Splenectomy for Visceral Leishmaniasis Out of an Endemic Region: A Case Report and Literature Review
Abstract
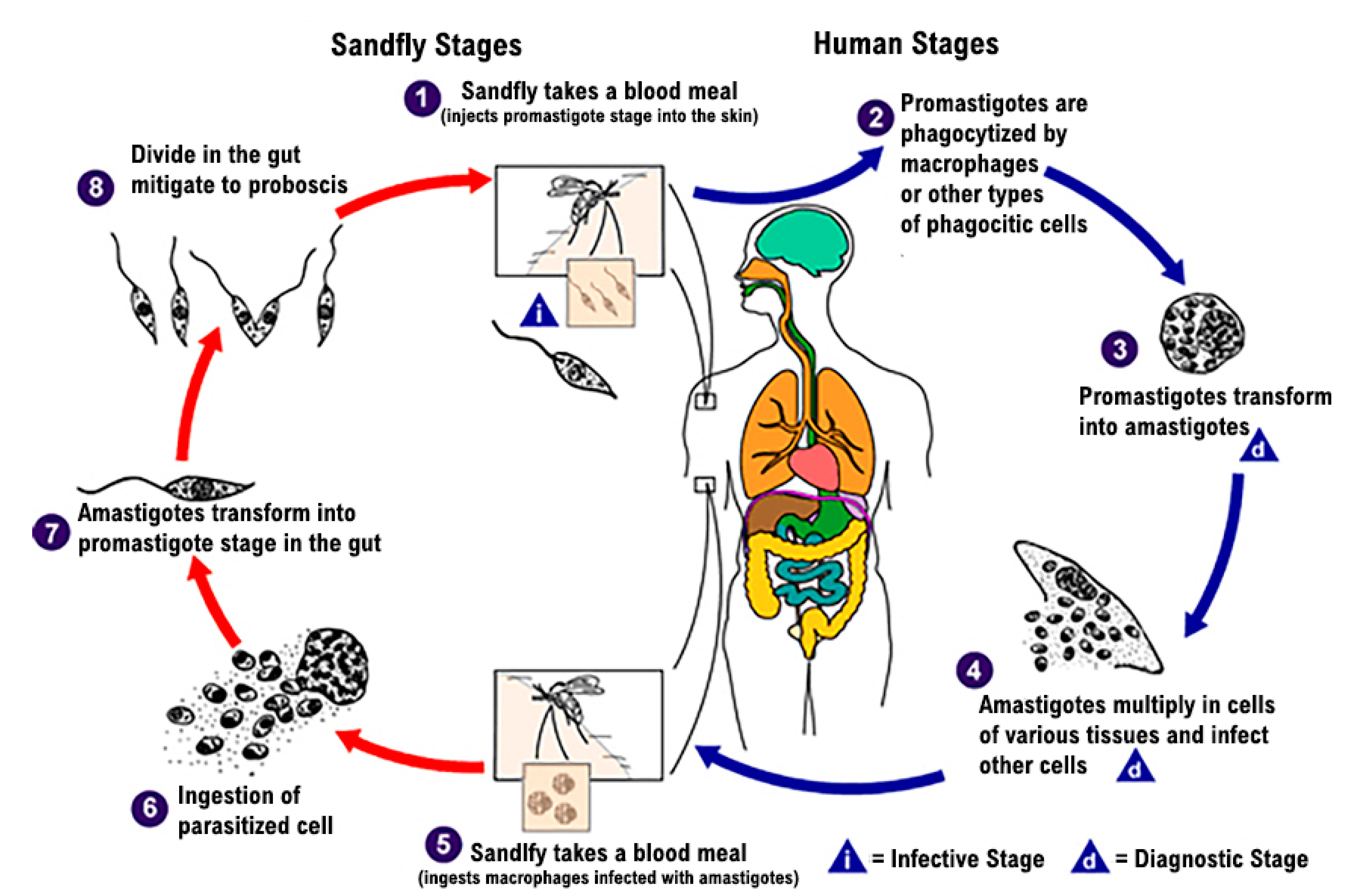
1. Introduction
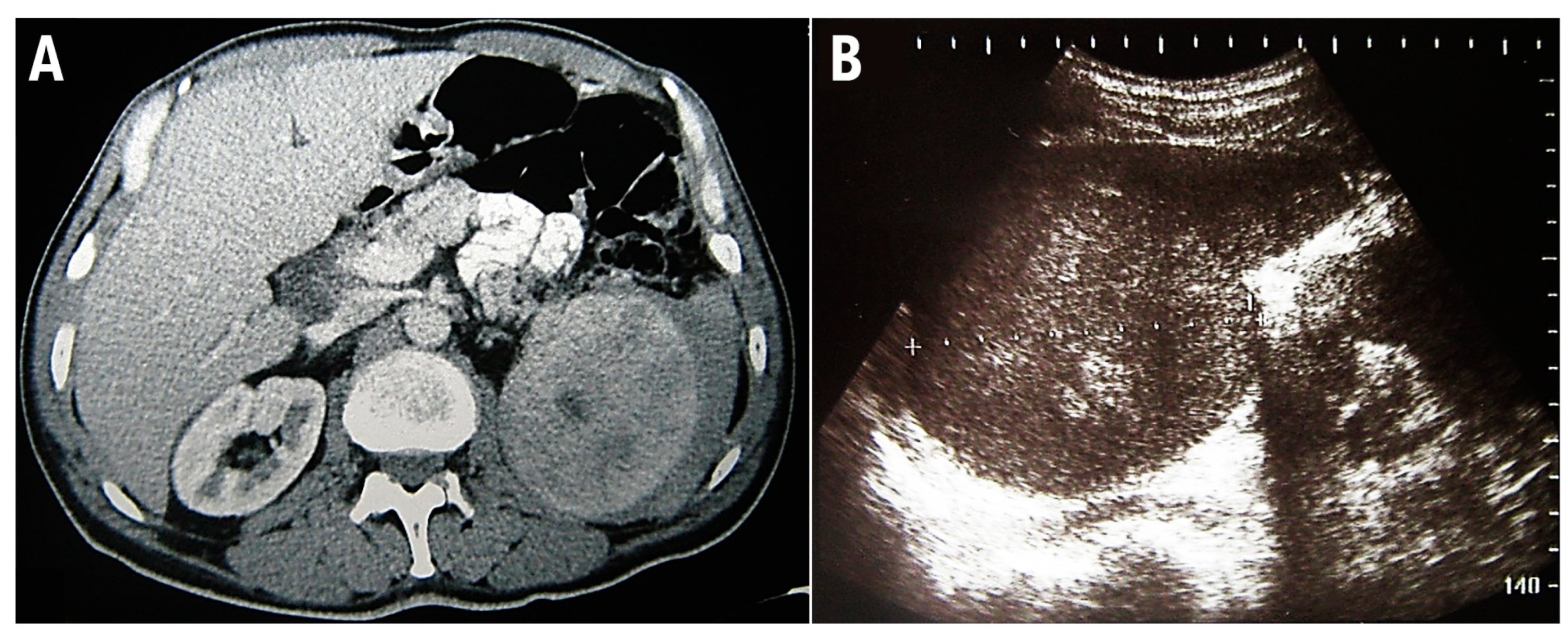
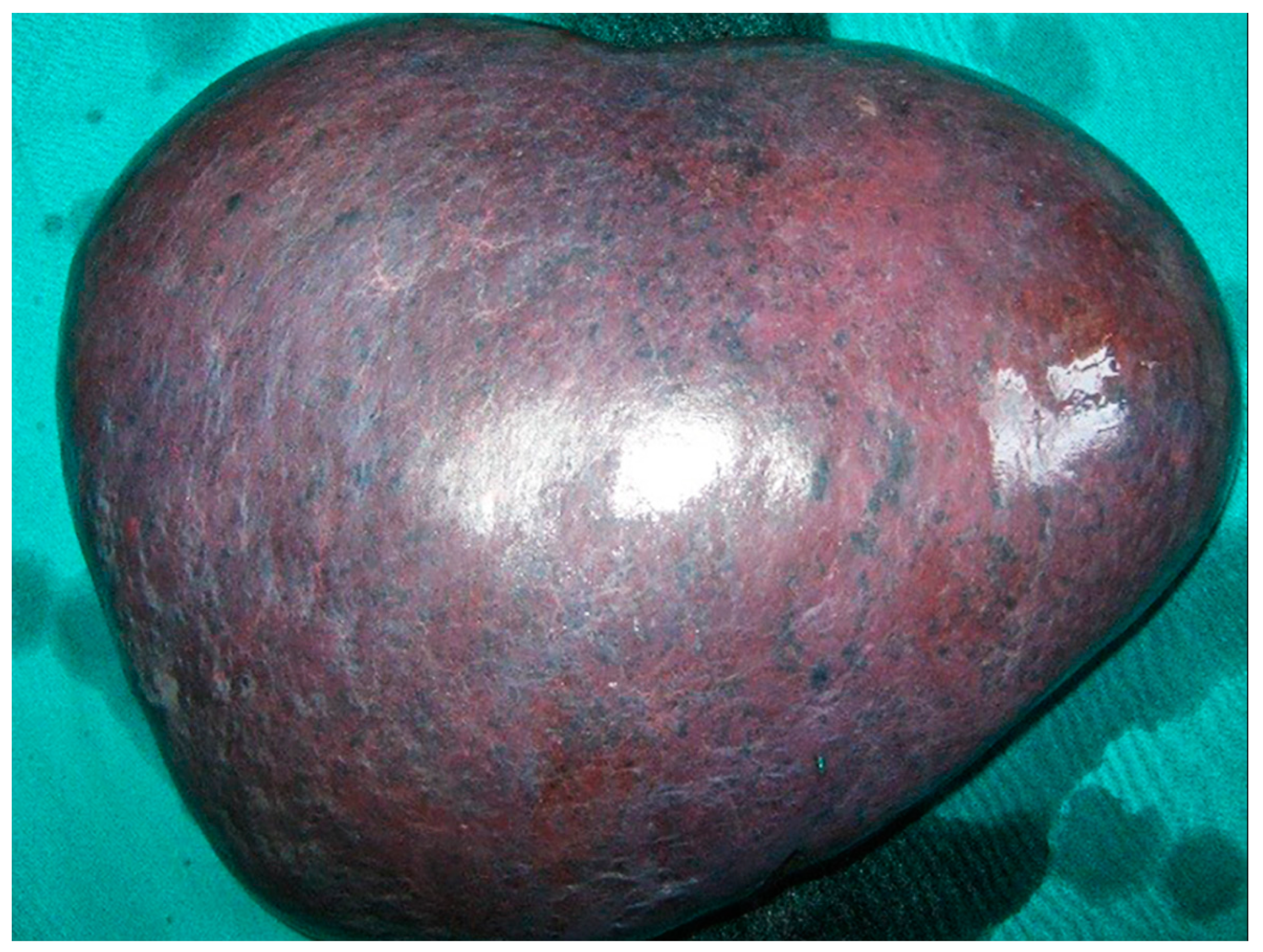
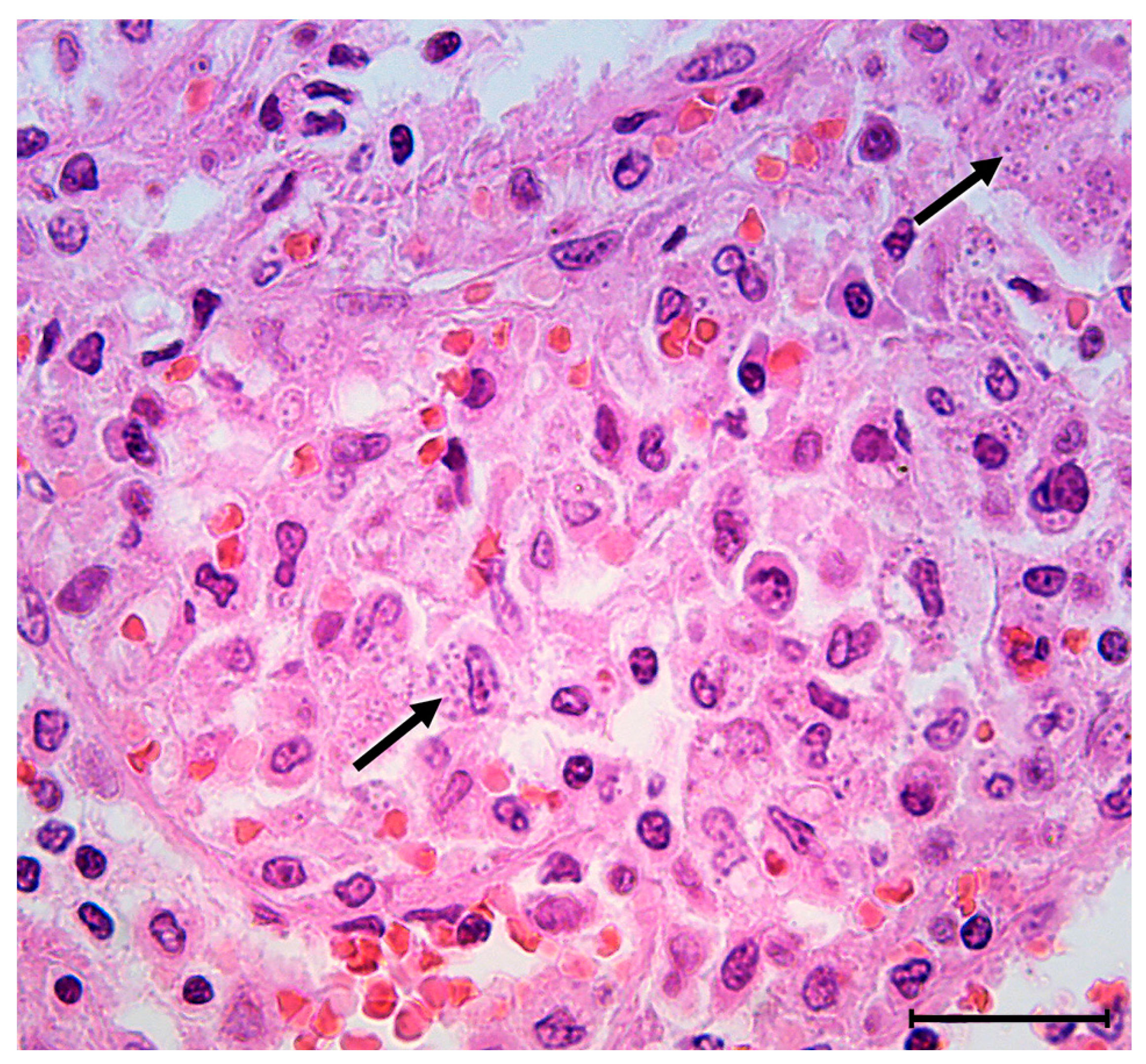
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. About Leishmaniasis. Available online: https://www.cdc.gov/parasites/leishmaniasis/gen_info/faqs.html (accessed on 19 May 2020).
- WHO. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 21 May 2021).
- WHO. Leishmaniasis in the WHO European Region. Available online: http://www.euro.who.int/__data/assets/pdf_file/0007/246166/Fact-sheet-Leishmaniasis-Eng.pdf?ua=1 (accessed on 4 October 2018).
- Miscevic, Z.; Milutinovic, M.; Ivovic, V. Fauna and distribution of sandflies (Diptera, Phlebotomidae) in Yugoslavia, Croatia, Macedonia and their role in the transmission of parasitic and viral diseases. Acta Vet. 1998, 48, 163–172. [Google Scholar]
- Kamhawi, S. Phlebotomine sand flies and Leishmania parasites: Friends or foes? Trends Parasitol. 2006, 22, 439–445. [Google Scholar] [CrossRef] [PubMed]
- CDC. Leishmania Species: Life Cycle. Available online: https://www.cdc.gov/parasites/leishmaniasis/biology.html (accessed on 18 February 2020).
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- den Boer, M.; Davidson, R.N. Treatment options for visceral leishmaniasis. Expert Rev. Anti-Infect. Ther. 2006, 4, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Cloots, K.; Burza, S.; Malaviya, P.; Hasker, E.; Kansal, S.; Mollett, G.; Chakravarty, J.; Roy, N.; Lal, B.K.; Rijal, S.; et al. Male predominance in reported Visceral Leishmaniasis cases: Nature or nurture? A comparison of population-based with health facility-reported data. PLoS Negl. Trop. Dis. 2020, 14, e0007995. [Google Scholar] [CrossRef] [PubMed]
- Dahal, P.; Singh-Phulgenda, S.; Olliaro, P.L.; Guerin, P.J. Gender disparity in cases enrolled in clinical trials of visceral leishmaniasis: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009204. [Google Scholar] [CrossRef] [PubMed]
- Eltoum, I.A.; Ali, M.S.; Zijlstra, E.E.; Ghalib, H.W.; Satti, M.M.H.; Eltoum, B.; El-Hassan, A.M. Congenital Kala-Azar and Leishmaniasis in the Placenta. Am. J. Trop. Med. Hyg. 1992, 46, 57–62. [Google Scholar] [CrossRef]
- Elamin, A.; Omer, M.I.A. Visceral Leishmaniasis in a 6-Week-Old Infant: Possible Congenital Transmission. Trop. Dr. 2016, 22, 133–135. [Google Scholar] [CrossRef]
- Meinecke, C.K.; Schottelius, J.; Oskam, L.; Fleischer, B. Congenital transmission of visceral leishmaniasis (Kala Azar) from an asymptomatic mother to her child. Pediatrics 1999, 104, e65. [Google Scholar] [CrossRef]
- Nyakundi, P.M.; Muigai, R.; Were, J.B.O.; Oster, C.N.; Gachihi, G.S.; Kirigi, G. Congenital visceral leishmaniasis: Case report. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 564. [Google Scholar] [CrossRef]
- Yadav, T.P.; Gupta, H.; Satteya, U.; Kumar, R.; Mittal, V. Congenital kala-azar. Ann. Trop. Med. Parasitol. 2016, 83, 535–537. [Google Scholar] [CrossRef] [PubMed]
- Symmers, W.S. Leishmaniasis acquired by contagion: A case of marital infection in Britain. Lancet 1960, 1, 127–132. [Google Scholar] [CrossRef]
- Guedes, D.L.; van Henten, S.; Cnops, L.; Adriaensen, W.; van Griensven, J. Sexual Transmission of Visceral Leishmaniasis: A Neglected Story. Trends Parasitol. 2020, 36, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Avila-Garca, M.; Mancilla, J.; Segura-Cervantes, E.; Galindo-Sevill, N. Transmission to Humans. In Leishmaniasis-Trends in Epidemiology, Diagnosis and Treatment; IntechOpen: London, UK, 2014. [Google Scholar]
- Cummins, D.; Amin, S.; Halil, O.; Chiodini, P.L.; Hewitt, P.E.; Radley-Smith, R. Visceral leishmaniasis after cardiac surgery. Arch. Dis. Child. 1995, 72, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Singh, S. Transfusion transmitted leishmaniasis: A case report and review of literature. Indian J. Med. Microbiol. 2006, 24, 165–170. [Google Scholar] [CrossRef]
- Herwaldt, B.L. Laboratory-acquired parasitic infections from accidental exposures. Clin. Microbiol. Rev. 2001, 14, 659–688. [Google Scholar] [CrossRef]
- Chappuis, F.; Sundar, S.; Hailu, A.; Ghalib, H.; Rijal, S.; Peeling, R.W.; Alvar, J.; Boelaert, M. Visceral leishmaniasis: What are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 2007, 5, 873–882. [Google Scholar] [CrossRef]
- Sheikha, A. Dyserythropoiesis in 105 patients with visceral leishmaniasis. Lab. Hematol. 2004, 10, 206–211. [Google Scholar]
- Bhatia, P.; Haldar, D.; Varma, N.; Marwaha, R.; Varma, S. A case series highlighting the relative frequencies of the common, uncommon and atypical/unusual hematological findings on bone marrow examination in cases of visceral leishmaniasis. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011035. [Google Scholar] [CrossRef]
- Chandra, H.; Chandra, S.; Kaushik, R.M. Visceral leishmaniasis with associated common, uncommon, and atypical morphological features on bone marrow aspirate cytology in nonendemic region. J. Trop. Med. 2013, 2013, 861032. [Google Scholar] [CrossRef]
- Costa, C.H.N.; Werneck, G.L.; Costa, D.L.; Holanda, T.A.; Aguiar, G.B.; Carvalho, A.S.; Cavalcanti, J.C.; Santos, L.S. Is severe visceral leishmaniasis a systemic inflammatory response syndrome? A case control study. Rev. Soc. Bras. Med. Trop. 2010, 43, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Coura-Vital, W.; Araujo, V.E.; Reis, I.A.; Amancio, F.F.; Reis, A.B.; Carneiro, M. Prognostic factors and scoring system for death from visceral leishmaniasis: An historical cohort study in Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3374. [Google Scholar] [CrossRef] [PubMed]
- Joist, J.; George, J. Hemostatic abnormalities in liver and renal disease. In Hemostasis and Thrombosis, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 973–995. [Google Scholar]
- Hedner, U.; Hirsh, J.; Hardner, V. Therapy with Antifibrinolytic Agents. In Hemostasis and Thrombosis; Lipincott Williams & Wilkins: London, UK, 2001; pp. 718–795. [Google Scholar]
- Vucelic, D.; Pesko, P.; Stojakov, D.; Sabljak, P.; Bjelovic, M.; Dunjic, M.; Ebrahimi, K.; Nenadic, B.; Velickovic, D.; Spica, B. Systemic hemostatic drugs. Acta Chir. Iugosl. 2007, 54, 177–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Federici, A.B. The use of desmopressin in von Willebrand disease: The experience of the first 30 years (1977–2007). Haemophilia 2008, 14 (Suppl. S1), 5–14. [Google Scholar] [CrossRef]
- Horstman, L.L.; Valle-Riestra, B.J.; Jy, W.; Wang, F.; Mao, W.; Ahn, Y.S. Desmopressin (DDAVP) acts on platelets to generate platelet microparticles and enhanced procoagulant activity. Thromb. Res. 1995, 79, 163–174. [Google Scholar] [CrossRef]
- Hezard, N.; Jallu, V.; Garnotel, R.; Kaplan, C.; Nguyen, P. DDAVP infusion can modify VASP phosphorylation in human platelets. J. Thromb. Haemost. 2004, 2, 672–673. [Google Scholar] [CrossRef]
- Mannucci, P.M. Hemostatic drugs. N. Engl. J. Med. 1998, 339, 245–253. [Google Scholar] [CrossRef]
- Vucelic, D.; Jesic, R.; Jovicic, S.; Zivotic, M.; Grubor, N.; Trajkovic, G.; Canic, I.; Elezovic, I.; Antovic, A. Comparison of standard fibrinogen measurement methods with fibrin clot firmness assessed by thromboelastometry in patients with cirrhosis. Thromb. Res. 2015, 135, 1124–1130. [Google Scholar] [CrossRef]
- Campos, M.A.G.; Moraes Filho, A.S.; Rego, G.; Silva, R.O.L.; Sousa, R.A.B.; Tchuisseu, Y.P.; Silva, G.E.B.; Gama, M.E.A. Is splenectomy an option for multiple relapses in a child with visceral leishmaniasis? A case report. Rev. Soc. Bras. Med. Trop. 2021, 54, e0748-2020. [Google Scholar] [CrossRef]
- Alon, D.; Chowers, M. Successful therapeutic splenectomy in an HIV patient with relapsing visceral leishmaniasis. Int. J. STD AIDS 2012, 23, 289–290. [Google Scholar] [CrossRef]
- Dutra, R.A.; Dutra, L.F.; Reis Mde, O.; Lambert, R.C. Splenectomy in a patient with treatment-resistant visceral leishmaniasis: A case report. Rev. Soc. Bras. Med. Trop. 2012, 45, 130–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Laboratory Test | Results Before Operation | Normal Value |
|---|---|---|
| APTT | 44.9 | 25–42 s |
| PT | 23.1 | 10.4–13 s |
| INR | 1.97 | 0.8–1.2 |
| Platelet count | 11 | 158–425 × 109 /L |
| Hemoglobin | 94.7 | 119–175 g/L |
| Fibrinogen | 3.8 | 1.8–3.5 g/L |
| FII | 66 | 70–120% |
| FV | 48 | 70–140% |
| FVII | 21 | 70–120% |
| FVIII:C | 94 | 70–150% |
| FIX | 87 | 70–120% |
| FX | 55 | 70–120% |
| FXI | 52 | 70–120% |
| FXIII | 34 | 70–140% |
| ROTEM Parameters | Before Operation | Reference Value |
|---|---|---|
| EXTEM CT | 75 | 38–79 s |
| EXTEM CFT | 412 | 34–159 s |
| EXTEM AA | 47 | 63–83° |
| EXTEM MCF | 35 | 50–72 mm |
| INTEM CT | 175 | 100–240 s |
| INTEM CFT | 405 | 30–110 s |
| INTEM AA | 52 | 70–83° |
| INTEM MCF | 33 | 50–72 mm |
| FIBTEM MCF | 16 | 9–25 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekic, N.; Tadic, B.; Djordjevic, V.; Basaric, D.; Micev, M.; Vucelic, D.; Mitrovic, M.; Grubor, N. Splenectomy for Visceral Leishmaniasis Out of an Endemic Region: A Case Report and Literature Review. Medicina 2022, 58, 184. https://doi.org/10.3390/medicina58020184
Lekic N, Tadic B, Djordjevic V, Basaric D, Micev M, Vucelic D, Mitrovic M, Grubor N. Splenectomy for Visceral Leishmaniasis Out of an Endemic Region: A Case Report and Literature Review. Medicina. 2022; 58(2):184. https://doi.org/10.3390/medicina58020184
Chicago/Turabian StyleLekic, Nebojsa, Boris Tadic, Vladimir Djordjevic, Dragan Basaric, Marjan Micev, Dragica Vucelic, Milica Mitrovic, and Nikola Grubor. 2022. "Splenectomy for Visceral Leishmaniasis Out of an Endemic Region: A Case Report and Literature Review" Medicina 58, no. 2: 184. https://doi.org/10.3390/medicina58020184
APA StyleLekic, N., Tadic, B., Djordjevic, V., Basaric, D., Micev, M., Vucelic, D., Mitrovic, M., & Grubor, N. (2022). Splenectomy for Visceral Leishmaniasis Out of an Endemic Region: A Case Report and Literature Review. Medicina, 58(2), 184. https://doi.org/10.3390/medicina58020184







