Diagnosis and Treatment of Primary Inflammatory Choriocapillaropathies (PICCPs): A Comprehensive Overview
Abstract
:1. Introduction
2. Diagnostic Imaging and Investigations
2.1. Fundus Autofluorescence (FAF)
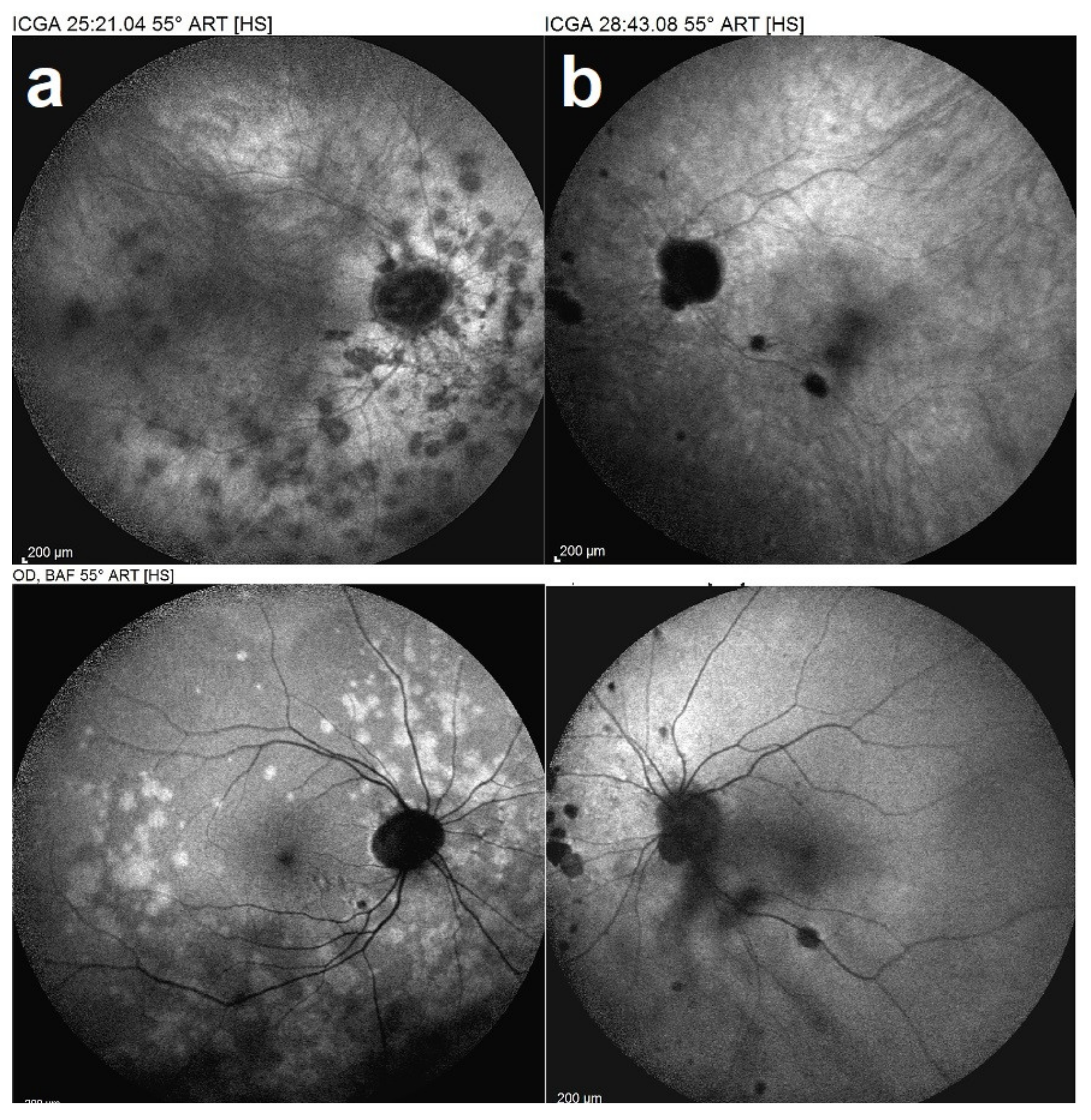
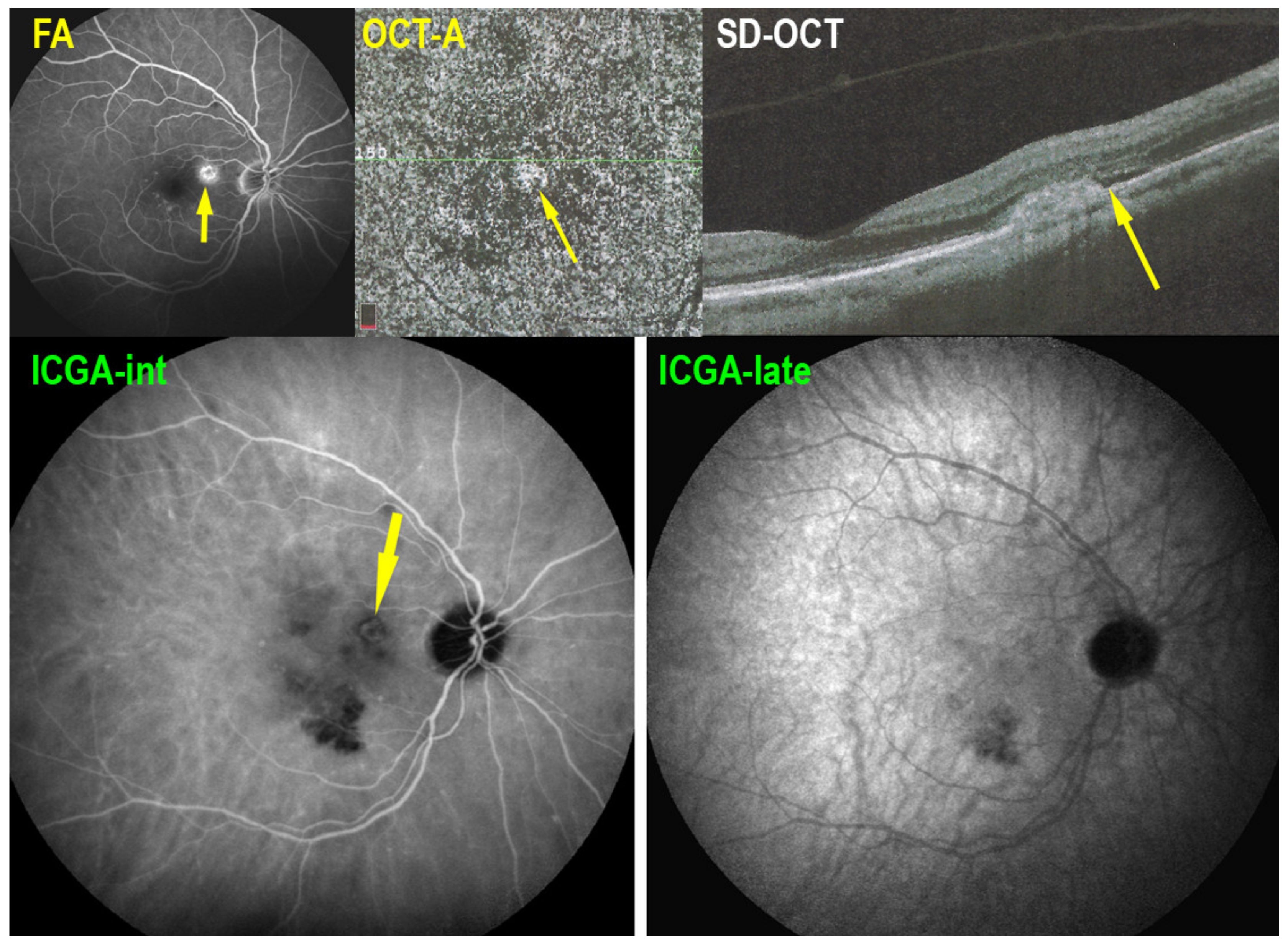
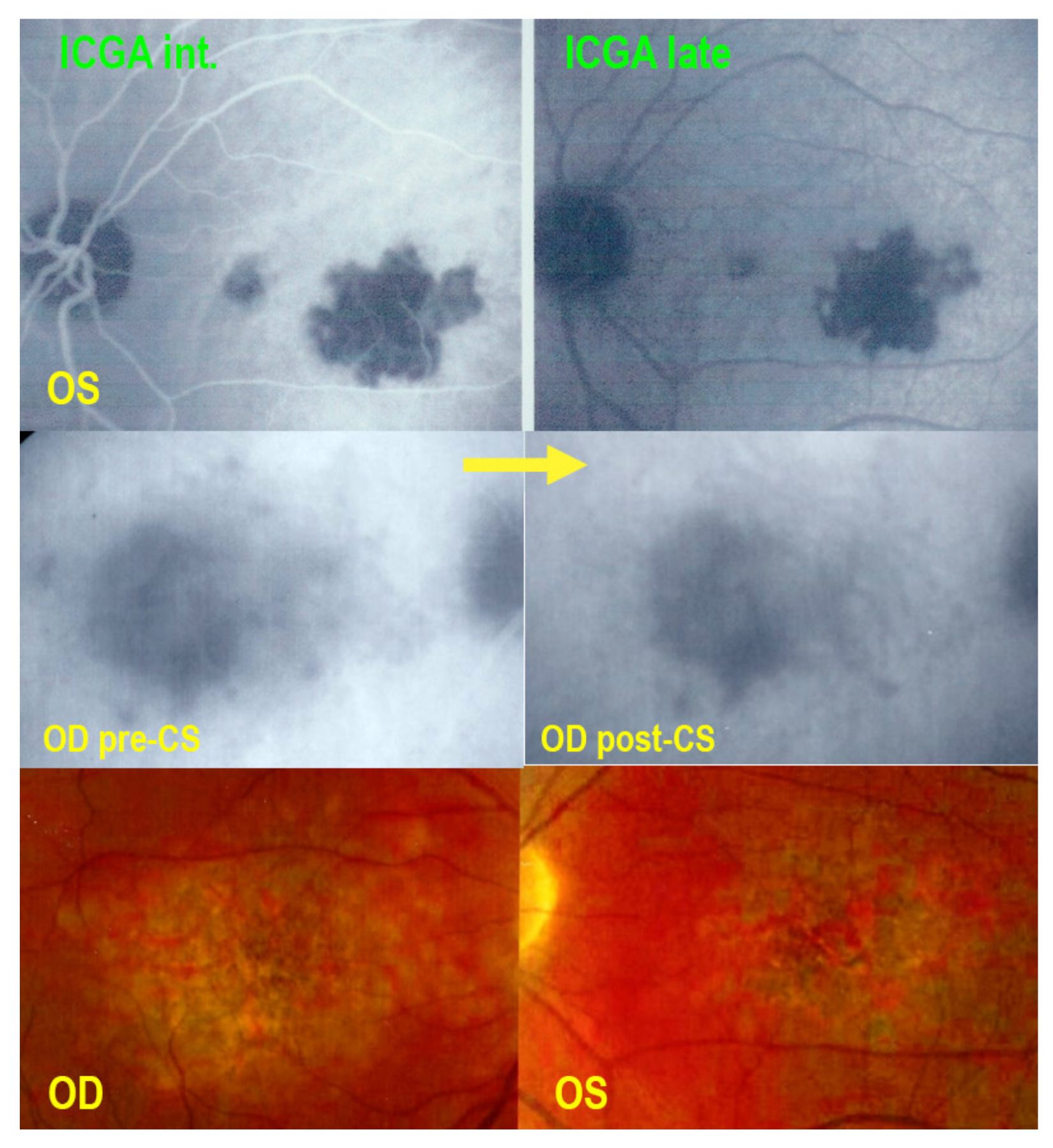
2.2. Indocyanine Green Angiography (ICGA)
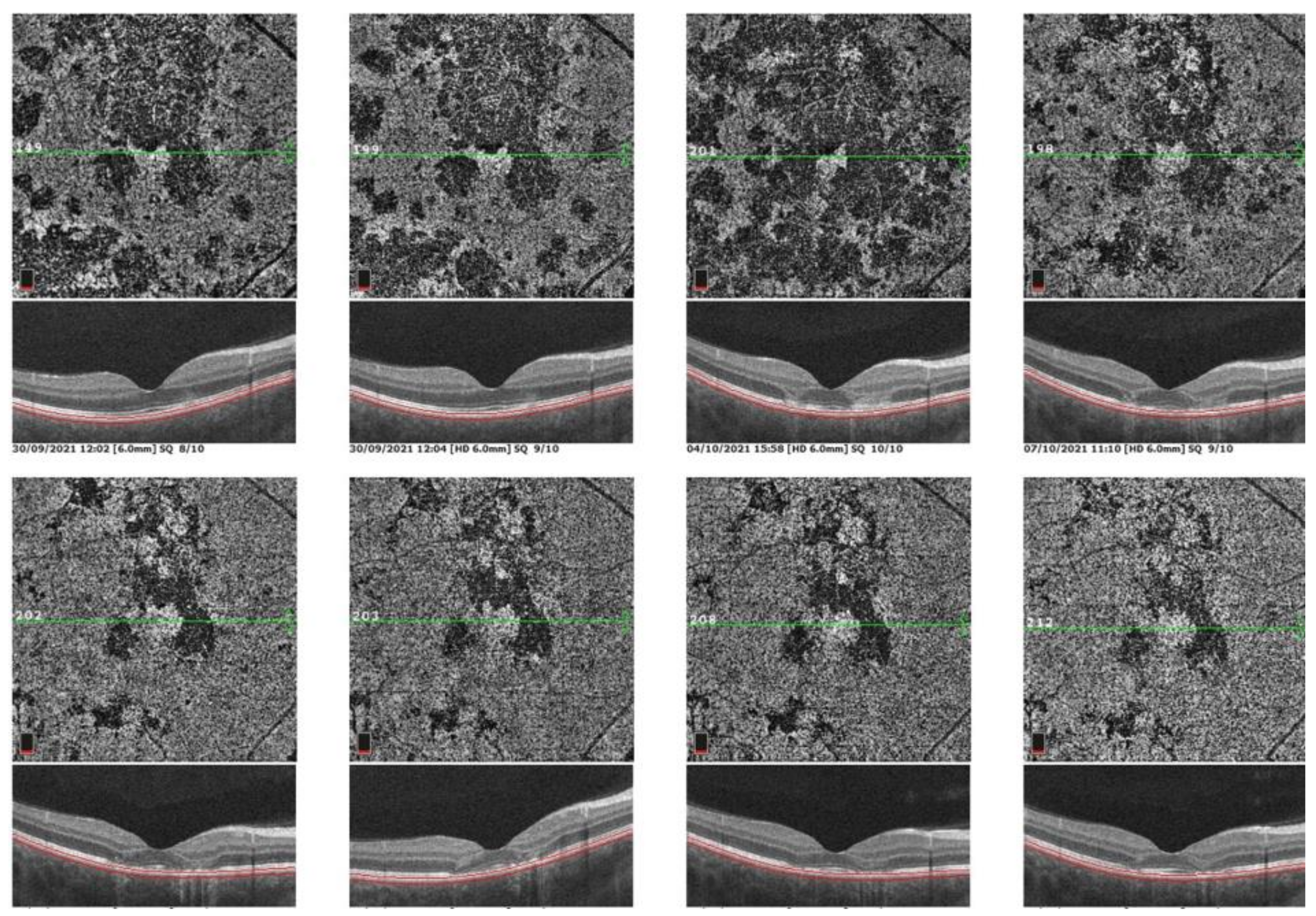
2.3. Spectral Domain-Optical Coherence Tomography (SD-OCT)
2.4. OCT Angiography (OCT-A)
2.5. Fluorescein Angiography (FA)
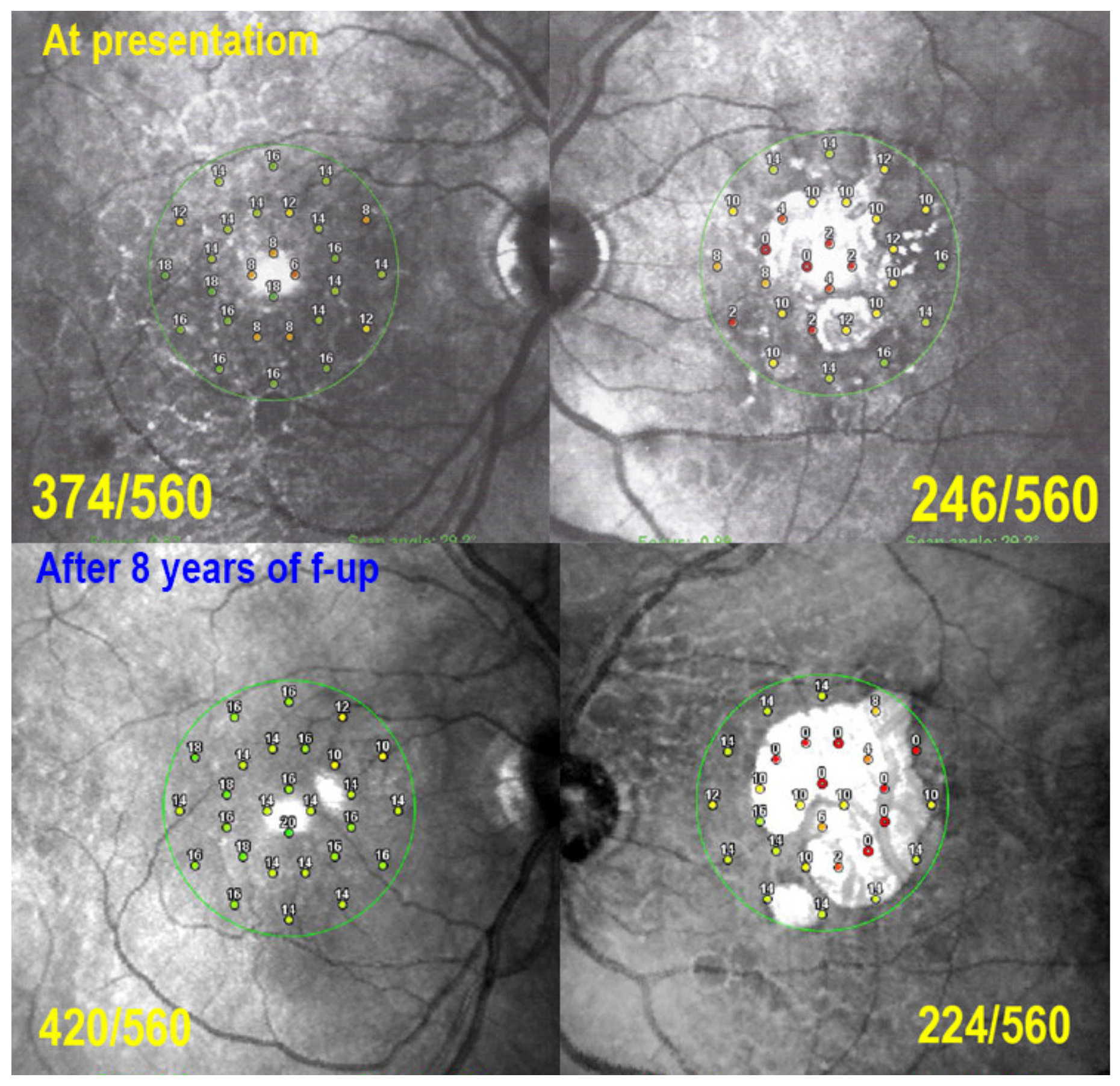
2.6. Visual Field Testing and Microperimetry
| MEWDS (Figure 1) | APMPPE (Figure 2) | MFC (Figure 3) | SC (Figure 4) | |
|---|---|---|---|---|
| ICGA | Intermediate-late hypofluorescence | Early to late hypofluorescence | Early to late hypofluorescence (scars and new lesions) | Hypofluorescence with perilesional hyperfluorescence |
| BL-FAF | Hyperautofluorescence of acute lesions | Hyperautofluorescence in active lesions/hypofluorescence of severe acute and atrophic lesions | Hyperautofluorescence (active lesions) Hypoautofluorescence (atrophic lesions/scars) | Active: Hypoautofluorescence surrounded by hyperautofluorescent halo Semi-Active: linear hypoautofluorescence around hyperautofluorescent lesion Inactive: Hypoautofluorescence |
| SD-OCT | Loss of photoreceptor OS Interruption and/or damage to IS/OS line | Thickening and denaturation of outer retina (hyperreflective area occupying the outer retina) | Loss of photoreceptors OS/Interruption and/or damage to IS/OS line atrophic lesions/ICNV | Hyperreflective accumulations in the outer retina/ICNV |
| OCT-A | Absent or subtle signs of choriocapillaris drop-out | Patchy choriocapillaris drop-out | Patchy Choriocapillaris drop-out/ICNV | Geographic areas of choriocapillaris drop-out/ICNVs |
| FA | Subtle early to late hyperfluorescence | Early hypofluorescence/late hyperfluorescence (staining and pooling) in severe non-perfusion | Early hypofluorescence/late hyperfluorescence of scared-atrophic lesions No FA signs of new lesions | Scars/atrophy: early hypofluorescence and late hyperfluorescence (window defect) surrounded by late more hyperfluorescent rim, if lesions are progressing |
3. Clinicopathology of PICCPs
4. Treatment
4.1. Corticosteroid Therapy
4.2. Immunomodulatory Agents
4.2.1. Antimetabolites
4.2.2. Calcineurin Inhibitors
Cyclosporine (CsA) (Sandimmun®)
Tacrolimus (FK 506) (Prograf®)
Strategy for Severe Choriocapillaritis
4.2.3. Biologic Agents
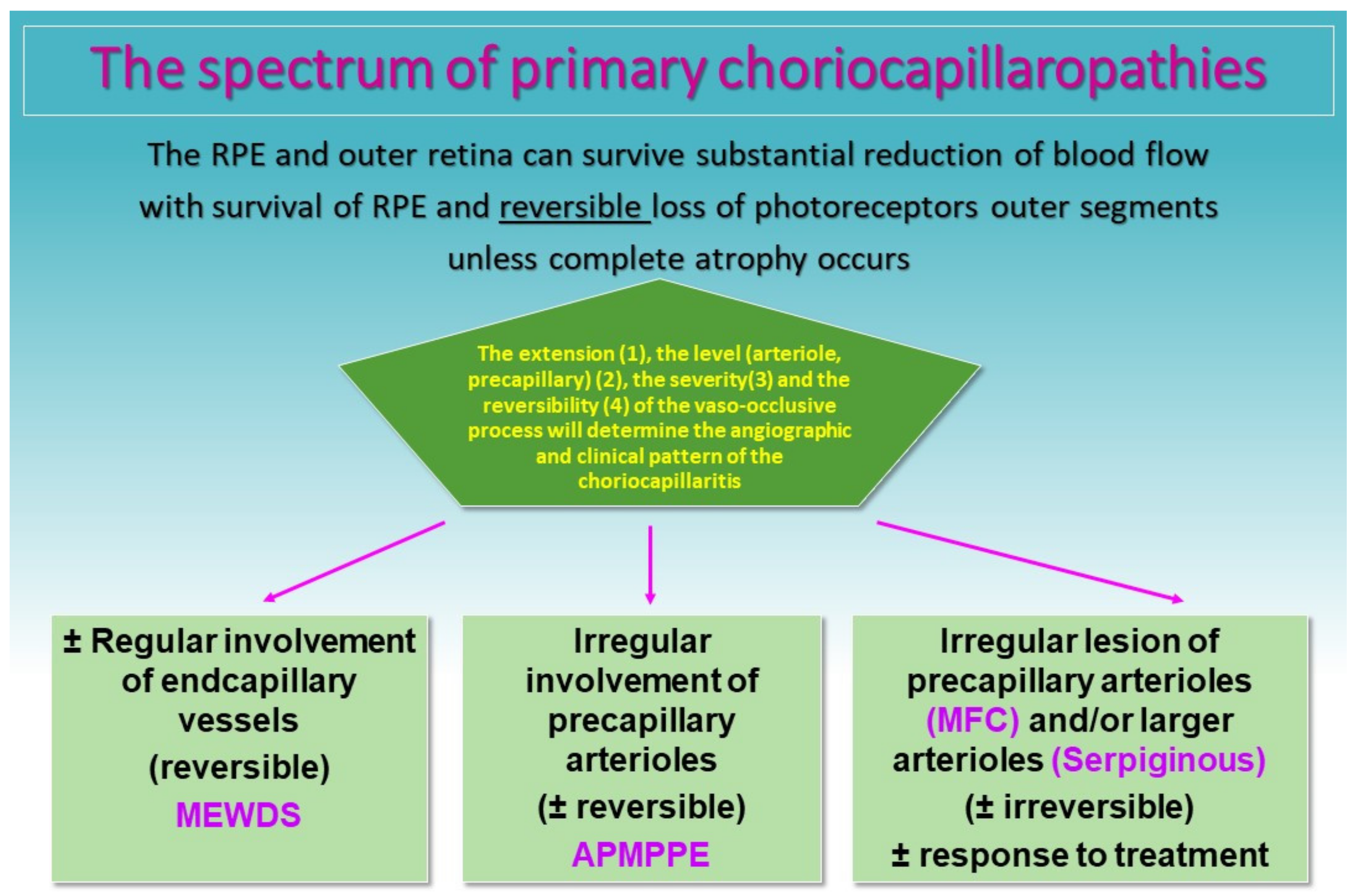
5. PICCPs Entities
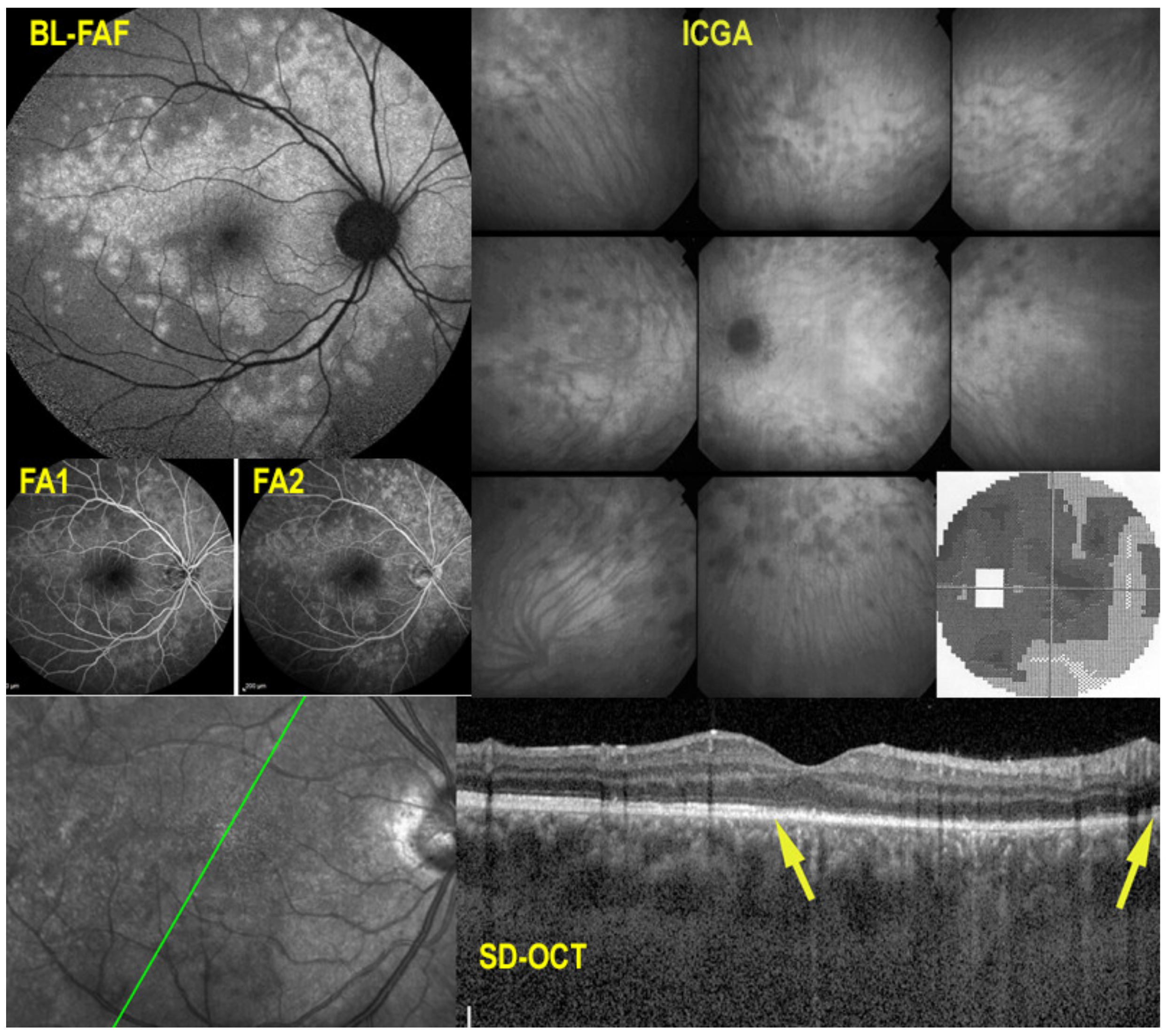
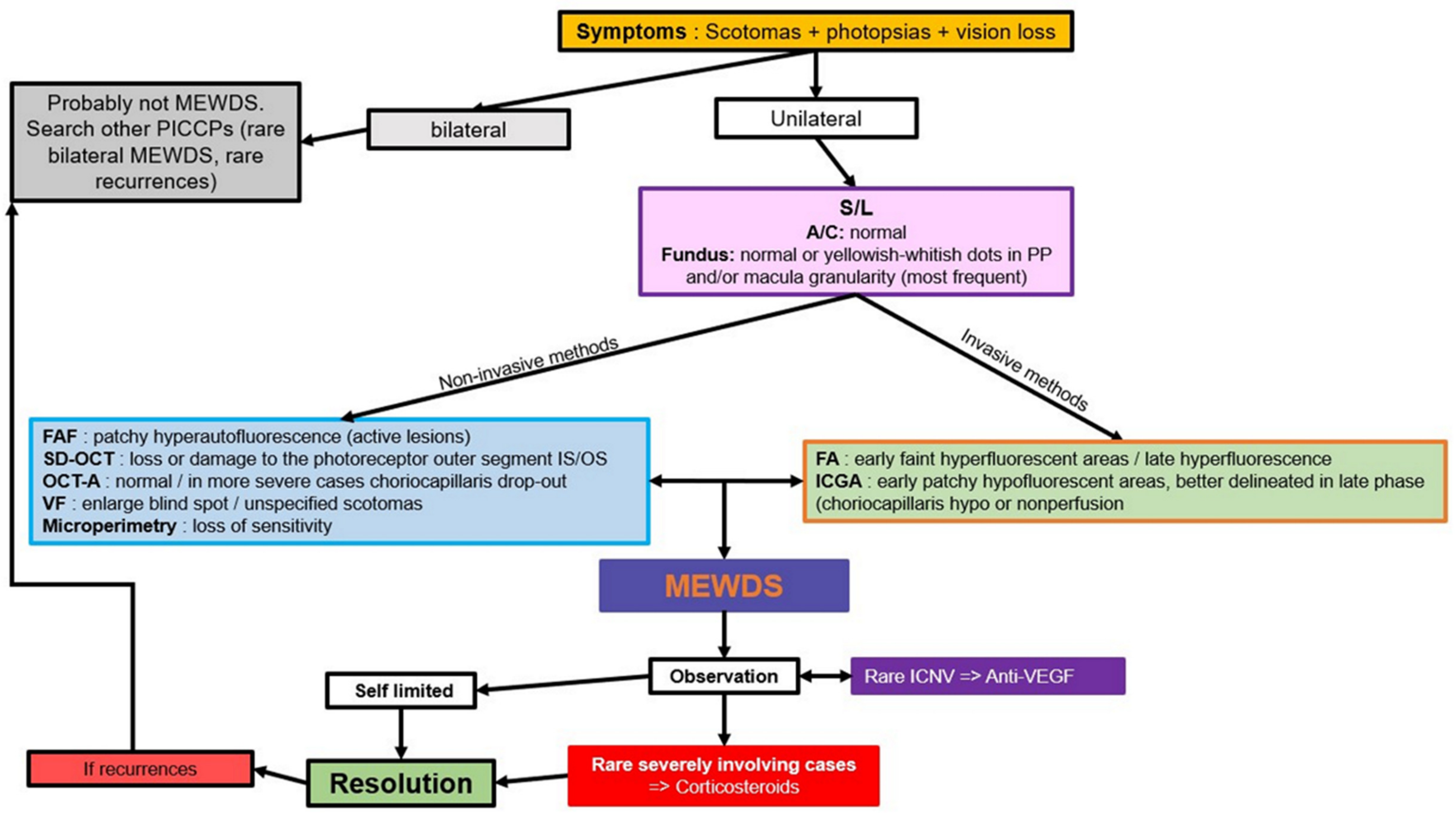
5.1. MEWDS
Illustrative MEWDS Case
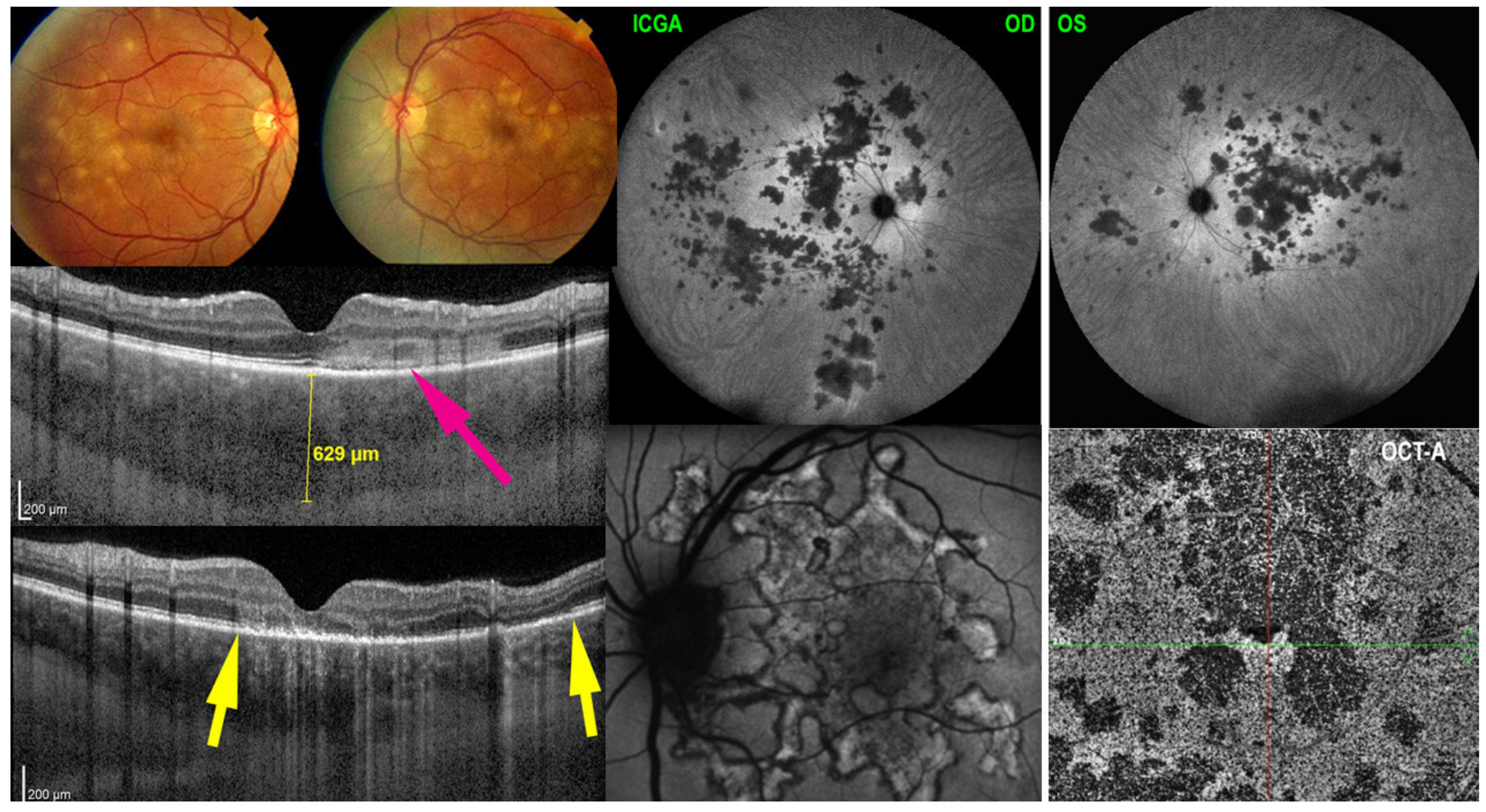
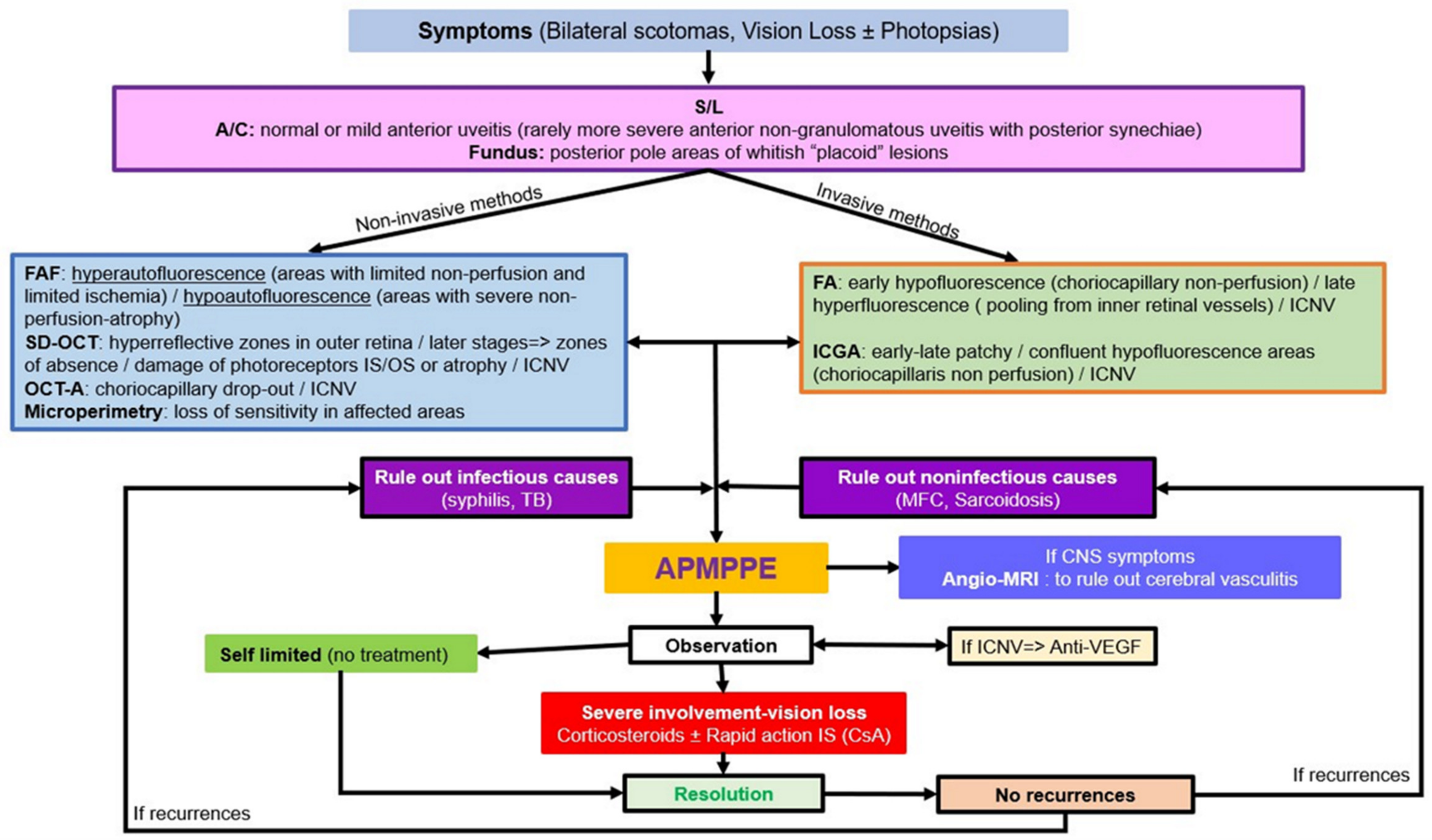
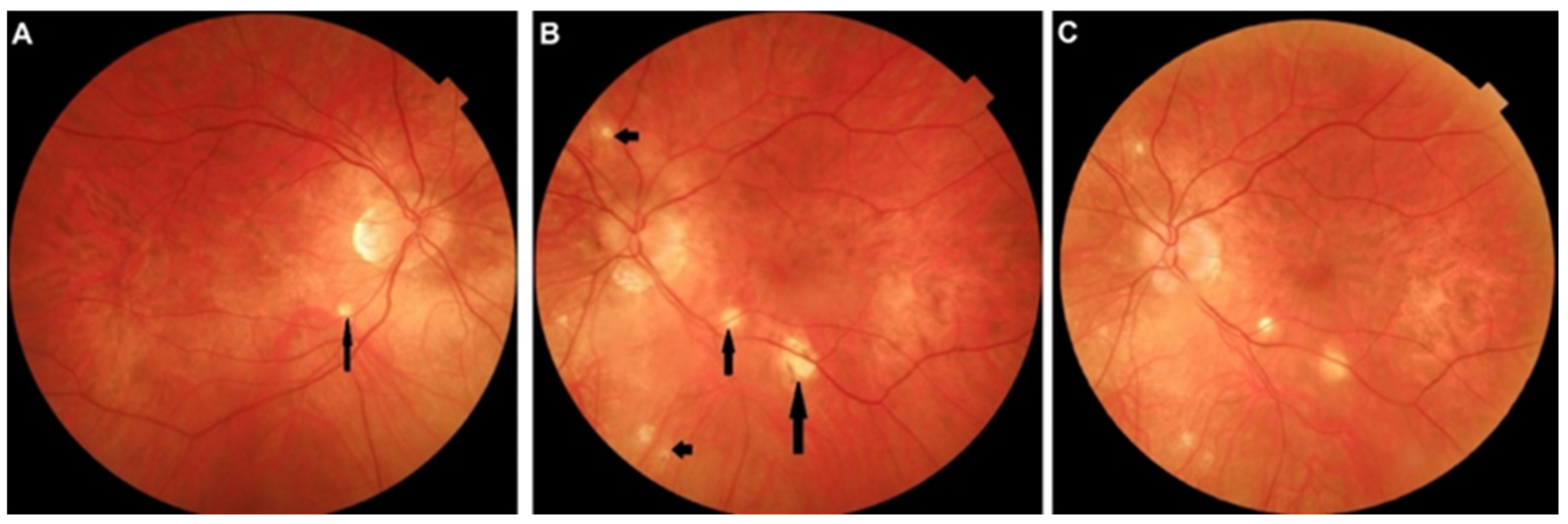
5.2. APMPPE
Illustrative APMPPE/AMIC Case
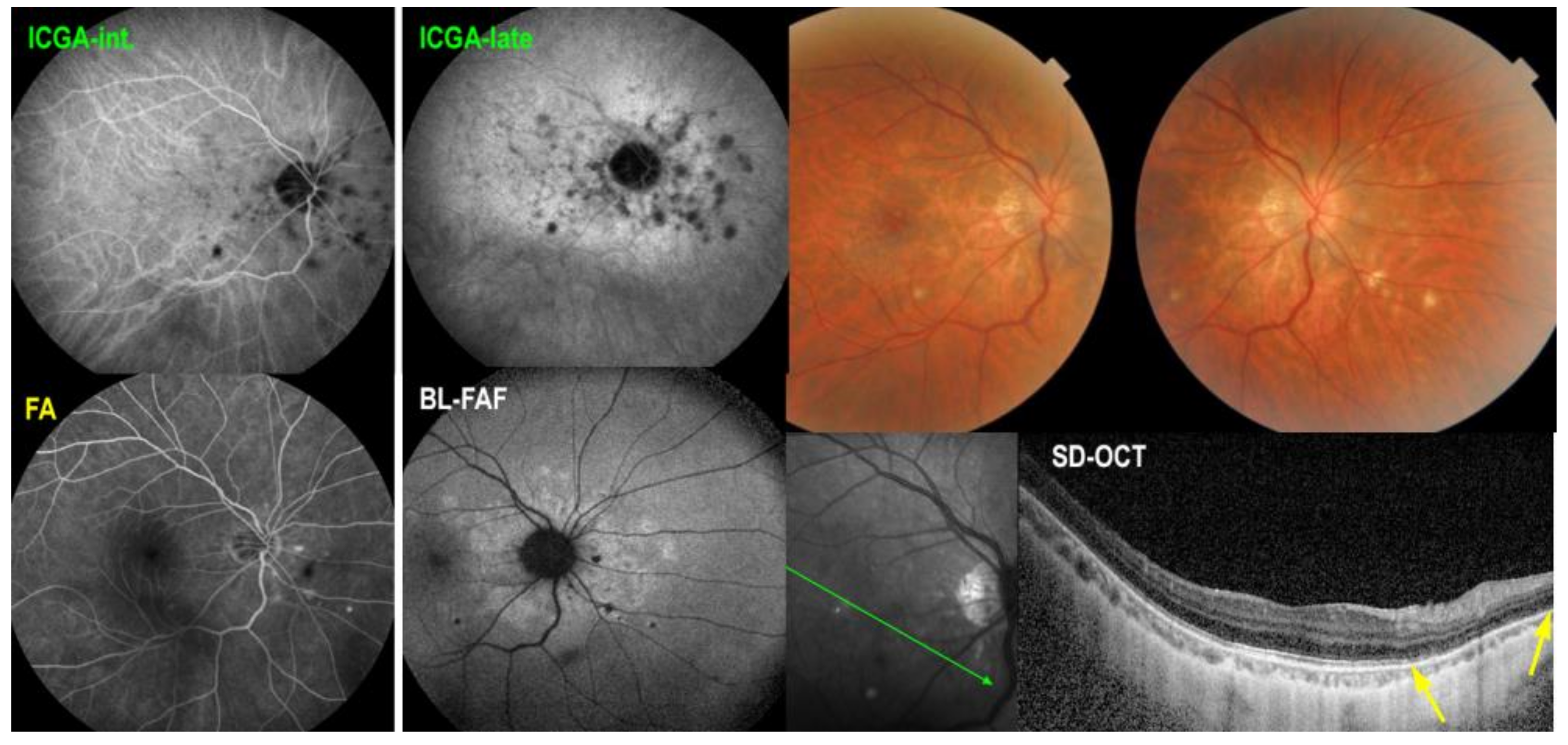
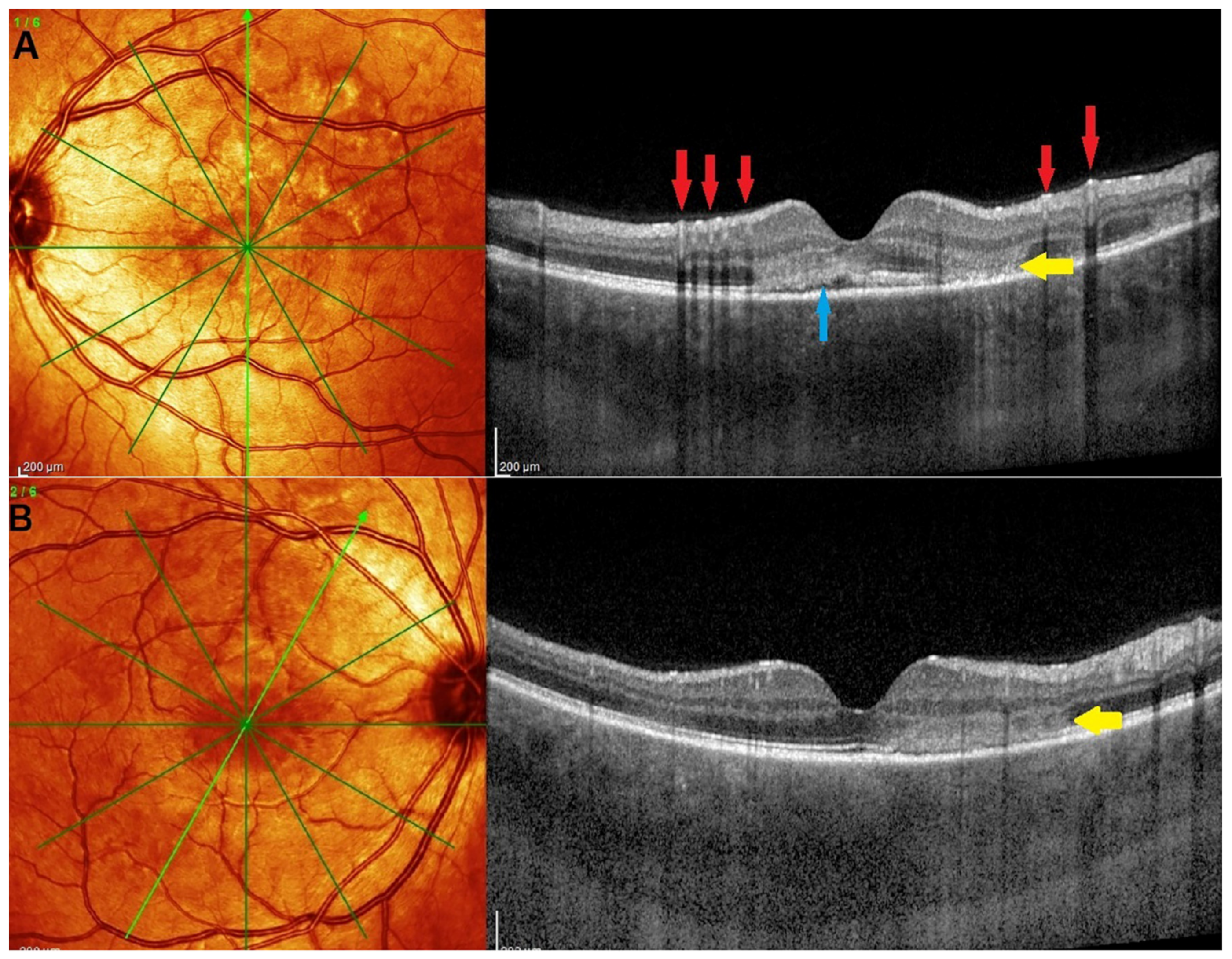
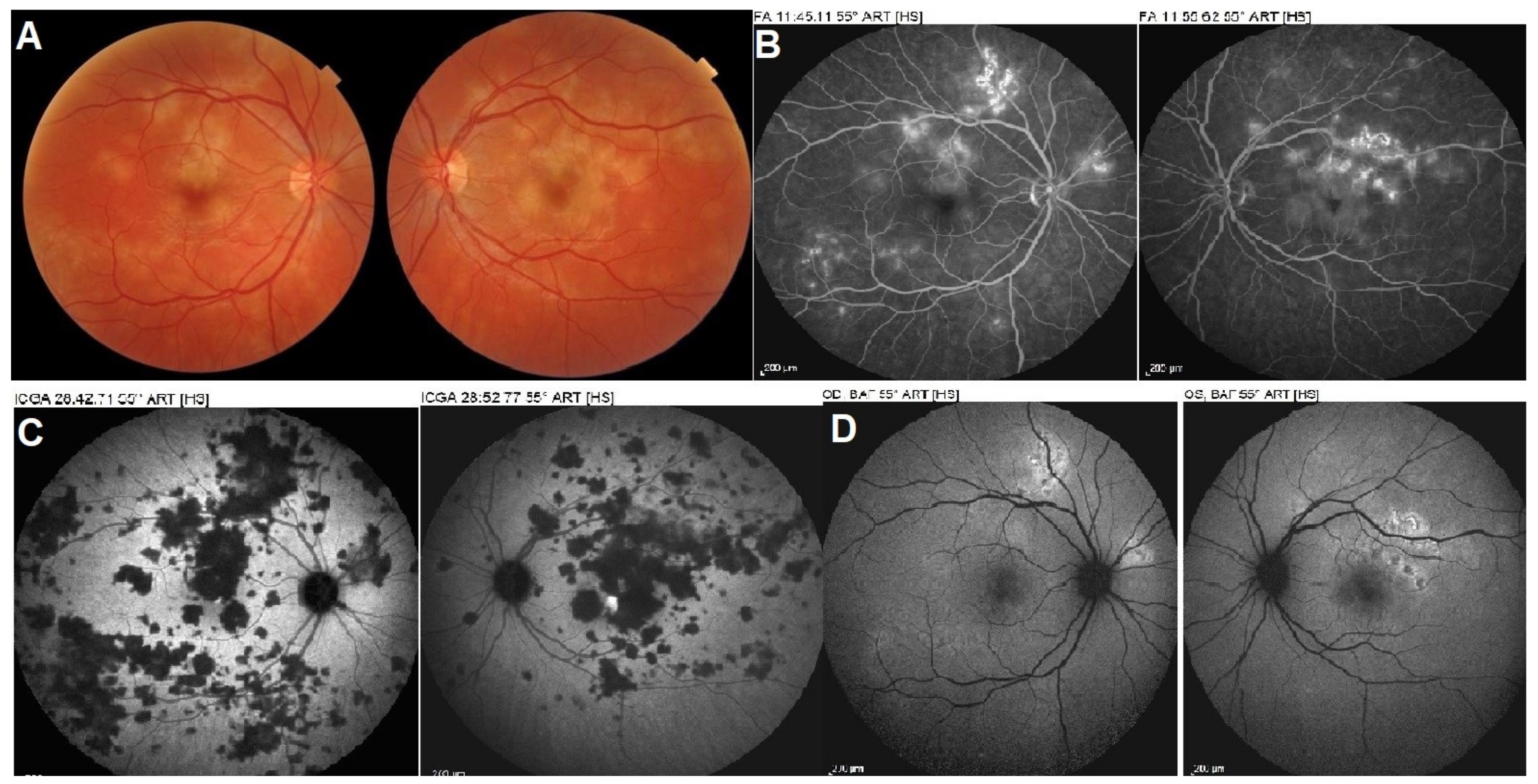
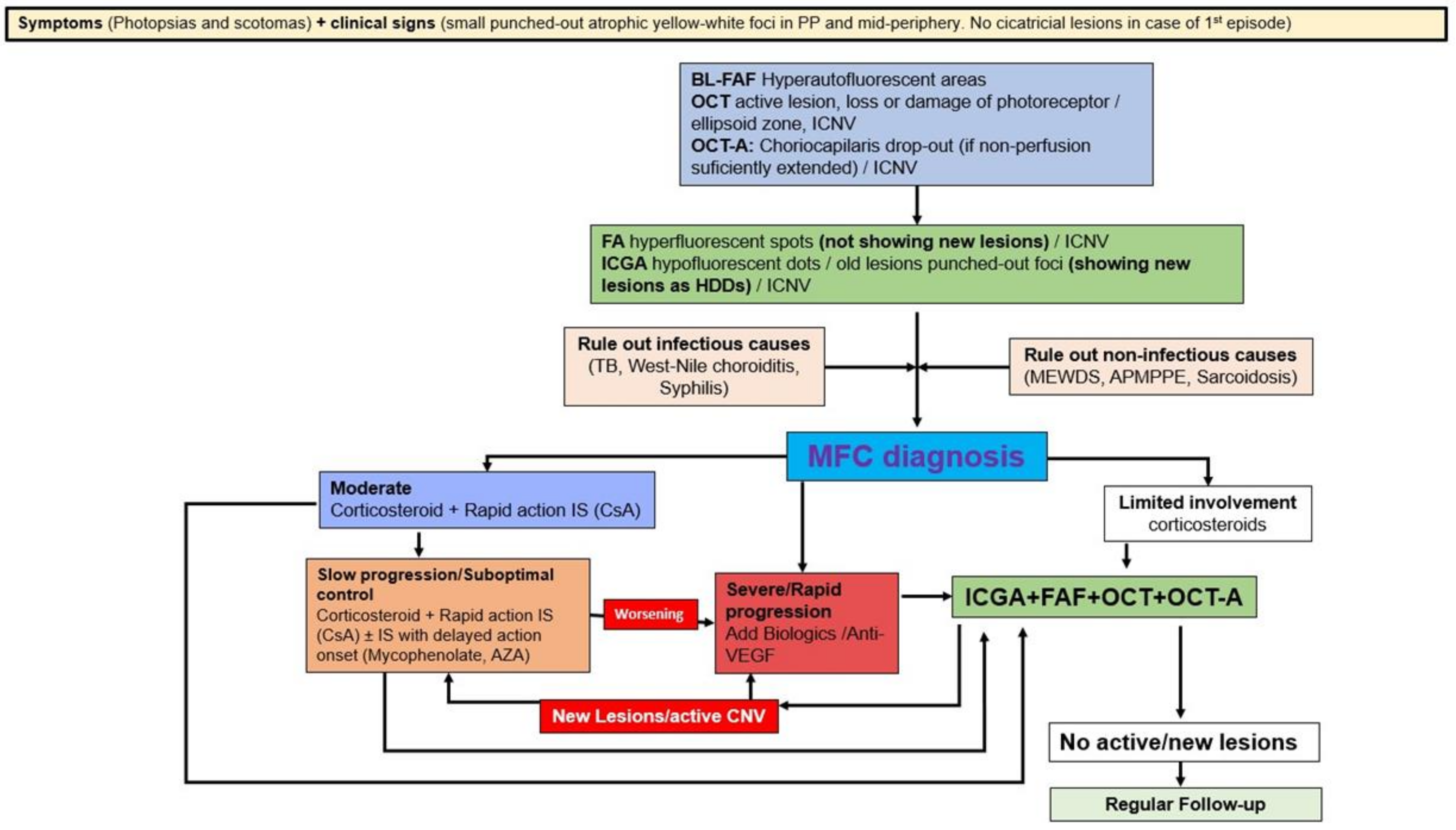
5.3. Idiopathic Multifocal Choroiditis (MFC/PIC)
Illustrative MFC Case
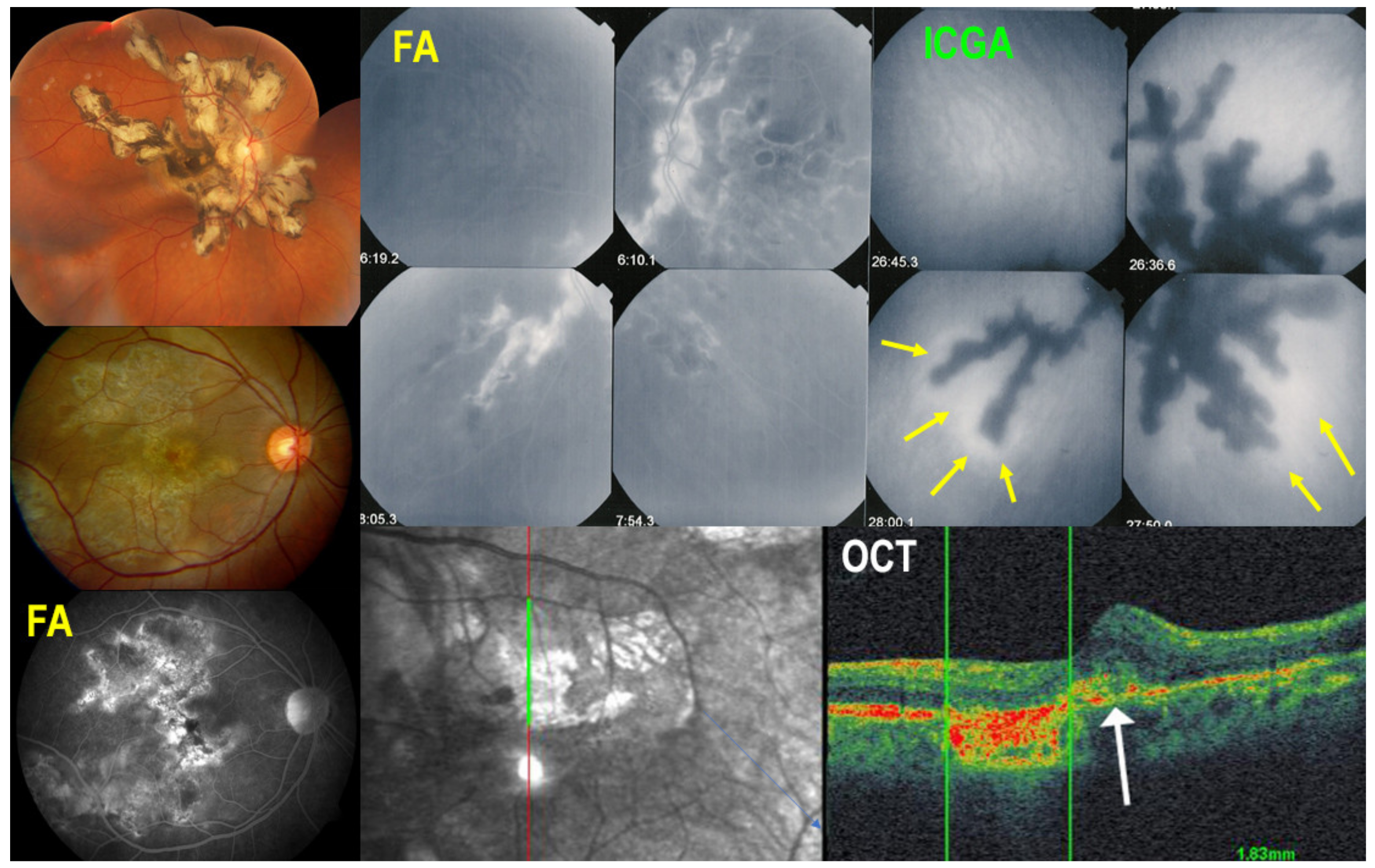
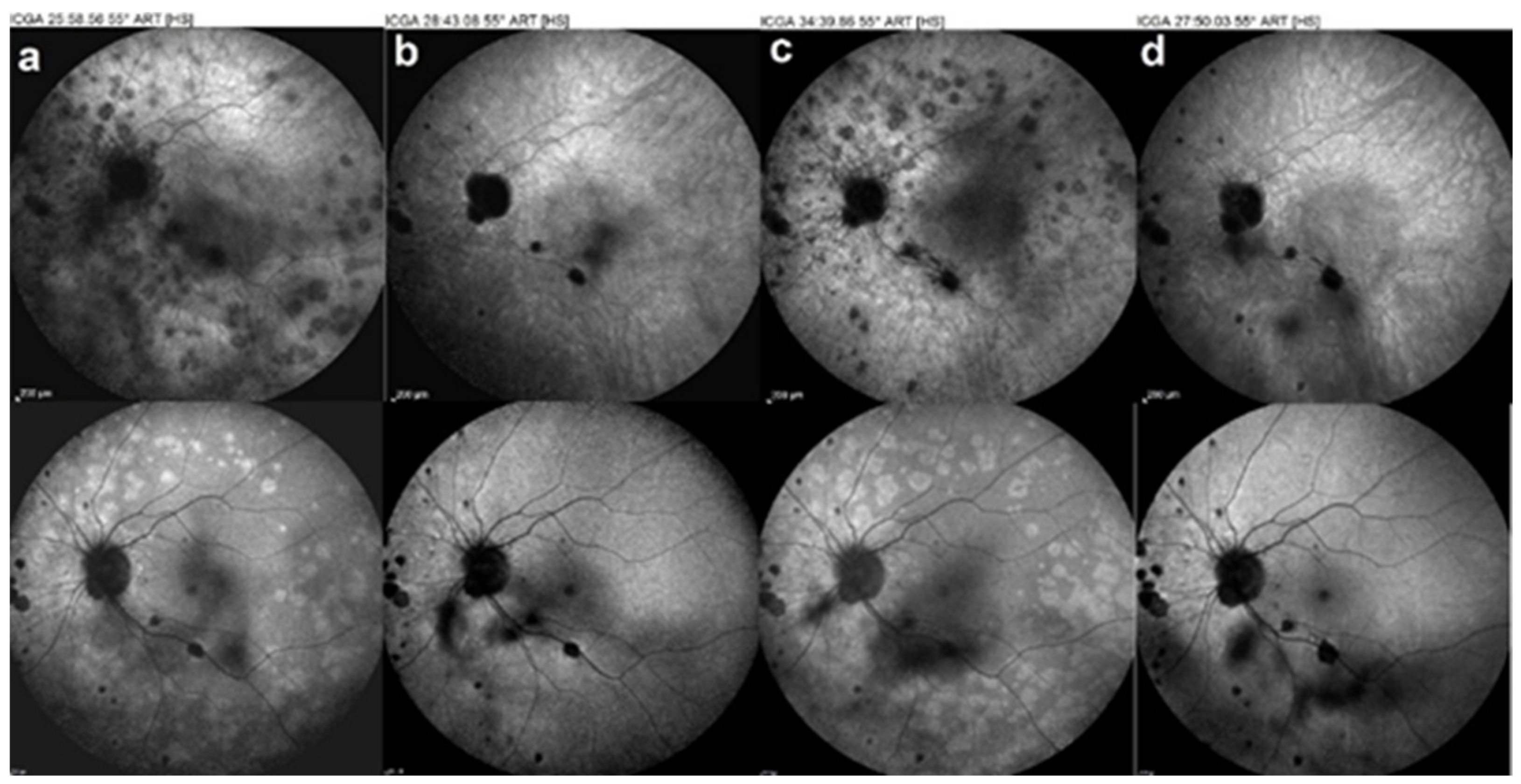
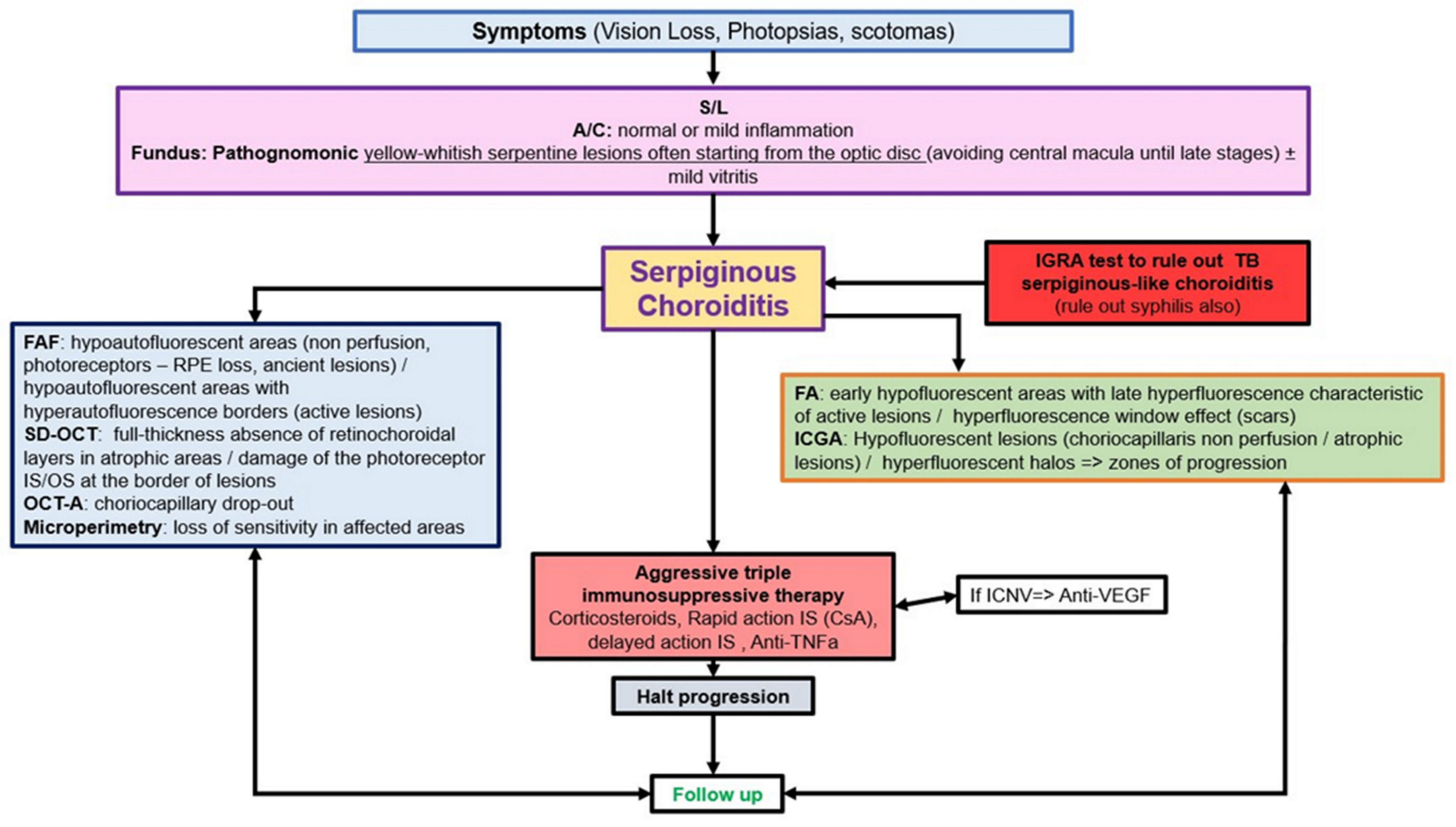
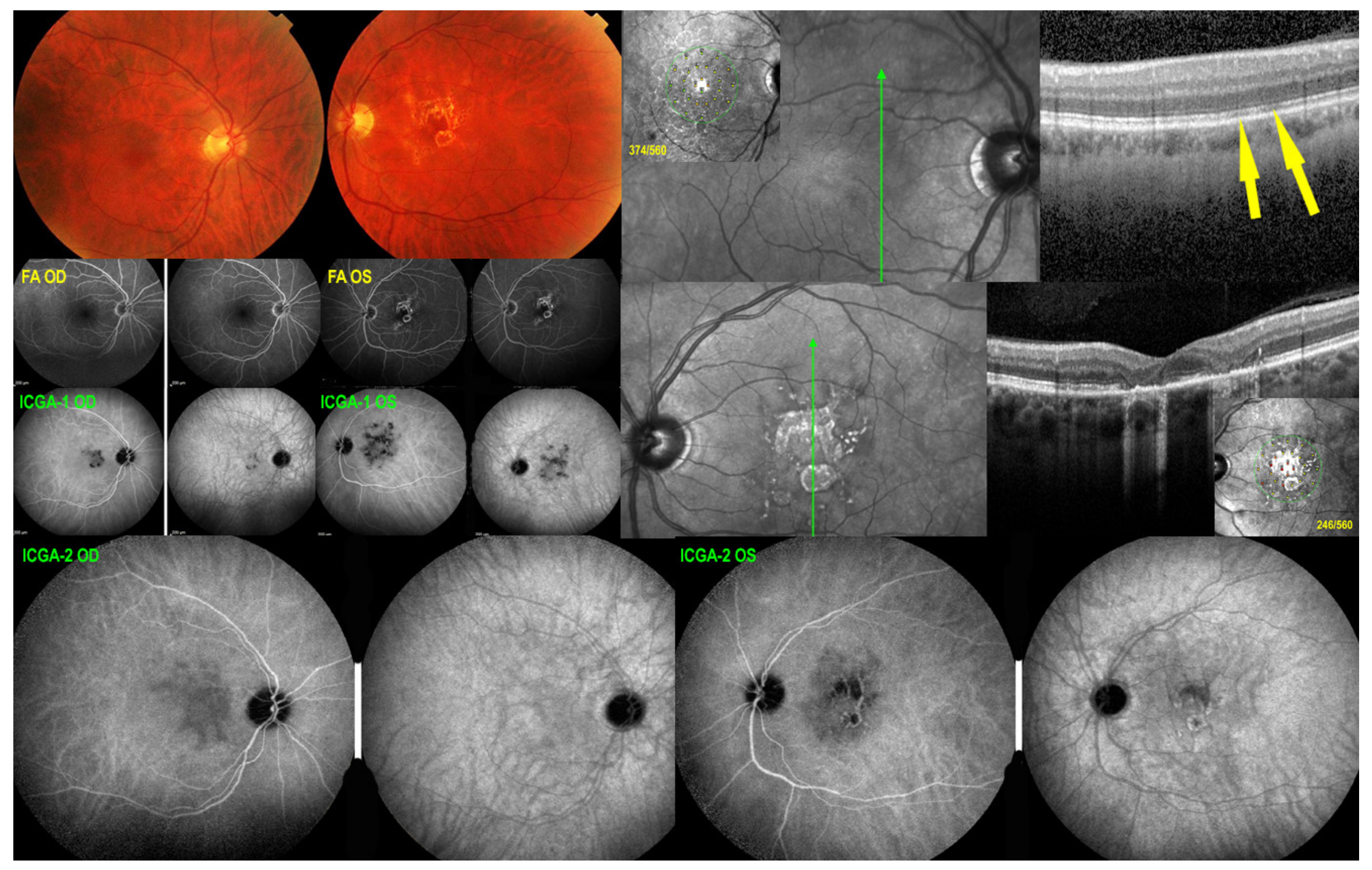
5.4. Serpiginous Choroiditis (SC)
Illustrative SC Case
5.5. Mixed-Intermediary/Overlapping and Non-Classifiable Forms of Choriocapillaritis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herbort, C.P., Jr.; Mantovani, A.; Tugal-Tutkun, I.; Papasavvas, I. Classification of Non-Infectious and/or Immune Mediated Choroiditis: A Brief Overview of the Essentials. Diagnostics 2021, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Neri, P.; Herbort, C.P., Jr.; Hedayatfar, A.; Tugal-Tutkun, I.; Cimino, L.; Urzua, C.A.; Papasavvas, I.; Takeuchi, M.; Lages, V. “White dot syndromes”, an inappropriate and outdated misnomer. Int. Ophthalmol. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Testi, I.; Modugno, R.L.; Pavesio, C. Multimodal imaging supporting the pathophysiology of white dot syndromes. J. Ophthalmic Inflamm. Infect. 2021, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.J.; Jampol, L.M. Recurrences and bilaterality in the multiple evanescent white-dot syndrome. Am. J. Ophthalmol. 1986, 101, 388–389. [Google Scholar] [CrossRef]
- Herbort, C.P., Jr.; Tugal-Tutkun, I.; Mantovani, A.; Neri, P.; Khairallah, M.; Papasavvas, I. Advances and potential new developments in imaging techniques for posterior uveitis Part 2: Invasive imaging methods. Eye 2021, 35, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Cimino, L.; Auer, C.; Herbort, C.P. Sensitivity of indocyanine green angiography for the follow-up of active inflammatory choriocapillaropathies. Ocul. Immunol. Inflamm. 2000, 8, 275–283. [Google Scholar] [CrossRef]
- Kuznetcova, T.; Jeannin, B.; Herbort, C.P. A case of overlapping choriocapillaritis syndromes: Multimodal imaging appraisal. J. Ophthalmic Vis. Res. 2012, 7, 67–75. [Google Scholar] [PubMed]
- Tugal-Tutkun, I.; Herbort, C.P., Jr.; Mantovani, A.; Neri, P.; Khairallah, M. Advances and potential new developments in imaging techniques for posterior uveitis. Part 1: Noninvasive imaging methods. Eye 2021, 35, 33–51. [Google Scholar] [CrossRef]
- Mantovani, A.; Giani, A.; Herbort, C.P., Jr.; Staurenghi, G. Interpretation of fundus autofluorescence changes in choriocapillaritis: A multi-modality imaging study. Graefe’s. Arch. Clin. Exp. Ophthalmol. 2016, 254, 1473–1479. [Google Scholar] [CrossRef]
- Herbort, C.P.; Papadia, M.; Mantovani, A. Classification of choroiditis based on inflammatory lesion process rather than fundus appearance: Enhanced comprehension through the ICGA concepts of the iceberg and jellyfish effects. Klin. Monbl. Augenheilkd. 2012, 229, 306–313. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Li, M.; Huang, Y. Late-phase hypercyanescence during indocyanine green angiography for assessment of myopic choroidal neovascularization. Eur. J. Ophthalmol. 2020, 31, 2578–2587. [Google Scholar] [CrossRef]
- Deutman, A.F.; Boen-Tan, T.N.; Oosterhuis, J.A. Proceedings: Acute posterior multifocal placoid pigment epitheliopathy. Ophthalmologica 1973, 167, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Ezra, D.B.; Forrester, J.V. Fundal white dots: The spectrum of a similar pathological process. Br. J. Ophthalmol. 1995, 79, 856–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, D.C. Endogenous uveitis: Current concepts of treatment. Mayo Clin. Proc. 1990, 65, 671–683. [Google Scholar] [CrossRef]
- Alfano, J.E. Changes in the intraocular pressue associated with systemic corticosteroid therapy. Am. J. Ophthalmol. 1963, 56, 245–247. [Google Scholar] [CrossRef]
- Kristensen, P. Posterior subcapsular cataract (P.S.C.) and systemic steroid therapy. Acta Ophthalmol. 1968, 46, 1025–1032. [Google Scholar] [CrossRef]
- Schalenbourg, A.; Leys, A.; De Courten, C.; Coutteel, C.; Herbort, C.P. Corticosteroid-induced central serous chorioretinopathy in patients with ocular inflammatory disorders. Klin. Monbl. Augenheilkd. 2002, 219, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Newell, F.W.; Krill, A.E. Treatment of uveitis with azathioprine (Imuran). Trans. Ophthalmol. Soc. UK 1967, 87, 499–511. [Google Scholar] [PubMed]
- Castiblanco, C.; Foster, C.S. Review of Systemic Immunosuppression for Autoimmune Uveitis. Ophthalmol. Ther. 2014, 3, 17–36. [Google Scholar] [CrossRef] [Green Version]
- Tavadia, S.M.; Mydlarski, P.R.; Reis, M.D.; Mittmann, N.; Pinkerton, P.H.; Shear, N.; Sauder, D.N. Screening for azathioprine toxicity: A pharmacoeconomic analysis based on a target case. J. Am. Acad. Dermatol. 2000, 42, 628–632. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Model List of Essential Medicines–22nd List, 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Chanaud, N.P.; Vistica, B.P.; Eugui, E.; Nussenblatt, R.B.; Allison, A.C.; Gery, I. Inhibition of experimental autoimmune uveoretinitis by mycophenolate mofetil, an inhibitor of purine metabolism. Exp. Eye Res. 1995, 61, 429–434. [Google Scholar] [CrossRef]
- Baltatzis, S.; Tufail, F.; Yu, E.N.; Vredeveld, C.M.; Foster, C.S. Mycophenolate mofetil as an immunomodulatory agent in the treatment of chronic ocular inflammatory disorders. Ophthalmology 2003, 110, 1061–1065. [Google Scholar] [CrossRef]
- Ortega, F.; Sánchez-Fructuoso, A.; Cruzado, J.M.; Gómez-Alamillo, J.C.; Alarcón, A.; Pallardó, L.; Morales, J.M.; Oliver, J.; Guinea, G.; MYVIDA Study Group. Gastrointestinal quality of life improvement of renal transplant recipients converted from mycophenolate mofetil to enteric-coated mycophenolate sodium drugs or agents: Mycophenolate mofetil and enteric-coated mycophenolate sodium. Transplantation 2011, 92, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Doycheva, D.; Zierhut, M.; Blumenstock, G.; Sobolewska, B.; Voykov, B.; Hohmann, J.; Spitzer, M.S.; Deuter, C. Mycophenolate sodium for the treatment of chronic non-infectious uveitis of childhood. Br. J. Ophthalmol. 2016, 100, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Azzi, J.R.; Sayegh, M.H.; Mallat, S.G. Calcineurin inhibitors: 40 years later, can’t live without. J. Immunol. 2013, 191, 5785–5791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nussenblatt, R.B.; Rodrigues, M.M.; Salinas-Carmona, M.C.; Gery, I.; Cevario, S.; Wacker, W. Modulation of experimental autoimmune uveitis with cyclosporin A. Arch. Ophthalmol. 1982, 100, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Nussenblatt, R.B.; Palestine, A.G.; Chan, C.C. Cyclosporine therapy for uveitis: Long-term followup. J. Ocul. Pharmacol. Ther. 1985, 1, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Nepovimova, E.; Kuca, K.; Wu, W. Cyclosporine A: Chemistry and Toxicity-A Review. Curr. Med. Chem. 2021, 28, 3925–3934. [Google Scholar] [CrossRef]
- Kawashima, H.; Fujino, Y.; Mochizuki, M. Effects of a new immunosuppressive agent, FK506, on experimental autoimmune uveoretinitis in rats. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1265–1271. [Google Scholar]
- Hogan, A.C.; McAvoy, C.E.; Dick, A.D.; Lee, R.W. Long-term efficacy and tolerance of tacrolimus for the treatment of uveitis. Ophthalmology 2007, 114, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Hooper, P.L.; Kaplan, H.J. Triple agent immunosuppression in serpiginous choroiditis. Ophthalmology 1991, 98, 944–951. [Google Scholar] [CrossRef]
- Barbari, A.; Stephan, A.; Masri, M.A.; Kamel, G.; Karam, A.; Mourad, N.; Kilani, H.; El Ghoul, B. Mycophenolic acid plasma trough level: Correlation with clinical outcome. Exp Clin Transplant. Exp. Clin. Transpl. 2005, 3, 355–360. [Google Scholar]
- Bodaghi, B.; Quoc, E.B.; Wechsler, B.; Tran, T.H.C.; Cassoux, N.; Huong, D.L.T.; Chosidow, O.; Herson, S.; Piette, J.-C.; LeHoang, P. Therapeutic use of infliximab in sight threatening uveitis: Retrospective analysis of efficacy, safety, and limiting factors. Ann. Rheum. Dis. 2005, 64, 962–964. [Google Scholar] [CrossRef] [PubMed]
- Seve, P.; Mennesson, E.; Grange, J.D.; Broussolle, C.; Kodjikian, L. Infliximab in serpiginous choroiditis. Acta Ophthalmol. 2010, 88, e342–e343. [Google Scholar] [CrossRef]
- Scheinfeld, N. Adalimumab (HUMIRA): A review. J. Drugs Dermatol. 2003, 2, 375–377. [Google Scholar] [PubMed]
- De Groot, E.L.; Ossewaarde-van Norel, J.; Ho, L.; Ninette, H.; de Boer, J.H. The efficacy of adalimumab in treating patients with central multifocal choroiditis. Am. J. Ophthalmol. Case Rep. 2020, 17, 100921. [Google Scholar] [CrossRef]
- Neri, P.; Ricci, F.; Giovannini, A.; Arapi, I.; De Felici, C.; Cusumano, A.; Mariotti, C. Successful treatment of an overlapping choriocapillaritis between multifocal choroiditis and acute zonal occult outer retinopathy (AZOOR) with adalimumab (Humira™). Int. Ophthalmol. 2014, 34, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Capote, A.C.; Jiménez, J.M.R.; Soto, M.L.; Gómez, C.R.; De Lucas, M.D.G. Effectiveness of adalimumab for refractory serpiginous choroiditis. Ocul. Immunol. Inflamm. 2014, 22, 405–408. [Google Scholar] [CrossRef] [Green Version]
- Wolf, D.; D’Haens, G.; Sandborn, W.J.; Colombel, J.-F.; Van Assche, G.; Robinson, A.M.; Lazar, A.; Zhou, Q.; Petersson, J.; Thakkar, R.B. Escalation to weekly dosing recaptures response in adalimumab-treated patients with moderately to severely active ulcerative colitis. Aliment. Pharm. Ther. 2014, 40, 486–497. [Google Scholar] [CrossRef] [Green Version]
- Jampol, L.M.; Sieving, P.A.; Pugh, D.; Fishman, G.A.; Gilbert, H. Multiple evanescent white dot syndrome. Arch. Ophthalmol. 1984, 102, 671–674. [Google Scholar] [CrossRef]
- Khochtali, S.; Dridi, T.; Abroug, N.; Ksiaa, I.; Lupidi, M.; Khairallah, M. Swept-Source Optical Coherence Tomography Angiography Shows Choriocapillaris Flow Reduction in Multiple Evanescent White Dot Syndrome. J. Curr. Ophthalmol. 2020, 32, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pichi, F.; Srivastava, S.K.; Chexal, S.; Lembo, A.; Lima, L.H.; Neri, P.; Chhablani, J.; Albini, T.A.; Nucci, P.; Freund, K.B.; et al. En face optical coherence tomography and optical tomography angiography of multiple evanescent white dot syndrome: New insights into pathogenesis. Retina 2016, 36 (Suppl. S1), S178–S188. [Google Scholar] [CrossRef] [PubMed]
- Gaudric, A.; Mrejen, S. Why the dots are black only in the late phase of the indocyanine green angiography in multiple evanescent white dot syndrome. Retin. Cases Brief Rep. 2017, 11 (Suppl. S1), S81–S85. [Google Scholar] [CrossRef]
- Lages, V.; Mantovani, A.; Papadia, M.; Herbort, C.P. MEWDS is a true primary choriocapillaritis and basic mechanisms do not seem to differ from other choriocapillaritis entities. J. Curr. Ophthalmol. 2018, 30, 281–286. [Google Scholar] [CrossRef]
- Mantovani, A.; Invernizzi, A.; Staurenghi, G.; Herbort, C.P., Jr. Multiple Evanescent White Dot Syndrome: A Multimodal Imaging Study of Foveal Granularity. Ocul. Immunol. Inflamm. 2019, 27, 141–147. [Google Scholar] [CrossRef]
- Papasavvas, I.; Mantovani, A.; Tugal-Tutkun, I.; Herbort, C.P., Jr. Multiple evanescent white dot syndrome (MEWDS): Update on practical appraisal, diagnosis and clinicopathology; a review and an alternative comprehensive perspective. J. Ophthalmic Inflamm. Infect. 2021, 11, 45. [Google Scholar] [CrossRef]
- Papadia, M.; Herbort, C.P. Idiopathic choroidal neovascularization as the inaugural sign of multiple evanescent white dot syndrome. Middle East Afr. J. Ophthalmol. 2010, 17, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, W.A.; Imes, R.K.; Goodman, D.; Hoyt, W.F. Acute idiopathic blind spot enlargement. A big blind spot syndrome without optic disc edema. Arch. Ophthalmol. 1988, 106, 44–49. [Google Scholar] [CrossRef]
- Hamed, L.A.; Schatz, N.J.; Glaser, J.S.; Gass, J.D.M. Idiopathic blind spot enlargement without optic disc edema. Arch. Ophthalmol. 1988, 106, 1030–1031. [Google Scholar] [CrossRef] [PubMed]
- Wyhinny, G.J.; Jackson, J.L.; Jampol, L.M.; Caro, N.C. Subretinal neovascularization following multiple evanescent white-dot syndrome. Arch. Ophthalmol. 1990, 108, 1384–1385. [Google Scholar] [CrossRef]
- McCollum, C.J.; Kimble, J.A. Peripapillary subretinal neovascularization associated with multiple evanescent white-dot syndrome. Arch. Ophthalmol. 1992, 110, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.R.; Addison, P.K.F.; Pavesio, C.E. Multifocal Evanescent White Dot Syndrome-like Phenotypes Associated with Inflammatory and Myopic Choroidal Neovascularization. Ocul. Immunol. Inflamm. 2021, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D.M. Acute Posterior Multifocal Placoid Pigment Epitheliopathy. Arch. Ophthalmol. 1968, 80, 171–185. [Google Scholar] [CrossRef]
- Deutman, A.F.; Oosterhuis, J.A.; Boen-Tan, T.N.; de Kerk, A.L.A. Acute posterior multifocal placoid pigment epitheliopathy. Pigment epitheliopathy or choriocapillaritis. Br. J. Ophthalmol. 1972, 56, 863–874. [Google Scholar] [CrossRef] [Green Version]
- Deutman, A.F.; Lion, F. Choriocapillaris nonpefusion in acute multifocal placoid pigment epitheliopathy. Am. J. Ophthalmol. 1977, 84, 652–657. [Google Scholar] [CrossRef]
- Howe, L.J.; Woon, H.; Graham, E.M.; Fitzke, F.; Bhandari, A.; Marshall, J. Choroidal hypoperfusion in acute posterior multifocal placoid pigment epitheliopathy. An indocyanine green angiography study. Ophthalmology 1995, 102, 790–798. [Google Scholar] [CrossRef]
- Daniele, S.; Daniele, C.; Orcidi, F.; Tavano, A. Progression of choroidal atrophy in acute posterior multifocal placoid pigment epitheliopathy. Ophthalmologica 1998, 212, 66–72. [Google Scholar] [CrossRef]
- Sulewski, M.E., Jr.; Kolomeyer, A.M.; Saran, B.R.; Brucker, A.J. A 15-Year-old boy with protracted vision loss from acute posterior multifocal placoid pigment epitheliopathy. Retin. Cases Brief Rep. 2021, 15, 756–759. [Google Scholar] [CrossRef]
- El-Markaby, H.S.; Mohammed, T.H.; El-Raggal, T.M. Acute posterior multifocal placoid pigment epitheliopathy: Role of TNF blocker in severe cases. Retina 2012, 32, 2102–2107. [Google Scholar] [CrossRef]
- Herbort, C.P., Jr.; Neri, P.; Papasavvas, I. Clinicopathology of non-infectious choroiditis: Evolution of its appraisal during the last 2-3 decades from "white dot syndromes" to precise classification. J. Ophthalmic Inflamm. Infect. 2021, 11, 43. [Google Scholar] [CrossRef]
- Herbort, C.P., Jr.; Papasavvas, I.; Mantovani, A. Choriocapillaris involvement in Acute Syphilis Posterior Placoid Chrioretinitis is responsible for functional impairment and points points towards an immunologic mechanism: A comprehensive clinicopathological approach. J. Curr. Ophthalmol. 2020, 32, 381–389. [Google Scholar] [CrossRef]
- Damato, B.E.; Nanjiani, M.; Foulds, W.S. Acute posterior multifocal placoid pigment epitheliopathy. A follow up study. Trans. Ophthalmol. Soc. UK 1983, 103, 517–522. [Google Scholar]
- Comu, S.; Verstraeten, T.; Rinkoff, J.S.; Busis, N.A. Neurological manifestations of acute posterior multifocal placoid pigment epitheliopathy. Stroke 1996, 27, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Tsang, B.K.; Chauhan, D.S.; Haward, R.; Whiteman, I.; Frayne, J.; McLean, C. Fatal ischemic stroke complicating acute multifocal placoid pigment epitheliopathy: Histopathological findings. J. Neuroophthalmol. 2014, 34, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.T.; Harlan, J.B.; Goldberg, M.F.; Dunn, J.P. Acute posterior multifocal placoid pigment epitheliopathy associated with a systemic necrotizing vasculitis. Retina 2003, 23, 64–68. [Google Scholar] [CrossRef]
- Essex, R.W.; Wong, J.; Jampol, L.M.; Dowler, J.; Bird, A.C. Idiopathic multifocal choroiditis: A comment on present and past nomenclature. Retina 2013, 33, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, R.F.; Gass, D.J. Multifocal choroiditis and panuveitis. A syndrome that mimics ocular histoplasmosis. Arch. Ophthalmol. 1984, 102, 1776–1784. [Google Scholar] [CrossRef]
- Fung, A.T.; Pal, S.; Yannuzzi, N.A.; Christos, P.; Cooney, M.; Slakter, J.S.; Klancnik, J.M., Jr.; Freund, K.B.; Cunningham, E.T., Jr.; Yannuzzi, L.A. Multifocal choroiditis without panuveitis: Clinical characteristics and progression. Retina 2014, 34, 98–107. [Google Scholar] [CrossRef]
- Papasavvas, I.; Neri, P.; Herbort, C.P., Jr. Idiopathic multifocal choroiditis (MFC): Aggessive and prolonged therapy with multiple immunosuppressive agents is needed to halt the progression of active disease. An offbeat review and a case series. J. Ophthalmic Inflamm. Infect. 2022, 12, 1–18. [Google Scholar] [CrossRef]
- Neri, P. Inflammatory choroidal neovascularization. In Uveitis Text and Imaging, 1st ed.; Gupta, A., Gupta, V., Herbort, C.P., Khairallah, M., Eds.; Jaypee: New Delhi, India, 2009; pp. 789–808. [Google Scholar]
- Borodoker, N.; Cunningham, E.T., Jr.; Yannuzzi, L.A.; Nicoletti, R. Peripheral curvilinear pigmentary streaks in multifocal choroiditis. Arch. Ophthalmol. 2002, 120, 520–521. [Google Scholar] [CrossRef]
- Reddy, C.V.; Brown, J.; Folk, J.C.; Kimura, A.E.; Gupta, S.; Walker, J. Enlarged blind spots in chorioretinal inflammatory disorders. Ophthalmology 1996, 103, 606–617. [Google Scholar] [CrossRef]
- Joondeph, B.C.; Tessler, H.H. Clinical course of multifocal choroiditis: Photographic and angiographic evidence of disease recurrence. Ann. Ophthalmol. 1991, 23, 424–429. [Google Scholar] [PubMed]
- De Groot, E.L.; ten Dam-van Loon, N.H.; de Boer, J.H.; Ossewaarde-van Norel, J. The efficacy of corticoid-sparing immunomodulatory therapy in treating patients with central multifocal choroiditis. Acta Ophthalmol. 2020, 98, 816–821. [Google Scholar] [CrossRef]
- Neri, P.; Pichi, F.; Pirani, V.; Arapi, I. Systemic Immunosuppression Is Highly Effective in the Long-term Control of Inflammatory non-infectious Uveitic Choroidal Neovascularization: A Comparative Study. Ocul Immunol Inflamm. 2021, 29, 1132–1136. [Google Scholar] [CrossRef]
- Papasavvas, I.; Jeannin, B.; Herbort, C.P., Jr. Tuberculosis-related serpiginous choroiditis: Aggressive therapy with dual concomitant of multiple anti-tubercular and multiple immunosuppressive agents is needed to halt the progression of the disease. J. Ophthalmic Immunol. Infect. 2021, 11. [Google Scholar]
- Piccolino, F.C.; Grosso, A.; Savini, E. Fundus autofluorescence in serpiginous choroiditis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Vonmoos, F.; Messerli, J.; Moser, H.R.; Prünte, C.; Flammer, J.; Haefliger, I.O. Immunosuppressive therapy in serpiginous choroiditis-case report and brief review of the literature. Klin. Monbl. Augenheilkd. 2001, 218, 394–397. [Google Scholar] [CrossRef]
- Akpek, E.K.; Baltatzis, S.; Yang, J.; Foster, C.S. Long-term immunosuppressive treatment of serpiginous choroiditis. Ocul. Immunol. Inflamm. 2001, 9, 153–167. [Google Scholar] [CrossRef]
- Lim, W.K.; Buggage, R.R.; Nussenblatt, R.B. Serpiginous choroiditis. Surv. Ophthalmol. 2005, 50, 231–244. [Google Scholar] [CrossRef]
- Sobaci, G.; Bayraktar, Z.; Bayer, A. Interferon alpha-2a treatment for serpiginous choroiditis. Ocul. Immunol. Inflamm. 2005, 13, 59–66. [Google Scholar] [CrossRef]
- Jones, B.E.; Jampol, L.M.; Yannuzzi, L.A.; Tittl, M.; Johnson, M.W.; Han, D.P.; Davis, J.L.; Williams, D.F. Relentless placoid chorioretinitis: A new entity or an unusual variant of serpiginous chorioretinitis? Arch. Ophthalmol. 2000, 118, 931–938. [Google Scholar]
- Jyotirmay, B.; Jafferji, S.S.; Sudharshan, S.; Kalpana, B. Clinical profile, treatment, and visual outcome of ampiginous choroiditis. Ocul. Immunol. Inflamm. 2010, 18, 46–51. [Google Scholar] [CrossRef]
- Bryan, R.G.; Freund, K.B.; Yannuzzi, L.A.; Spaide, R.F.; Huang-Sheau, J.; Costa, D.L. Multiple evanescent white dot syndrome in patients with multifocal choroiditis. Retina 2002, 22, 317–322. [Google Scholar] [CrossRef]
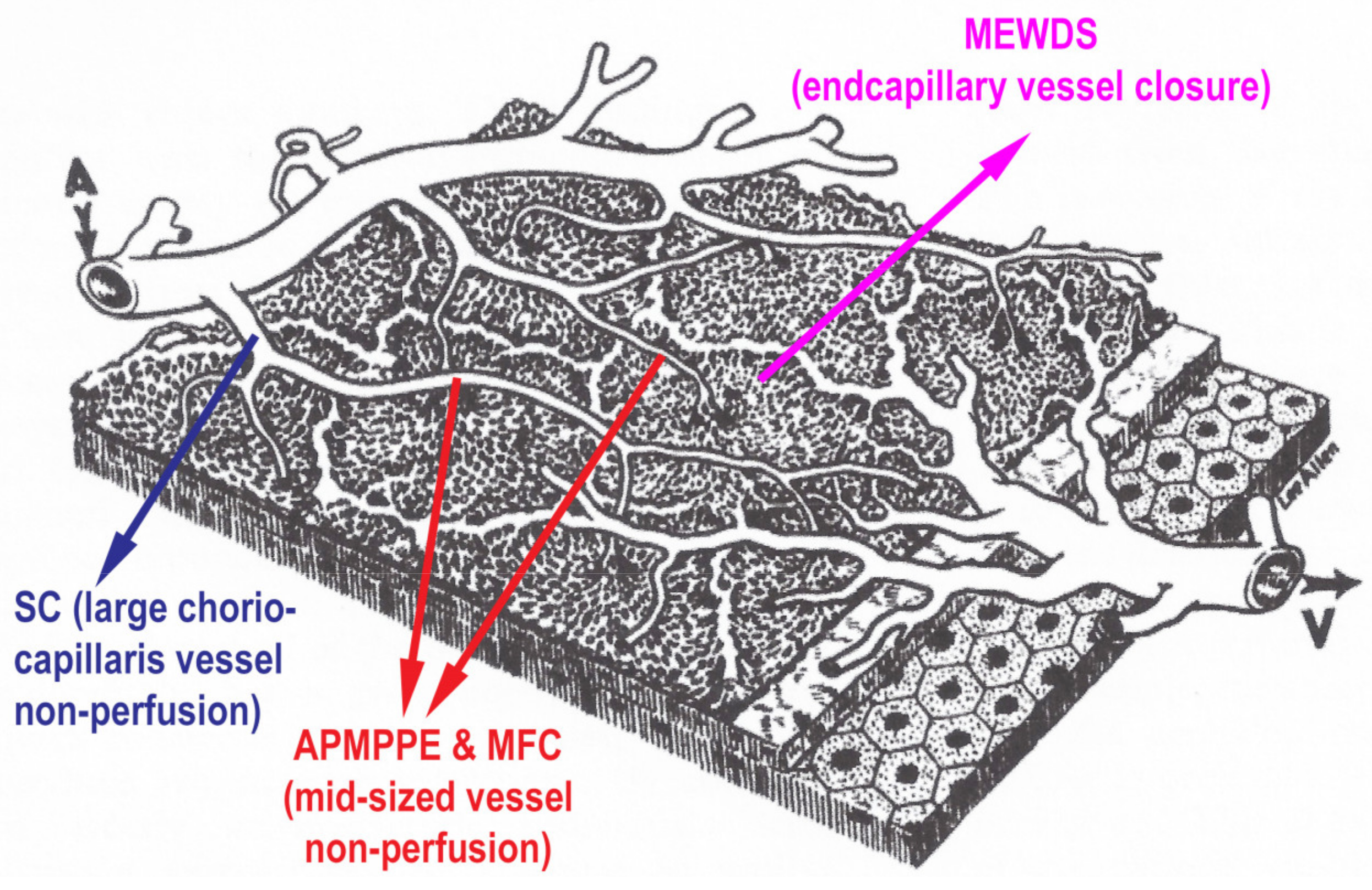
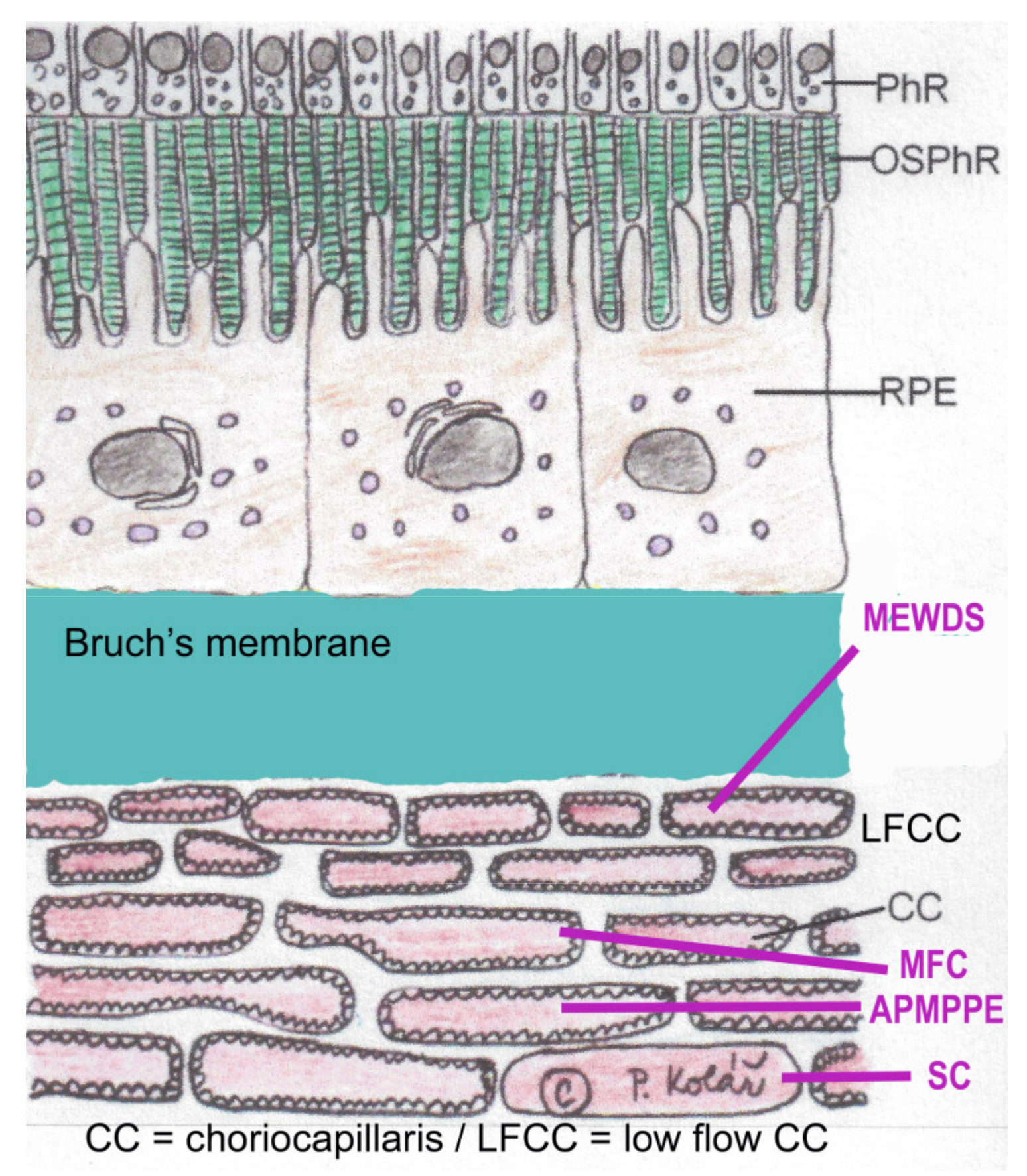
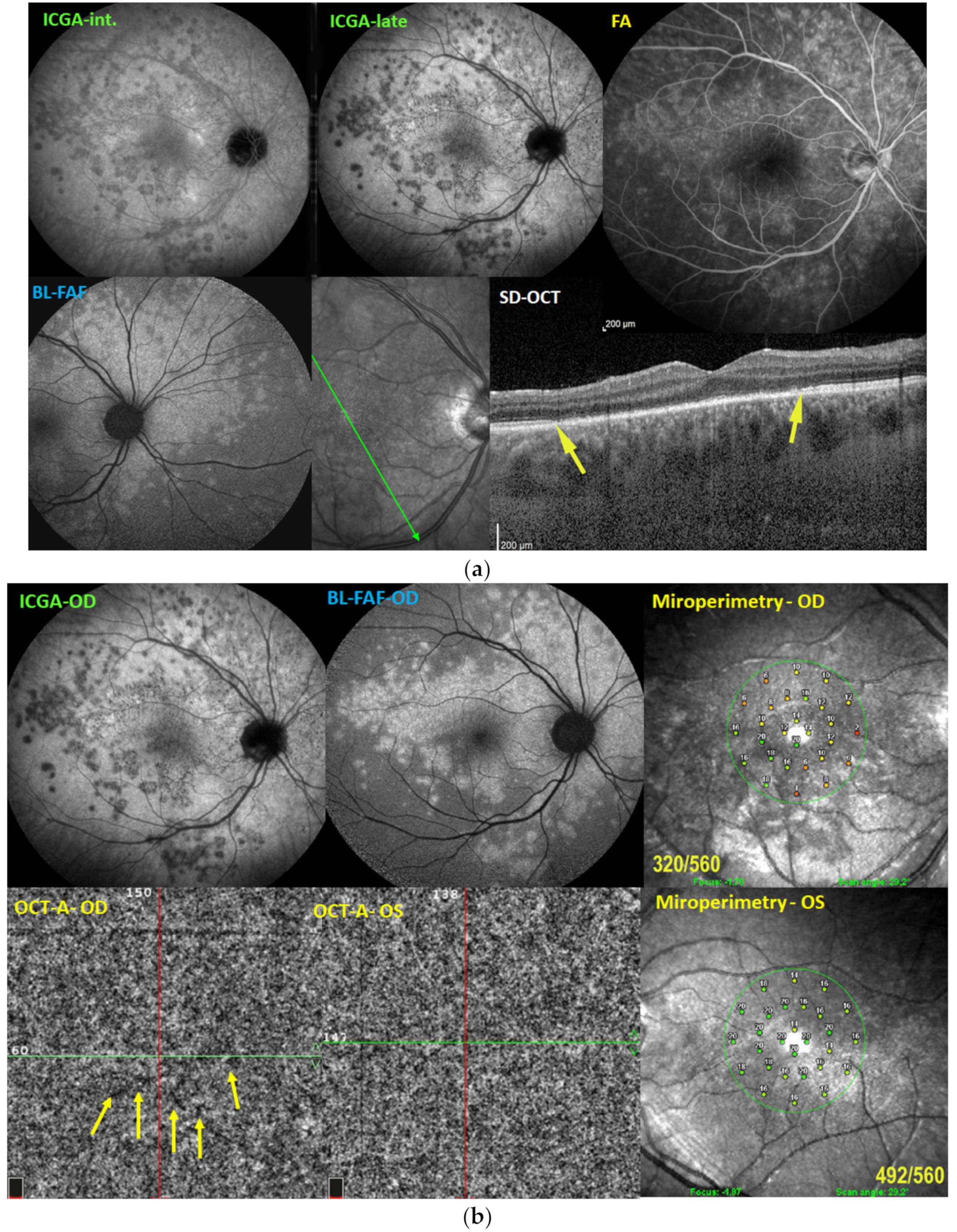
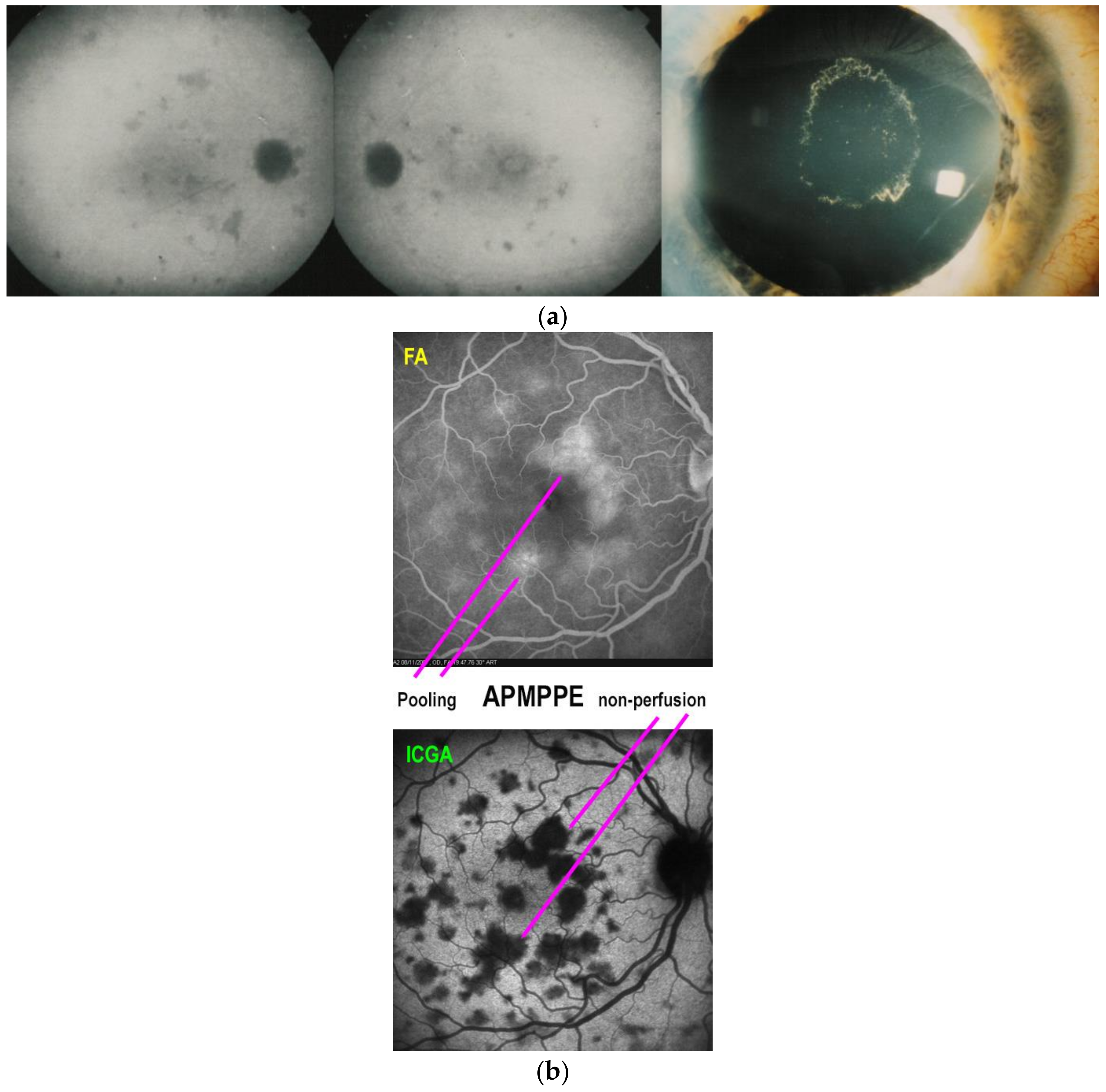
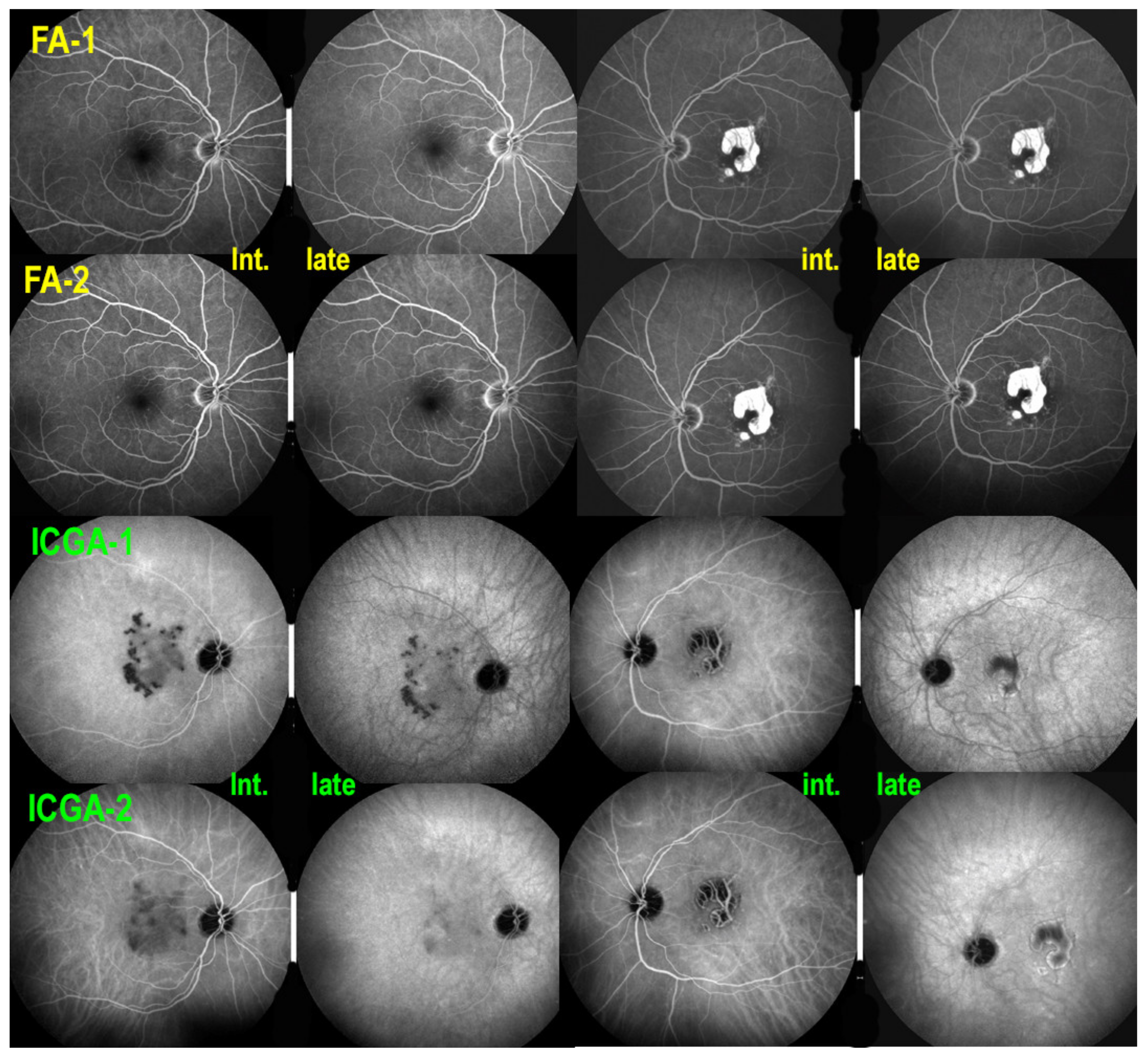
| MEWDS | APMPPE/AMIC | MFC/PIC | Serpiginous | |
|---|---|---|---|---|
| Age (y) | 20–50 | 20–40 | 20–50 | 30–50 |
| Gender | F | F = M | F | F = M |
| Laterality | Unilateral * | Bilateral/asymmetric | Bilateral/asymmetric | Bilateral/asymmetric |
| Myopia | Tendency towards myopia | Not important | Myopia is usual | Not important |
| Mechanism of Action | Dose | Side Effects | |
|---|---|---|---|
| Infliximab [35] | Chimeric monoclonal antibody, bound to both transmembrane and soluble form of TNF-a. Kills cells that express TNF-a | iv 5–20 mg/kg/day Loading dose at 0, 2, 4 weeks then every 6–10 weeks |
|
| Adalimumab [36] | Human monoclonal antibody, same as infliximab | SC 40 mg every 2 weeks (in severe cases interval can decrease to 7–10 days [40]) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papasavvas, I.; Herbort, C.P., Jr. Diagnosis and Treatment of Primary Inflammatory Choriocapillaropathies (PICCPs): A Comprehensive Overview. Medicina 2022, 58, 165. https://doi.org/10.3390/medicina58020165
Papasavvas I, Herbort CP Jr. Diagnosis and Treatment of Primary Inflammatory Choriocapillaropathies (PICCPs): A Comprehensive Overview. Medicina. 2022; 58(2):165. https://doi.org/10.3390/medicina58020165
Chicago/Turabian StylePapasavvas, Ioannis, and Carl P. Herbort, Jr. 2022. "Diagnosis and Treatment of Primary Inflammatory Choriocapillaropathies (PICCPs): A Comprehensive Overview" Medicina 58, no. 2: 165. https://doi.org/10.3390/medicina58020165
APA StylePapasavvas, I., & Herbort, C. P., Jr. (2022). Diagnosis and Treatment of Primary Inflammatory Choriocapillaropathies (PICCPs): A Comprehensive Overview. Medicina, 58(2), 165. https://doi.org/10.3390/medicina58020165






