Vitamin D3 Serum Levels in Periodontitis Patients: A Case–Control Study
Abstract
:1. Introduction
1.1. Background
1.2. Vitamin D3 and Its Association with Periodontal Health and Periodontitis
1.3. Classification of Periodontal and Peri-Implant Diseases and Conditions
1.4. Study Objective
1.5. Trial Design
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Statistical Methods
3. Results
3.1. Adverse Events and Safety Monitoring
3.2. Study Population
3.3. Serum Levels of Vitamin D3 in Patients with Periodontitis vs. Healthy Controls
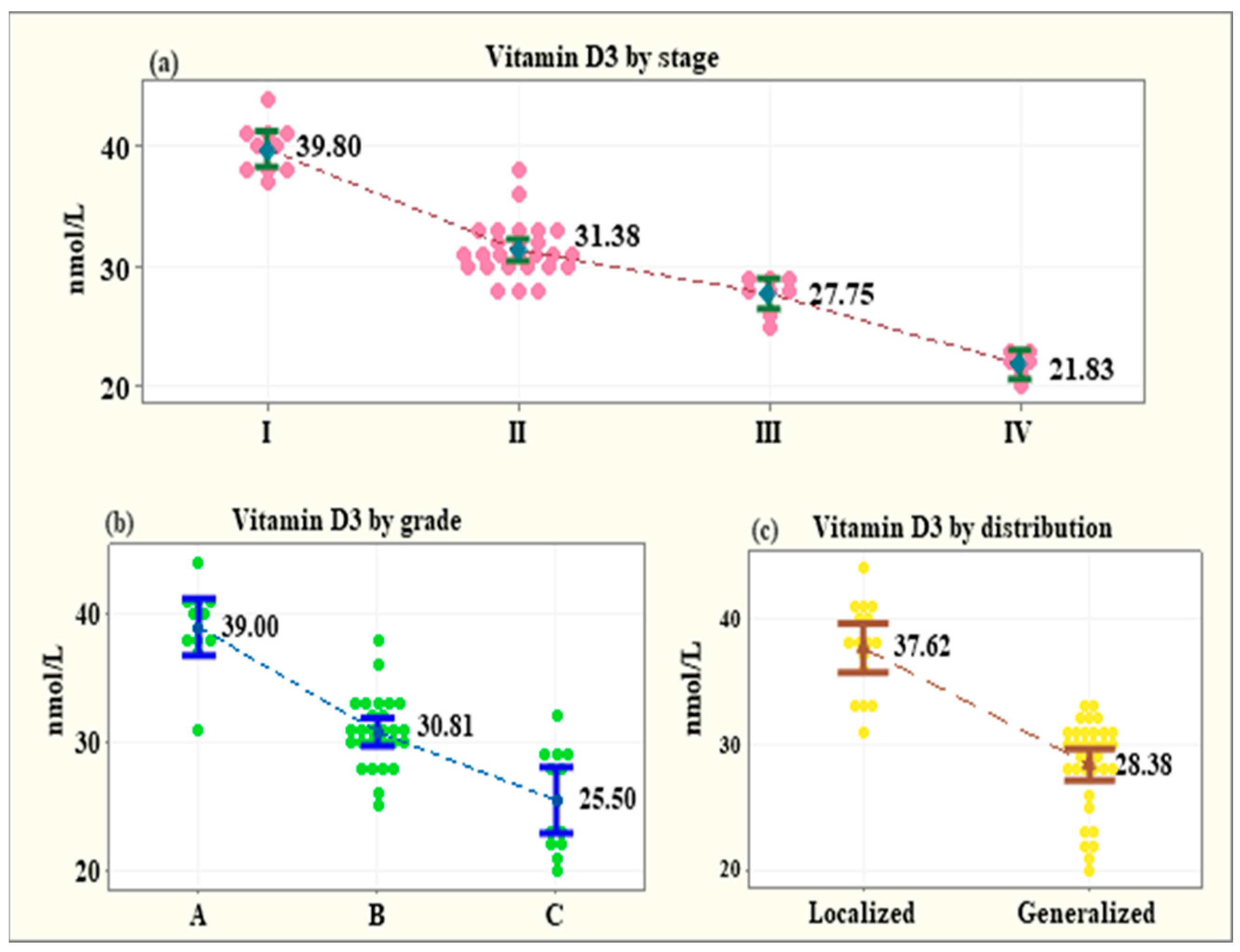
3.4. Serum Levels of Vitamin D3 across Subgroups of the Patients with Periodontitis
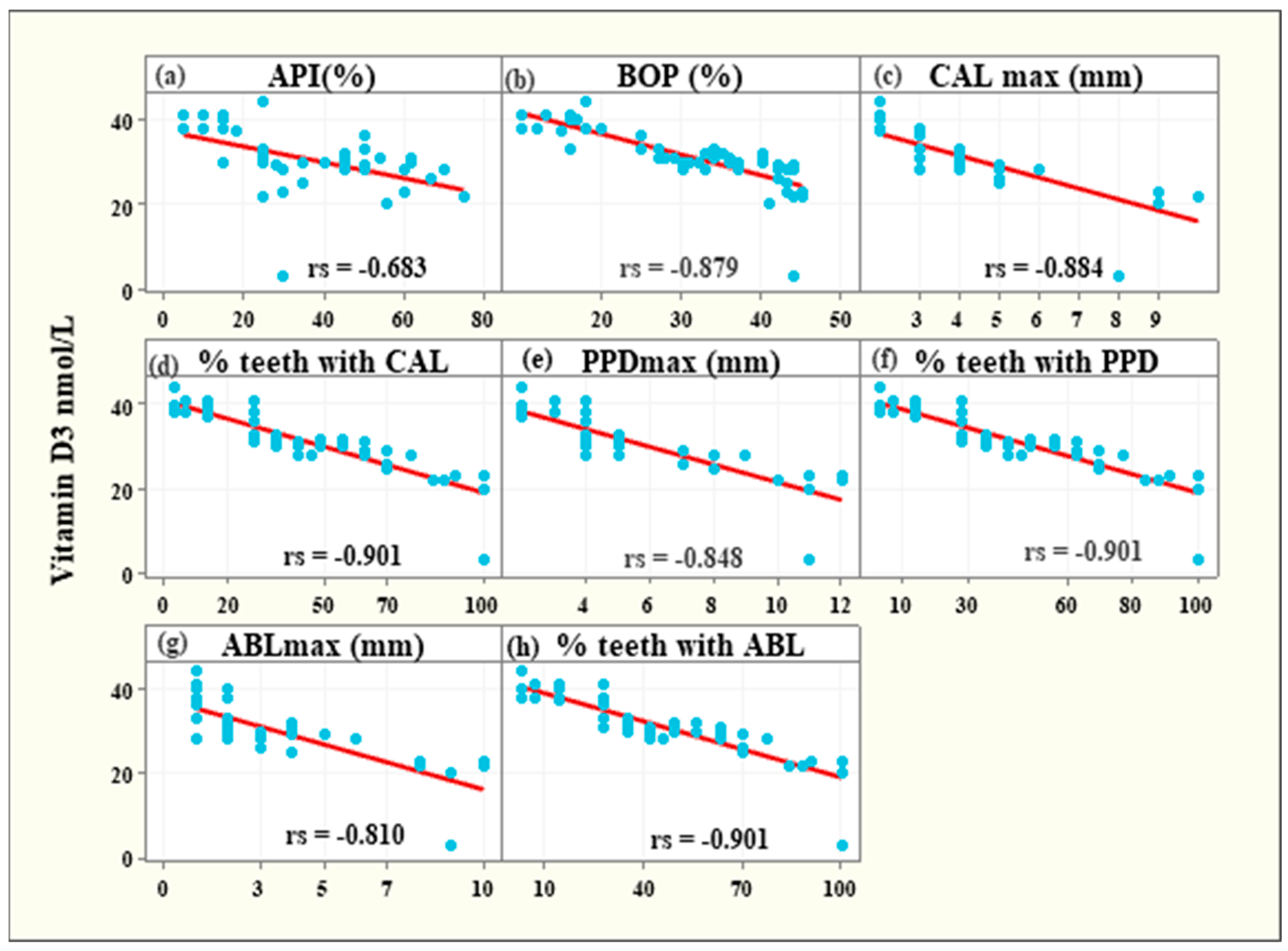
3.5. Associations between Vitamin D3 Levels and Clinical Parameters among Periodontitis Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontology 2000 2012, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Weinberg, A. Role of bacteria in health and disease of periodontal tissues. Periodontology 2000 2006, 40, 50–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and chronic diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80 (Suppl. 6), 1678S–1688S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, C.; Badenhoop, K. Vitamin D and type 1 diabetes mellitus: State of the art. Trends Endocrinol. Metab. 2005, 16, 261–266. [Google Scholar] [CrossRef]
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif. Tissue Int. 2020, 106, 58–75. [Google Scholar] [CrossRef] [Green Version]
- Kamen, D.L.; Tangpricha, V. Vitamin D and molecular actions on the immune system: Modulation of innate and autoimmunity. J. Mol. Med. 2010, 88, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Glerup, H.; Mikkelsen, K.; Poulsen, L.; Hass, E.; Overbeck, S.; Thomsen, J.; Charles, P.; Eriksen, E.F. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J. Intern. Med. 2000, 247, 260–268. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; Fuleihan, G.E.-H.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, 23–54. [Google Scholar] [CrossRef] [Green Version]
- Jagelavičienė, E.; Vaitkevičienė, I.; Šilingaitė, D.; Šinkūnaitė, E.; Daugėlaitė, G. The Relationship between Vitamin D and periodontal pathology. Medicina 2018, 54, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011, 55, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Joshipura, K.; Willett, W. Nutrition and health: Guidelines for dental practitioners. Oral Dis. 2009, 15, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Joshipura, K.J.; Dawson-Hughes, B.; Bischoff-Ferrari, H.A. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am. J. Clin. Nutr. 2004, 80, 108–113. [Google Scholar]
- Dietrich, T.; Nunn, M.; Dawson-Hughes, B.; Bischoff-Ferrari, H.A. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am. J. Clin. Nutr. 2005, 82, 575–580. [Google Scholar] [CrossRef]
- Dragonas, P.; El-Sioufi, I.; Bobetsis, Y.A.; Madianos, P.N. Association of Vitamin D With Periodontal Disease: A Narrative Review. Oral Health Prev. Dent. 2020, 18, 103–114. [Google Scholar] [CrossRef]
- Perić, M.; Cavalier, E.; Toma, S.; Lasserre, J.F. Serum vitamin D levels and chronic periodontitis in adult, Caucasian population—A systematic review. J. Periodontal Res. 2018, 53, 645–656. [Google Scholar] [CrossRef]
- Pinto, J.P.N.S.; Goergen, J.; Muniz, F.W.M.G.; Haas, A.N. Vitamin D levels and risk for periodontal disease: A systematic review. J. Periodontal Res. 2018, 53, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Khammissa, R.A.G.; Ballyram, R.; Jadwat, Y.; Fourie, J.; Lemmer, J.; Feller, L. Vitamin D deficiency as it relates to oral immunity and chronic periodontitis. Int. J. Dent. 2018, 2018, 7315797. [Google Scholar] [CrossRef] [Green Version]
- Tonetti, M.S.; Sanz, M. Implementation of the new classification of periodontal diseases: Decision-making algorithms for clinical practice and education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Lange, D.E.; Plagmann, H.C.; Eenboom, A.; Promesberger, A. Klinische Bewertungsverahren zur Objektivierung der Mundhygiene [Clinical methods for the objective evaluation of oral hygiene]. Dtsch Zahnarztl Z. 1977, 32, 44–47. (In German) [Google Scholar] [PubMed]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar] [PubMed]
- Bonnet, C.; Rabbani, R.; Moffatt, M.E.K.; Kelekis-Cholakis, A.; Schroth, R.J. The relation between periodontal disease and vitamin D. J. Can. Dent. Assoc. 2019, 85, j4. [Google Scholar]
- Madi, M.; Pavlic, V.; Mongith Alammar, S.; Mohammad Alsulaimi, L.; Shaker Alotaibi, R.; Mohammed AlOtaibi, G.; Zakaria, O. The association between vitamin D level and periodontal disease in Saudi population, a preliminary study. Saudi Dent. J. 2021, 33, 595–600. [Google Scholar] [CrossRef]
- Machado, V.; Lobo, S.; Proença, L.; Mendes, J.J.; Botelho, J. Vitamin D and periodontitis: A systematic review and meta-analysis. Nutrients 2020, 12, 2177. [Google Scholar] [CrossRef]
- Balci Yuce, H.; Gokturk, O.; Aydemir Turkal, H.; Inanir, A.; Benli, I.; Demir, O. Assessment of local and systemic 25-hydroxy-vitamin D, RANKL, OPG, and TNF levels in patients with rheumatoid arthritis and periodontitis. J. Oral Sci. 2017, 59, 397–404. [Google Scholar] [CrossRef]
- Lee, M.R.; Han, S.J.; Kim, H.E.; Choi, J.S. Relationship between Vitamin D Deficiency and Periodontitis in Korean Adults Aged ≥60 Years: Analysis of Data from the Korea National Health and Nutrition Examination Survey (2013–2014). Int. J. Environ. Res. Public Health 2021, 18, 4181. [Google Scholar] [CrossRef]
- Isola, G.; Palazzo, G.; Polizzi, A.; Murabito, P.; Giuffrida, C.; Lo Gullo, A. Association of Systemic Sclerosis and Periodontitis with Vitamin D Levels. Nutrients 2021, 13, 705. [Google Scholar] [CrossRef]
- Kim, H.; Shin, M.H.; Yoon, S.J.; Kweon, S.S.; Lee, Y.H.; Choi, C.K.; Kim, O.; Kim, Y.J.; Chung, H.; Kim, O.S. Low serum 25-hydroxyvitamin D levels, tooth loss, and the prevalence of severe periodontitis in Koreans aged 50 years and older. J. Periodontal Implant Sci. 2020, 50, 368–378. [Google Scholar] [CrossRef]
- Alzahrani, A.A.H.; Alharbi, R.A.; Alzahrani, M.S.A.; Sindi, M.A.; Shamlan, G.; Alzahrani, F.A.; Albanghali, M.A.; Sindi, A.A.A. Association between periodontitis and vitamin D status: A case-control study. Saudi J. Biol. Sci. 2021, 28, 4016–4021. [Google Scholar] [CrossRef]
- Costantini, E.; Sinjari, B.; Piscopo, F.; Porreca, A.; Reale, M.; Caputi, S.; Murmura, G. Evaluation of Salivary Cytokines and Vitamin D Levels in Periodontopathic Patients. Int. J. Mol. Sci. 2020, 21, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, A.A.; Kolte, A.P.; Kolte, R.A.; Chari, S.; Gupta, M.; Pakhmode, R. Evaluation and comparison of serum vitamin D and calcium levels in periodontally healthy, chronic gingivitis and chronic periodontitis in patients with and without diabetes mellitus—A cross-sectional study. Acta Odontol. Scand. 2019, 77, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Ketharanathan, V.; Torgersen, G.R.; Petrovski, B.É.; Preus, H.R. Radiographic alveolar bone level and levels of serum 25-OH-Vitamin D3 in ethnic Norwegian and Tamil periodontitis patients and their periodontally healthy controls. BMC Oral Health 2019, 19, 83. [Google Scholar] [CrossRef] [PubMed]

| Variable | Periodontitis | Healthy Controls | p-Value |
|---|---|---|---|
| N = 50 | N = 50 | ||
| Age | |||
| Mean ± SD | 49.08 ± 10.14 | 49.28 ± 10.78 | 0.924 t |
| Minimum–Maximum | 25–65 | 29–62 | |
| Sex n (%) | |||
| 25 (50%) | 24 (48%) | 0.841 χ2 | |
| Male | 25 (50%) | 26 (52%) | |
| Female | |||
| BMI (Body Mass Index) | |||
| Mean ± SD | 22.07 ± 3.74 | 21.26 ± 4.18 | 0.724 t |
| Minimum–Maximum | 18.65–24.35 | 19.00–24.25 | |
| BOP (Bleeding on Probing) | |||
| Mean ± SD | 31% ± 11% | 31% ± 10% | 0.001 t |
| Minimum–Maximum | 15–45% | 12–47% | |
| PPD (Periodontal Pocket Depth) | |||
| Mean ± SD | 5.52 ± 2.64 | 2 ± 1 | 0.001 t |
| Minimum–Maximum | 4–12 | 0–2 | |
| CAL (Clinical Attachment Level) | |||
| Mean ± SD | 4.14 ± 2.15 | 0 | 0.001 t |
| Minimum–Maximum | 2–10 | 0 |
| Groups | N | Mean | Mean Diff. | p-Value |
|---|---|---|---|---|
| (SD) | (95% CI) | |||
| Overall | ||||
| o Periodontitis | 50 | 31.34 (5.62) | 8.3 | <0.001 ! |
| o Healthy controls | 50 | 39.64 (8.77) | (5.37 to 11.22) | |
| Male | ||||
| o Periodontitis | 25 | 31.64 (5.67) | 8.23 | 0.001 ! |
| o Healthy controls | 24 | 39.88 (9.69) | (3.61 to 12.85) | |
| Female | ||||
| o Periodontitis | 25 | 31.04 (5.67) | 8.38 | |
| o Healthy controls | 26 | 39.42 (7.99) | (4.46 to 12.29) | <0.001 ! |
| Age < 50 years | ||||
| o Periodontitis | 22 | 32.50 (5.58) | 7.63 | 0.002 ! |
| o Healthy controls | 22 | 40.14 (9.39) | (2.93 to 12.33) | |
| Age > 50 years | ||||
| o Periodontitis | 28 | 30.43 (5.58) | 8.82 | <0.001 ! |
| o Healthy controls | 28 | 39.25 (8.38) | (5.00 to 12.63) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska-Czyz, I.; Firkova, E. Vitamin D3 Serum Levels in Periodontitis Patients: A Case–Control Study. Medicina 2022, 58, 585. https://doi.org/10.3390/medicina58050585
Olszewska-Czyz I, Firkova E. Vitamin D3 Serum Levels in Periodontitis Patients: A Case–Control Study. Medicina. 2022; 58(5):585. https://doi.org/10.3390/medicina58050585
Chicago/Turabian StyleOlszewska-Czyz, Iwona, and Elena Firkova. 2022. "Vitamin D3 Serum Levels in Periodontitis Patients: A Case–Control Study" Medicina 58, no. 5: 585. https://doi.org/10.3390/medicina58050585
APA StyleOlszewska-Czyz, I., & Firkova, E. (2022). Vitamin D3 Serum Levels in Periodontitis Patients: A Case–Control Study. Medicina, 58(5), 585. https://doi.org/10.3390/medicina58050585






