53 | <0.001 | 0.26 | <0.001 |
| BNP, pg/mL | 0.32 | 0.002 | 0.12 | n.s. |
| Hs-TnI, ng/L | 0.01 | n.s. | 0.01 | n.s. |
| PCT, ng/mL | 0.16 | n.s. | 0.21 | 0.01 |
| Death | 0.15 | 0.05 | 0.35 | <0.001 |
| AUC | SE | LCL (98%) | UCL (98%) | |
|---|---|---|---|---|
| SOFA | 0.68 #, $, ° | 0.05 | 0.57 | 0.79 |
| NEWS | 0.68 #, $, ° | 0.04 | 0.57 | 0.79 |
| REMS | 0.73 *, §, ° | 0.04 | 0.63 | 0.82 |
| PenKid | 0.60 *, §, #, $ | 0.06 | 0.46 | 0.73 |
| Bio-ADM | 0.73 *, §, ° | 0.04 | 0.63 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casalboni, S.; Valli, G.; Terlizzi, F.; Mastracchi, M.; Fidelio, G.; De Marco, F.; Bernardi, C.; Chieruzzi, A.; Curcio, A.; De Cicco, F.; et al. 30 Days Mortality Prognostic Value of POCT Bio-Adrenomedullin and Proenkephalin in Patients with Sepsis in the Emergency Department. Medicina 2022, 58, 1786. https://doi.org/10.3390/medicina58121786
Casalboni S, Valli G, Terlizzi F, Mastracchi M, Fidelio G, De Marco F, Bernardi C, Chieruzzi A, Curcio A, De Cicco F, et al. 30 Days Mortality Prognostic Value of POCT Bio-Adrenomedullin and Proenkephalin in Patients with Sepsis in the Emergency Department. Medicina. 2022; 58(12):1786. https://doi.org/10.3390/medicina58121786
Chicago/Turabian StyleCasalboni, Silvia, Gabriele Valli, Ferdinando Terlizzi, Marina Mastracchi, Giacomo Fidelio, Francesca De Marco, Caterina Bernardi, Anastasia Chieruzzi, Alessia Curcio, Francesco De Cicco, and et al. 2022. "30 Days Mortality Prognostic Value of POCT Bio-Adrenomedullin and Proenkephalin in Patients with Sepsis in the Emergency Department" Medicina 58, no. 12: 1786. https://doi.org/10.3390/medicina58121786
APA StyleCasalboni, S., Valli, G., Terlizzi, F., Mastracchi, M., Fidelio, G., De Marco, F., Bernardi, C., Chieruzzi, A., Curcio, A., De Cicco, F., Colella, N., Papasidero, I. D., Tartarone, E., Ruggieri, M. P., & Di Somma, S. (2022). 30 Days Mortality Prognostic Value of POCT Bio-Adrenomedullin and Proenkephalin in Patients with Sepsis in the Emergency Department. Medicina, 58(12), 1786. https://doi.org/10.3390/medicina58121786







