Clinical Manifestations and Outcomes of Tubercular Uveitis in Taiwan—A Ten-Year Multicenter Retrospective Study
Abstract
:1. Introduction
2. Material and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Glassroth, J.; Robins, A.G.; Snider, D.E., Jr. Tuberculosis in the 1980s. N. Engl. J. Med. 1980, 302, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Baydur, A. The spectrum of extrapulmonary tuberculosis. West J. Med. 1977, 126, 253–262. [Google Scholar] [PubMed]
- Gupta, A.; Gupta, V. Tubercular Posterior Uveitis. Int. Ophthalmol. Clin. 2005, 45, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Sen, H.N.; Colyer, M.; Zapor, M.; Wroblewski, K. Update on ocular tuberculosis. Curr. Opin. Ophthalmol. 2012, 23, 551–556. [Google Scholar] [CrossRef]
- Lou, S.M.; Montgomery, P.A.; Larkin, K.L.; Winthrop, K.; Zierhut, M.; Rosenbaum, J.T. Diagnosis and Treatment for Ocular Tuberculosis among Uveitis Specialists: The International Perspective. Ocul. Immunol. Inflamm. 2015, 23, 32–39. [Google Scholar] [CrossRef]
- Albert, D.M.; Raven, M.L. Ocular Tuberculosis. Microbiol. Spectr. 2016, 4, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Pounder, J.I.; Aldous, W.K.; Woods, G.L. Comparison of real-time polymerase chain reaction using the Smart Cycler and the Gen-Probe amplified Mycobacterium tuberculosis direct test for detection of M. tuberculosis complex in clinical specimens. Diagn. Microbiol. Infect. Dis. 2006, 54, 217–222. [Google Scholar] [CrossRef]
- Sharma, P.; Bansal, R.; Gupta, V.; Gupta, A. Diagnosis of tubercular uveitis by quantitative polymerase chain reaction. J. Ophthalmic Inflamm. Infect. 2010, 1, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Gunasekeran, D.V.; Gupta, B.; Cardoso, J.; Pavesio, C.E.; Agrawal, R. Visual Morbidity and Ocular Complications in Presumed Intraocular Tuberculosis: An Analysis of 354 Cases from a Non-Endemic Population. Ocul. Immunol. Inflamm. 2017, 26, 865–869. [Google Scholar] [CrossRef]
- Ang, M.; Vasconcelos-Santos, D.V.; Sharma, K.; Accorinti, M.; Sharma, A.; Gupta, A.; Rao, N.A.; Chee, S.-P. Diagnosis of Ocular Tuberculosis. Ocul. Immunol. Inflamm. 2016, 26, 208–216. [Google Scholar] [CrossRef]
- Gupta, B.; Agrawal, R.; Swampillai, A.J.; Lim, R.H.; Kee, A.; Gunasekaran, D.; Pavesio, C. Ocular manifestations of tuberculosis: An update. Expert Rev. Ophthalmol. 2016, 11, 145–154. [Google Scholar] [CrossRef]
- Gunasekeran, D.V.; Agrawal, R.; Agarwal, A.; Carreno, E.; Raje, D.; Aggarwal, K.; Kon, O.M.; Nguyen, Q.D.; Pavesio, C.; Gupta, V.; et al. THE Collaborative Ocular Tuberculosis Study (COTS)-1: A multinational review of 251 patients with tubercular retinal vasculitis. Retina 2019, 39, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Gupta, A.; Rao, N.A. Intraocular tuberculosis—an update. Surv Ophthalmol. 2007, 52, 561–587. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bansal, R.; Gupta, V.; Sharma, A.; Bambery, P. Ocular Signs Predictive of Tubercular Uveitis. Am. J. Ophthalmol. 2010, 149, 562–570. [Google Scholar] [CrossRef]
- Nayak, S.; Basu, S.; Singh, M.K. Presumed Tubercular Retinal Vasculitis with Serpiginous-like Choroiditis in the Other Eye. Ocul. Immunol. Inflamm. 2011, 19, 361–362. [Google Scholar] [CrossRef]
- Bansal, R.; Gupta, A.; Gupta, V.; Dogra, M.R.; Sharma, A.; Bambery, P. Tubercular Serpiginous-Like Choroiditis Presenting as Multifocal Serpiginoid Choroiditis. Ophthalmology 2012, 119, 2334–2342. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, V.; Arora, S.; Dogra, M.R.; Bambery, P.P. CR-positive tubercular retinal vasculitis: Clinical characteristics and management. Retina 2001, 21, 435–444. [Google Scholar] [CrossRef]
- Shakarchi, F.A. Mode of presentations and management of presumed tuberculous uveitis at a referral center. Iraqi. Postgrad. Med. J. 2015, 14, 91–95. [Google Scholar]
- Gupta, A.; Sharma, A.; Bansal, R.; Sharma, K. Classification of Intraocular Tuberculosis. Ocul. Immunol. Inflamm. 2014, 23, 7–13. [Google Scholar] [CrossRef]
- Nora, R.L.D.; Van Velthoven, M.E.J.; Dam-Van Loon, N.H.T.; Misotten, T.; Bakker, M.; Van Hagen, M.P.; Rothova, A. Clinical manifestations of patients with intraocular inflammation and positive QuantiFERON-TB Gold In-Tube test in a country nonendemic for tuberculosis. Am. J. Ophthalmol. 2014, 157, 754–761. [Google Scholar] [CrossRef]
- Gan, W.-L.; Jones, N.P. Serpiginous-like choroiditis as a marker for tuberculosis in a non-endemic area. Br. J. Ophthalmol. 2013, 97, 644–647. [Google Scholar] [CrossRef]
- Nayak, S.; Acharjya, B. Mantoux test and its interpretation. Indian Dermatol. Online J. 2012, 3, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-C.; Lin, C.-J.; Chen, H.-S.; Lee, T.-L. Negative Mantoux test in a patient with definite pulmonary and ocular tuberculosis. Taiwan J. Ophthalmol. 2015, 5, 182–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Agrawal, R.; Gunasekaran, D.V.; Raje, D.; Gupta, B.; Aggarwal, K.; Murthy, S.L.; Westcott, M.; Chee, S.P.; McCluskey, P.; et al. The Collaborative Ocular Tuberculosis Study (COTS)-1 Report 3: Polymerase Chain Reaction in the Diagnosis and Management of Tubercular Uveitis: Global Trends. Ocul. Immunol. Inflamm. 2017, 27, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Wong, W.L.; Kiew, S.Y.; Li, X.; Chee, S.P. Prospective head-to-head study comparing two commercial interferon-gamma release assays for the diagnosis of tuberculous uveitis. Am. J. Ophthalmol. 2014, 157, 1306–1314.e4. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Sibley, C.H.; Lin, P. Retinal vasculitis. Curr. Opin. Rheumatol. 2016, 28, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.; Gunasekeran, D.V.; Gonzalez-Lopez, J.J.; Cardoso, J.; Gupta, B.; Addison, P.K.; Westcott, M.; Pavesio, C.E.F. Peripheral retinal vasculitis: Analysis of 110 consecutive cases and a contemporary reappraisal of tubercular etiology. Retina 2017, 37, 112–117. [Google Scholar] [CrossRef] [PubMed]
- El-Asrar, A.M.; Al-Kharashi, S.A. Full panretinal photocoagulation and early vitrectomy improve prognosis of retinal vasculitis associated with tuberculoprotein hypersensitivity (Eales’ disease). Br. J. Ophthalmol. 2002, 86, 1248–1251. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Nayak, S.; Padhi, T.R.; Das, T. Progressive ocular inflammation following anti-tubercular therapy for presumed ocular tuberculosis in a high-endemic setting. Eye 2013, 27, 657–662. [Google Scholar] [CrossRef] [Green Version]
- Kee, A.R.; Gonzalez-Lopez, J.J.; Al-Hity, A.; Gupta, B.; Lee, C.S.; Gunasekeran, D.V.; Jayabalan, N.; Grant, R.; Kon, O.M.; Gupta, V.; et al. Anti-tubercular therapy for intraocular tuberculosis: A systematic review and meta-analysis. Surv. Ophthalmol. 2016, 61, 628–653. [Google Scholar] [CrossRef] [Green Version]
- Tomkins-Netzer, O.; Leong, B.C.; Zhang, X.; Lightman, S.; McCluskey, P.J.; Lee, A.; Leahy, K.; Zagora, S.; Younan, C.; Fung, A.; et al. Effect of Antituberculous Therapy on Uveitis Associated with Latent Tuberculosis. Am. J. Ophthalmol. 2018, 190, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Testi, I.; Bodaghi, B.; Barisani-Asenbauer, T.; McCluskey, P.; Agarwal, A.; Kempen, J.H.; Gupta, A.; Smith, J.R.; de Smet, M.D.; et al. Collaborative Ocular Tuberculosis Study Consensus Group. Collaborative Ocular Tuberculosis Study Consensus Guidelines on the Management of Tubercular Uveitis-Report 2: Guidelines for Initiating Antitubercular Therapy in Anterior Uveitis, Intermediate Uveitis, Panuveitis, and Retinal Vasculitis. Ophthalmology 2021, 128, 277–287. [Google Scholar] [PubMed]
- Kroesen, V.M.; Gröschel, M.I.; Martinson, N.; Zumla, P.S.A.; Maeurer, M.; van der Werf, T.; Vilaplana, C. Non-Steroidal Anti-inflammatory Drugs as Host-Directed Therapy for Tuberculosis: A Systematic Review. Front. Immunol. 2017, 8, 772. [Google Scholar] [CrossRef]
- Nau, R.; Sörgel, F.; Eiffert, H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthony, R.M.; Hertog, A.D.; Mansjö, M.; Werngren, J. New insights into the mechanism of action of pyrazinamide, implications for susceptibility testing, and future regimens. Int. J. Mycobacteriol. 2016, 5 (Suppl. 1), S71–S72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koul, P.A. Ocular toxicity with ethambutol therapy: Timely recaution. Lung India 2015, 32, 1–3. [Google Scholar] [CrossRef]
- Murdoch, I.E.; Morris, S.; Cousens, S.N. People and eyes: Statistical approaches in ophthalmology. Br. J. Ophthalmol. 1998, 82, 971–973. [Google Scholar] [CrossRef] [Green Version]
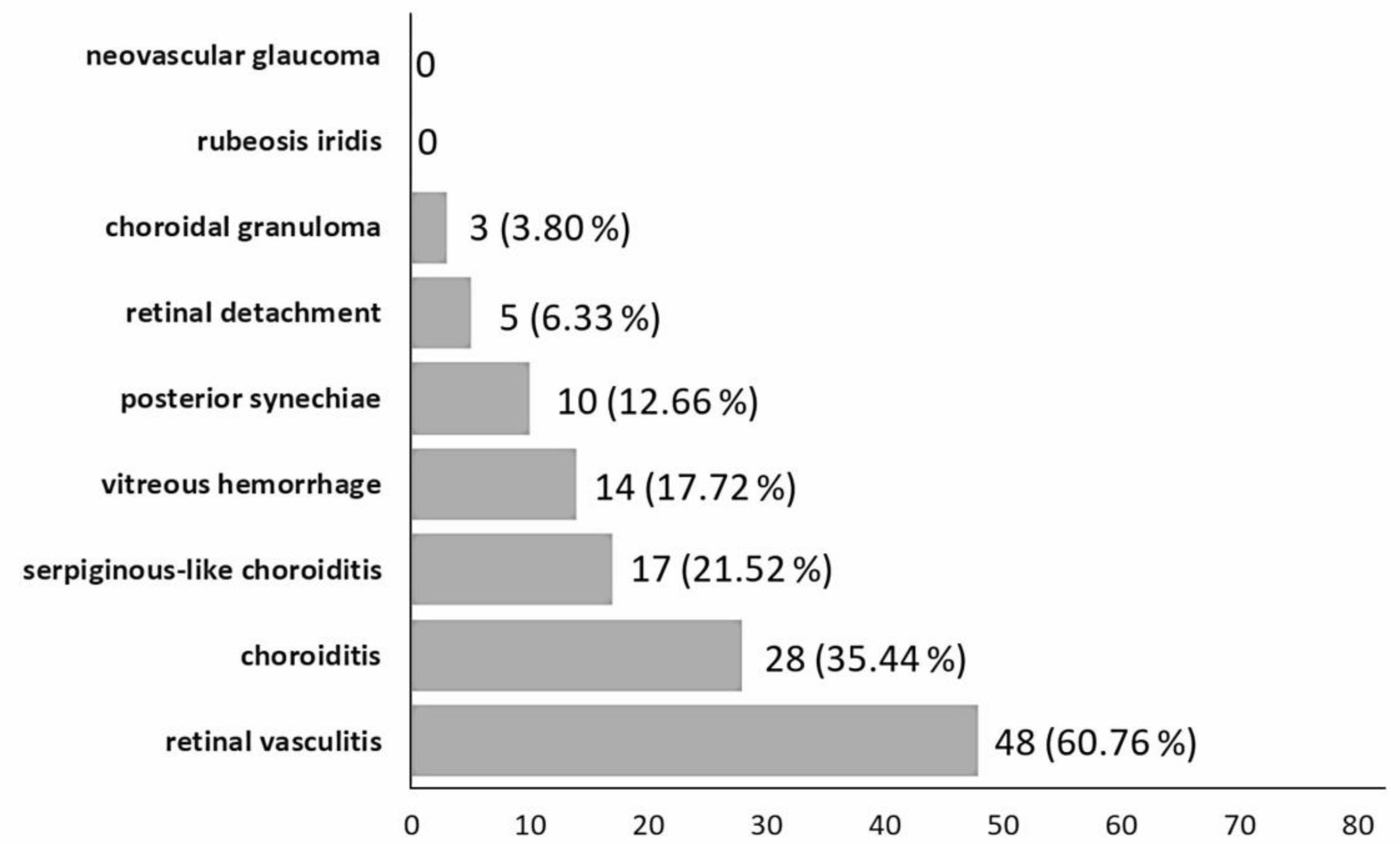

| No. of Eyes | 79 (51 Patients) |
|---|---|
| Male/female (% of male) | 24/27 (47.06%) |
| Mean age (years ± SD) | 48.9 ± 16.4 |
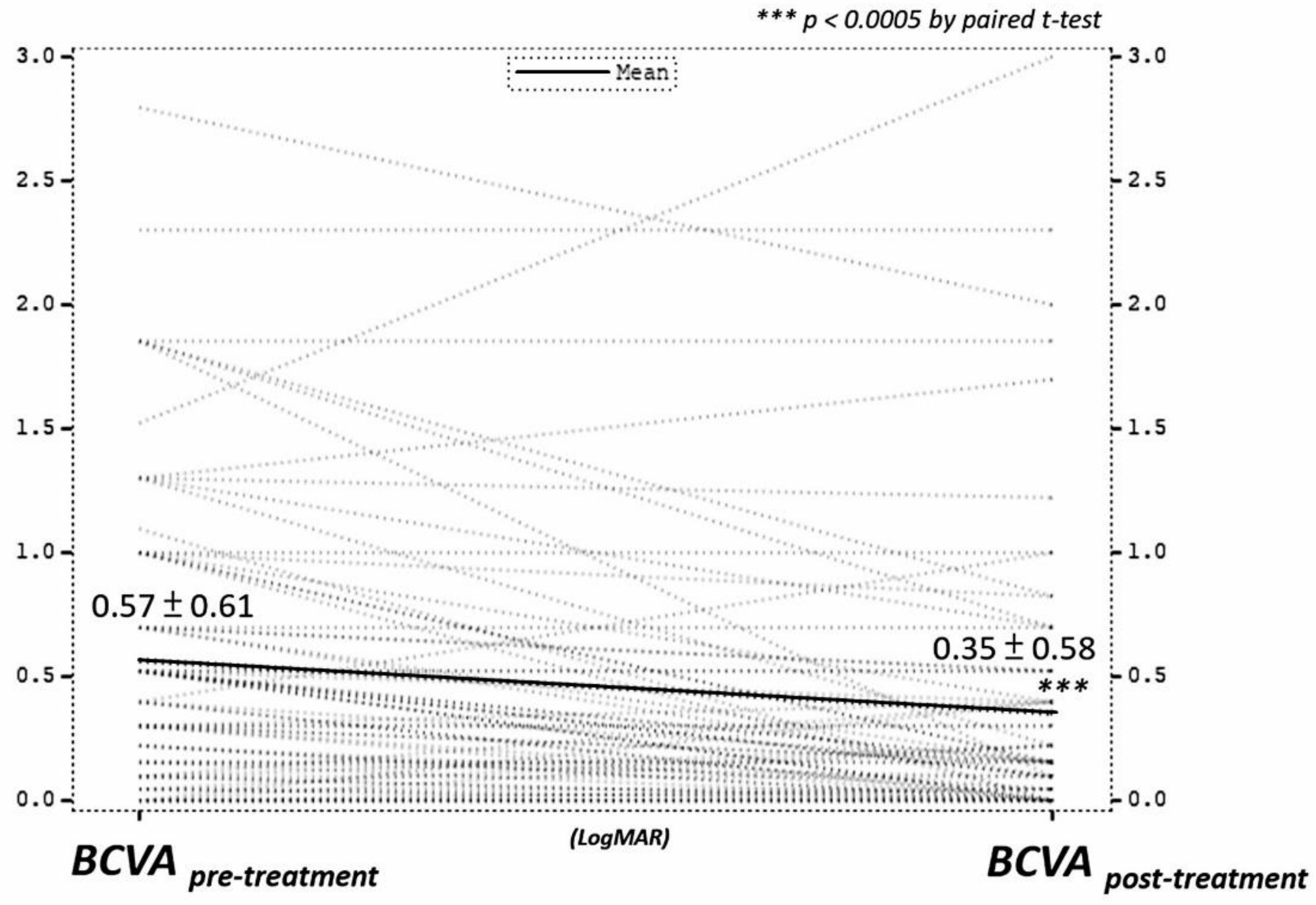
| Mean BCVA at presentation (LogMAR) | 0.57 ± 0.61 |
| Mean BCVA at last F/U (LogMAR) | 0.35 ± 0.58 |
| Mean change of BCVA (LogMAR) | −0.21 ± 0.45 |
| No. of recurrence (%) | 7 (8.86%) |
| Follow-up period (months) | 18.20 ± 24.05 |
| Diagnostic Tests | No. of Positive Test | No. of Eyes | % | |||||
|---|---|---|---|---|---|---|---|---|
| MT | BAL | IFPCR | CXR | CCT | IGRA | |||
| + | + | + | + | + | 5 | 2 | 3% | |
| + | + | + | 3 | 7 | 9% | |||
| + | + | + | 3 | 1 | 1% | |||
| + | + | 2 | 1 | 1% | ||||
| + | + | 2 | 2 | 3% | ||||
| + | + | 2 | 4 | 5% | ||||
| + | + | 2 | 9 | 11% | ||||
| + | + | 2 | 2 | 3% | ||||
| + | + | 2 | 1 | 1% | ||||
| + | + | 2 | 2 | 3% | ||||
| + | 1 | 40 | 51% | |||||
| + | 1 | 1 | 1% | |||||
| + | 1 | 5 | 6% | |||||
| + | 1 | 2 | 3% | |||||
| 79 | 100% | |||||||
| Analysis of Variance | |||||
|---|---|---|---|---|---|
| Source | DF | Sum of Squares | Mean Square | F Value | Pr > F |
| Model | 4 | 4.02949 | 1.00737 | 6.86 | 0.0001 |
| Error | 57 | 8.37522 | 0.14693 | ||
| Corrected Total | 61 | 12.40471 | |||
| Variable | Parameter Estimate | Standard Error | Type II SS | F Value | Pr > F |
| Intercept | 0.05795 | 0.09698 | 0.05246 | 0.36 | 0.5525 |
| ValmFirst | −0.27612 | 0.08888 | 1.418 | 9.65 | 0.0029 |
| TxEtha | 0.2987 | 0.10758 | 1.13278 | 7.71 | 0.0074 |
| TxPyra | −0.40127 | 0.11489 | 1.79237 | 12.2 | 0.0009 |
| TxVitr | −0.39099 | 0.18499 | 0.65637 | 4.47 | 0.0389 |
| Model R2 | 0.3248 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-J.; Hsia, N.-Y.; Hwang, D.-K.; Hwang, Y.-S.; Chang, Y.-C.; Lee, Y.-C.; Hsu, Y.-R.; Yeh, P.-T.; Lin, C.-P.; Chen, H.-F.; et al. Clinical Manifestations and Outcomes of Tubercular Uveitis in Taiwan—A Ten-Year Multicenter Retrospective Study. Medicina 2022, 58, 376. https://doi.org/10.3390/medicina58030376
Lin C-J, Hsia N-Y, Hwang D-K, Hwang Y-S, Chang Y-C, Lee Y-C, Hsu Y-R, Yeh P-T, Lin C-P, Chen H-F, et al. Clinical Manifestations and Outcomes of Tubercular Uveitis in Taiwan—A Ten-Year Multicenter Retrospective Study. Medicina. 2022; 58(3):376. https://doi.org/10.3390/medicina58030376
Chicago/Turabian StyleLin, Chun-Ju, Ning-Yi Hsia, De-Kuang Hwang, Yih-Shiou Hwang, Yo-Chen Chang, Yueh-Chang Lee, Yung-Ray Hsu, Po-Ting Yeh, Chang-Ping Lin, Hsi-Fu Chen, and et al. 2022. "Clinical Manifestations and Outcomes of Tubercular Uveitis in Taiwan—A Ten-Year Multicenter Retrospective Study" Medicina 58, no. 3: 376. https://doi.org/10.3390/medicina58030376
APA StyleLin, C.-J., Hsia, N.-Y., Hwang, D.-K., Hwang, Y.-S., Chang, Y.-C., Lee, Y.-C., Hsu, Y.-R., Yeh, P.-T., Lin, C.-P., Chen, H.-F., Jan, W.-C., Chiang, W.-Y., & Tsai, M.-L. (2022). Clinical Manifestations and Outcomes of Tubercular Uveitis in Taiwan—A Ten-Year Multicenter Retrospective Study. Medicina, 58(3), 376. https://doi.org/10.3390/medicina58030376






