D-Ribose-Induced Glycation and Its Attenuation by the Aqueous Extract of Nigella sativa Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Plant Extract
2.2. Phytochemical Analysis
2.2.1. Determination of Total Phenolic Content (TPC)
2.2.2. Determination of Total Flavonoid Content (TFC)
2.3. Enzyme Inhibition Assays
2.3.1. Inhibition of α-Amylase
2.3.2. Inhibition of α-Glucosidase
2.4. In Vitro Glycation of BSA Induced by D-Ribose
2.4.1. Investigation of Hyperchromicity Utilizing UV–Vis Spectroscopy
NSAE-treated sample/Absorbance of glycated sample × 100.
2.4.2. Fourier Transform Infrared Spectroscopy
2.4.3. Measurement of Browning
2.4.4. Determination of Ketoamine
2.4.5. Determination of Protein Carbonyl Content
2.4.6. Determination of Thiol Group
2.4.7. Total Fluorescent AGEs Formation Using Fluorescence Spectroscopy
NSAE-treated BSA)/Fluorescence of glycated BSA × 100.
2.4.8. Individual AGEs Formation
2.5. In Vitro Aggregation of BSA Induced by D-Ribose
2.5.1. Congo Red Assay
2.5.2. Thioflavin T Assay
2.5.3. Measurement of Amyloid Fibril Inhibition Using SDS-Polyacrylamide Gel Electrophoresis
2.6. In Vitro Glycation of Plasmid DNA
2.7. Statistical Analysis
3. Results
3.1. Plant Material Extraction
3.2. Phytochemical Analysis
3.2.1. Total Phenolic Content
3.2.2. Total Flavonoid Content
3.3. Enzyme Inhibition Assays
3.3.1. Inhibition Assay for α-Amylase Activity (DNSA)
3.3.2. Inhibition Assay for α-Glucosidase Activity
3.4. Analysis of Physicochemical Processes and Characterization
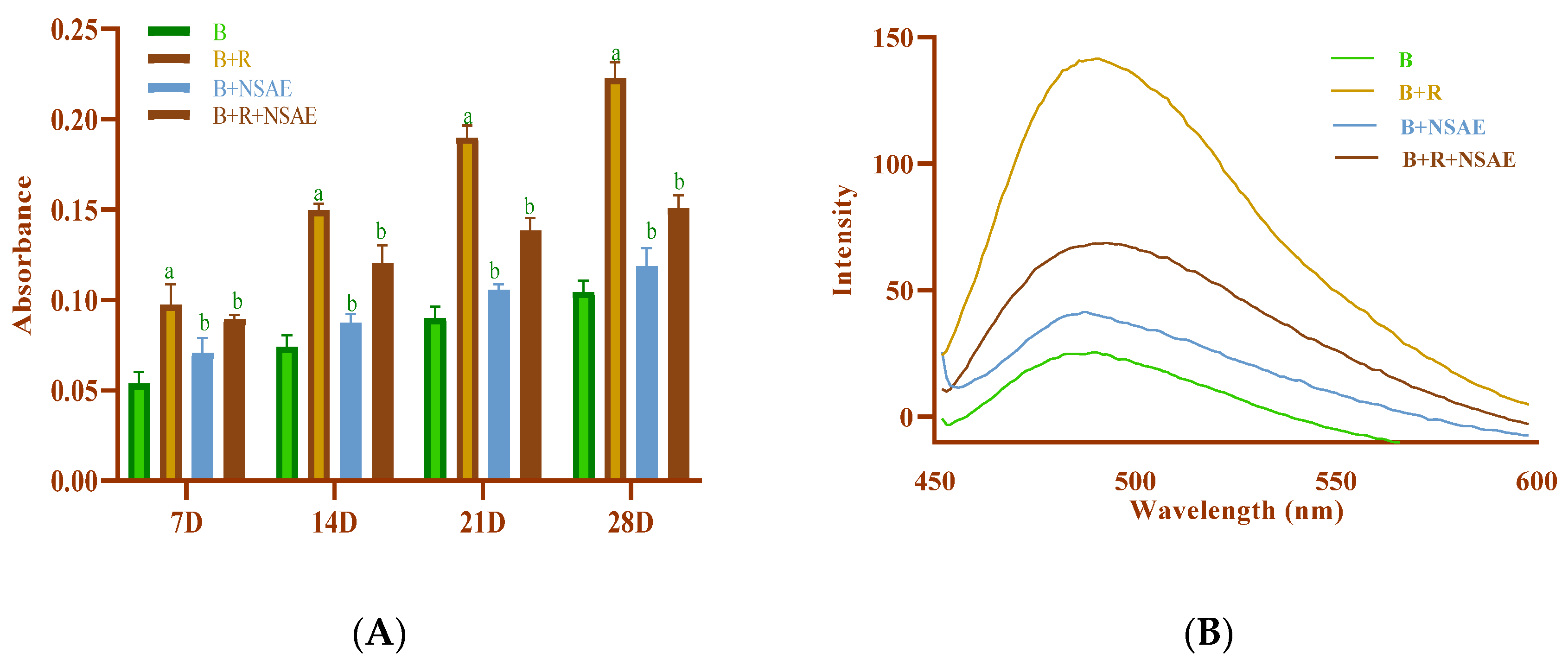
3.4.1. Estimation of Hyperchromicity Using UV–Visible Spectroscopy
3.4.2. Fourier Transform Infrared (FTIR) Spectrum
3.4.3. Measurement of Browning
3.4.4. Determination of Ketoamine Content
3.4.5. Determination of Protein Carbonyl Content
3.4.6. Determination of Thiol Group
3.4.7. Total Fluorescent AGEs Formation
3.4.8. Individual AGE Formation
3.5. In Vitro Aggregation of BSA Induced by D-Ribose
3.5.1. Congo Red Assay
3.5.2. Thioflavin T Assay
3.5.3. Measurement of Amyloid Inhibition Using SDS-Polyacrylamide Gel Electrophoresis
3.6. In Vitro Glycation of Plasmid DNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khangholi, S.; Majid, F.A.A.; Berwary, N.J.A.; Ahmad, F.; Aziz, R.B.A. The Mechanisms of Inhibition of Advanced Glycation End Products Formation through Polyphenols in Hyperglycemic Condition. Planta Medica 2015, 82, 32–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 2020, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, A.N.; Duncan, A.; Kruimer, D.; Venn, B.J. Glycated Albumin Is Associated with Body Mass Index in Euglycemic Adults but Is Not Predictive of Postprandial Blood Glucose Response. J. Clin. Lab. Anal. 2017, 31, e22085. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yu, L.; Wang, Y.; Wei, Y.; Xu, Y.; He, T.; He, R. D-Ribose Contributes to the Glycation of Serum Protein. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, L.; Chen, J.; Ge, L.; He, R.Q. Rapid Glycation with D-Ribose Induces Globular Amyloid-like Aggregations of BSA with High Cytotoxicity to SH-SY5Y Cells. BMC Cell Biol. 2009, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Friedman, E.A. Advanced glycation end-products in diabetic nephropathy. Nephrol. Dial. Transplant. 1999, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Thilavech, T.; Marnpae, M.; Mäkynen, K.; Adisakwattana, S. Phytochemical Composition, Antiglycation, Antioxidant Activity and Methylglyoxal-Trapping Action of Brassica Vegetables. Plant Foods Hum. Nutr. 2021, 76, 340–346. [Google Scholar] [CrossRef]
- Rubab, U.; Kumar, D.; Farah, M.; Al-Anazi, K.; Ali, M.; Ali, A. Inhibitory Roles of Nigella Sativa Seed Extracts on in Vitro Glycation and Aggregation. Pharmacogn. Mag. 2021, 17, 220. [Google Scholar] [CrossRef]
- Dabeer, S.; Rather, M.A.; Rasool, S.; Rehman, M.U.; Alshahrani, S.; Jahan, S.; Rashid, H.; Halawi, M.; Khan, A. Chapter 1—History and Traditional Uses of Black Seeds (Nigella sativa); Khan, A., Rehman, M., Seeds, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–28. ISBN 9780128244623. [Google Scholar] [CrossRef]
- Chaudhry, Z.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Sumrra, S.H. Chapter 13—Cumin. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–178. [Google Scholar]
- Ali, A. Herbs That Heal: The Philanthropic Behaviour of Nature. Ann. Phytomedicine Int. J. 2020, 9. [Google Scholar] [CrossRef]
- Balyan, P.; Khan, J.; Ali, A. Therapeutic Potential of Nigella Sativa in the Prevention of Aggregation and Glycation of Proteins. In Black Seeds (Nigella sativa): Pharmacological and Therapeutic Applications; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- El Rabey, H.A.; Al-Seeni, M.N.; Bakhashwain, A.S. The Antidiabetic Activity of Nigella sativa and Propolis on Streptozotocin-Induced Diabetes and Diabetic Nephropathy in Male Rats. Evidence-based complementary and alternative medicine. Evidence-Based Complement. Altern. Med. 2017, 2017, 5439645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balyan, P.; Ali, A. Comparative analysis of the biological activities of different extracts of Nigella sativa L. seeds. Ann. Phytomedicine Int. J. 2022, 11, 577–587. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An Appraisal of Eighteen Commonly Consumed Edible Plants as Functional Food Based on Their Antioxidant and Starch Hydrolase Inhibitory Activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Sathiavelu, A.; Sangeetha, S.; Archit, R.; Mythili, S. In Vitro Anti-Diabetic Activity of Aqueous Extract of the Medicinal Plants Nigella Sativa, Eugenia Jambolana, Andrographis Paniculata and Gymnema Sylvestre. Int. J. Drug Dev. Res. 2013, 5, 323–328. [Google Scholar]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of Inhibition of α-Amylase and α-Glucosidase by Aqueous Extract of Morinda Lucida Benth Leaf. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nabi, R.; Alvi, S.S.; Khan, R.H.; Ahmad, S.; Ahmad, S.; Khan, M.S. Antiglycation Study of HMG-R Inhibitors and Tocotrienol against Glycated BSA and LDL: A Comparative Study. Int. J. Biol. Macromol. 2018, 116, 983–992. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Antiglycation and Antiaggregation Potential of Thymoquinone. Nat. Volatiles Essent. Oils 2019, 6, 25–33. [Google Scholar]
- Johnson, R.N.; Metcalf, P.A.; Baker, J.R. Fructosamine: A New Approach to the Estimation of Serum Glycosylprotein. An Index of Diabetic Control. Clin. Chim. Acta 1983, 127, 87–95. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Ni, M.; Zhang, G.; Liao, Y.; Hu, X.; Zhang, Y.; Gong, D. The Inhibition of Oleanolic Acid on Protein Non-Enzymatic Glycation. LWT 2020, 125, 109253. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Rabbani, N. Detection of Oxidized and Glycated Proteins in Clinical Samples Using Mass Spectrometry—A User’s Perspective. Biochim. Et Biophys. Acta Gen. Subj. 2014, 1840, 818–829. [Google Scholar] [CrossRef]
- Ali, A.; More, T.A.; Hoonjan, A.K.; Sivakami, S. Antiglycating Potential of Acesulfame Potassium: An Artificial Sweetener. Appl. Physiol. Nutr. Metab. 2017, 42, 1054–1063. [Google Scholar] [CrossRef]
- Tupe, R.S.; Agte, V.V. Role of Zinc along with Ascorbic Acid and Folic Acid during Long-Term in Vitro Albumin Glycation. Br. J. Nutr. 2009, 103, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shahu, R.; Balyan, P.; Kumari, S.; Ghodmare, R.; Jobby, R.; Jha, P. Antioxidation and Antiglycation Properties of a Natural Sweetener: Stevia Rebaudiana. Sugar Tech 2022, 24, 563–575. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Madhusankha, G.D.M.P.; Navaratne, S.B. Phytochemical Analysis of Indian and Ethiopian Black Cumin Seeds (Nigella Sativa). Agric. Res. Technol. Open Access J. 2018, 17, 1–6. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Chobot, A.; Górowska-Kowolik, K.; Sokołowska, M.; Jarosz-Chobot, P. Obesity and Diabetes—Not Only a Simple Link between Two Epidemics. Diabetes/Metabolism Res. Rev. 2018, 34, e3042. [Google Scholar] [CrossRef]
- Naumov, D.G. Structure and Evolution of Mammalian Maltase-Glucoamylase and Sucrase-Isomaltase Genes. Mol. Biol. 2007, 41, 1056–1068. [Google Scholar] [CrossRef]
- Chesne, S.; Rondeau, P.; Armenta, S.; Bourdon, E. Effects of Oxidative Modifications Induced by the Glycation of Bovine Serum Albumin on Its Structure and on Cultured Adipose Cells. Biochimie 2006, 88, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Bohlooli, M.; Ghaffari-Moghaddam, M.; Khajeh, M.; Aghashiri, Z.; Sheibani, N.; Moosavi-Movahedi, A.A. Acetoacetate Promotes the Formation of Fluorescent Advanced Glycation End Products (AGEs). J. Biomol. Struct. Dyn. 2016, 34, 2658–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arasteh, A.; Farahi, S.; Habibi-Rezaei, M.; Moosavi-Movahedi, A.A. Glycated Albumin: An Overview of the In Vitro Models of an In Vivo Potential Disease Marker. J. Diabetes Metab. Disord. 2014, 13, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Moinuddin; Dixit, K.; Shahab, U.; Alam, K.; Ali, A. Genotoxicity and Immunogenicity of DNA-Advanced Glycation End Products Formed by Methylglyoxal and Lysine in Presence of Cu2+. Biochem. Biophys. Res. Commun. 2011, 407, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A Review on Therapeutic Potential of Nigella Sativa: A Miracle Herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Tiji, S.; Bouhrim, M.; Addi, M.; Drouet, S.; Lorenzo, J.M.; Hano, C.; Bnouham, M.; Mimouni, M. Linking the Phytochemicals and the α-Glucosidase and α-Amylase Enzyme Inhibitory Effects of Nigella sativa Seed Extracts. Foods 2021, 10, 1818. [Google Scholar] [CrossRef]
- Sobhi, W.; Stevigny, C.; Duez, P.; Calderon, B.B.; Atmani, D.; Benboubetra, M.P. Effect of Lipid Extracts of Nigella Sativa L. Seeds on the Liver ATP Reduction and Alpha-Glucosidase Inhibition. Pak. J. Pharm. Sci. 2016, 29, 111–117. [Google Scholar]
- Khan, M.S.; Dwivedi, S.; Priyadarshini, M.; Tabrez, S.; Siddiqui, M.A.; Jagirdar, H.; Al-Senaidy, A.M.; Al-Khedhairy, A.A.; Musarrat, J. Ribosylation of Bovine Serum Albumin Induces ROS Accumulation and Cell Death in Cancer Line (MCF-7). Eur. Biophys. J. 2013, 42, 811–818. [Google Scholar] [CrossRef]
- Dorsey, P.G.; Greenspan, P. Inhibition of nonenzymatic protein glycation by pomegranate and other fruit juices. J. Med. Food 2014, 17, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Narasimhan, A.; Chinnaiyan, M. Ferulic acid exerts its antidiabetic effect by modulating insulin signalling molecules in the liver of high fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2009, 40, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, J.; Yang, W.; Chen, S.; Liu, D.; Fang, H.; Zhang, H.; Ye, X. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Ganeshpurkar, A.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Glycolytic enzyme inhibitory and antiglycation potential of rutin. Futur. J. Pharm. Sci. 2017, 3, 158–162. [Google Scholar] [CrossRef]
- Adefegha, S.S.; Oboh, G.; Ejakpovi, I.I.; Oyeleye, S.I. Antioxidant and antidiabetic effects of gallic and protocatechuic acids: A structure–function perspective. Comp. Clin. Pathol. 2015, 24, 1579–1585. [Google Scholar] [CrossRef]
- Aazza, S.; El-Guendouz, S.; Miguel, M.G. Antioxidant, anti-inflammatory and anti-hyperglycaemic activities of essential oils from thymbra capitata, thymus albicans, thymus caespititius, thymus carnosus, thymus lotocephalus and thymus mastichina from Portugal. Nat. Prod. Commun. 2016, 11, 1029–1038. [Google Scholar] [CrossRef] [Green Version]
- Dalli, M.; Daoudi, N.E.; Azizi, S.; Benouda, H.; Bnouham, M.; Gseyra, N. Chemical composition analysis using HPLC-UV/GC-MS and Inhibitory Activity of Different Nigella sativa fractions on pancreatic α-amylase and intestinal glucose absorption. BioMed Res. Int. 2021, 2021, 9979419. [Google Scholar] [CrossRef]
- Toma, C.C.; Olah, N.K.; Vlase, L.; Mogosan, C.; Mocan, A. Comparative studies on polyphenolic composition, antioxidant, and diuretic effects of Nigella sativa L. (Black cumin) and Nigella damascene L. (Lady-in-a-mist) seeds. Molecules 2015, 20, 9560. [Google Scholar] [CrossRef] [Green Version]
- Eze, F.N.; Tola, A.J. Protein glycation and oxidation inhibitory activity of Centella Asiatica Phenolics (CAP) in glucose-mediated bovine serum albumin glycoxidation. Food Chem. 2020, 332, 127302. [Google Scholar] [CrossRef]
- Jha, P.; Momin, A.R.; Kumar, D.; Ali, A. reversal of glycoxidative damage of DNA and protein by antioxidants. Ann. Phytomedicine Int. J. 2018, 7, 101–105. [Google Scholar] [CrossRef]
- Mohammed, A.K. Ameliorative effect of black seed (Nigella sativa L.) on the toxicity of aluminum in rabbits. Iraqi J. Veter-Med. 2010, 34, 110–116. [Google Scholar] [CrossRef]
- Al-Ghamdi, M.S. Protective effect of Nigella sativa seeds against carbon tetrachloride-induced liver damage. Am. J. Chin. Med. 2003, 31, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Dollah, M.A.; Parhizkar, S.; Latiff, L.A.; Bin Hassan, M.H. Toxicity effect of Nigella sativa on the liver function of rats. Adv. Pharm. Bull. 2013, 3, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalli, M.; Daoudi, N.E.; Abrigach, F.; Azizi, S.-E.; Bnouham, M.; Kim, B.; Gseyra, N. In vitro α-amylase and hemoglobin glycation inhibitory potential of Nigella sativa essential oil, and molecular docking studies of its principal components. Front. Pharmacol. 2022, 13, 1036129. [Google Scholar] [CrossRef]
- Losso, J.N.; Bawadi, H.A.; Chintalapati, M. Inhibition of the formation of advanced glycation end products by thymoquinone. Food Chem. 2011, 128, 55–61. [Google Scholar] [CrossRef]
- Mahmood, T.; Nims; Moin, S.; Faizy, A.F.; Naseem, S.; Aman, S. Nigella Sativa as an Antiglycating Agent for Human Serum Albumin. Int. J. Sci. Res. 2012, 2, 25–27. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef] [PubMed]











| Test Sample | TPC (mg/GAE g DW) | TFC (mg QE/g DW) |
|---|---|---|
| Nigella sativa | 228.18 ± 0.013 | 191.644 ± 0.031 |
| % Inhibition | Browning | Ketoamine Content | Protein Carbonyl Content | Thiol Group | Congo Red Assay |
|---|---|---|---|---|---|
| AG | 37.41%, | 39.44% | 47.34% | 47.90% | 52.97% |
| NSAE | 27.34% | 27.23% | 31.86% | 38.69% | 40.06% |
| Band I | Band II | ||||
|---|---|---|---|---|---|
| Lane | Integrated Density | Percent Increase/Decrease | Lane | Integrated Density | Percent Increase/Decrease |
| 1 | 149,351 | 0 | 1 | 119,767 | 0 |
| 2 | 160,126 | +7.21 | 2 | 81,765 | −31.73 |
| 3 | 134,268 | +10.10 | 3 | 91,443 | −23.65 |
| 4 | 144,438 | +3.29 | 4 | 85,715 | −28.43 |
| 5 | 128,280 | +14.11 | 5 | 93,658 | −21.80 |
| 6 | 144,551 | +3.21 | 6 | 111,581 | −6.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balyan, P.; Ola, M.S.; Alhomida, A.S.; Ali, A. D-Ribose-Induced Glycation and Its Attenuation by the Aqueous Extract of Nigella sativa Seeds. Medicina 2022, 58, 1816. https://doi.org/10.3390/medicina58121816
Balyan P, Ola MS, Alhomida AS, Ali A. D-Ribose-Induced Glycation and Its Attenuation by the Aqueous Extract of Nigella sativa Seeds. Medicina. 2022; 58(12):1816. https://doi.org/10.3390/medicina58121816
Chicago/Turabian StyleBalyan, Prairna, Mohammad Shamsul Ola, Abdullah S. Alhomida, and Ahmad Ali. 2022. "D-Ribose-Induced Glycation and Its Attenuation by the Aqueous Extract of Nigella sativa Seeds" Medicina 58, no. 12: 1816. https://doi.org/10.3390/medicina58121816








