Tibial Stem Extension versus Standard Configuration in Total Knee Arthroplasty: A Biomechanical Assessment According to Bone Properties
Abstract
:1. Introduction
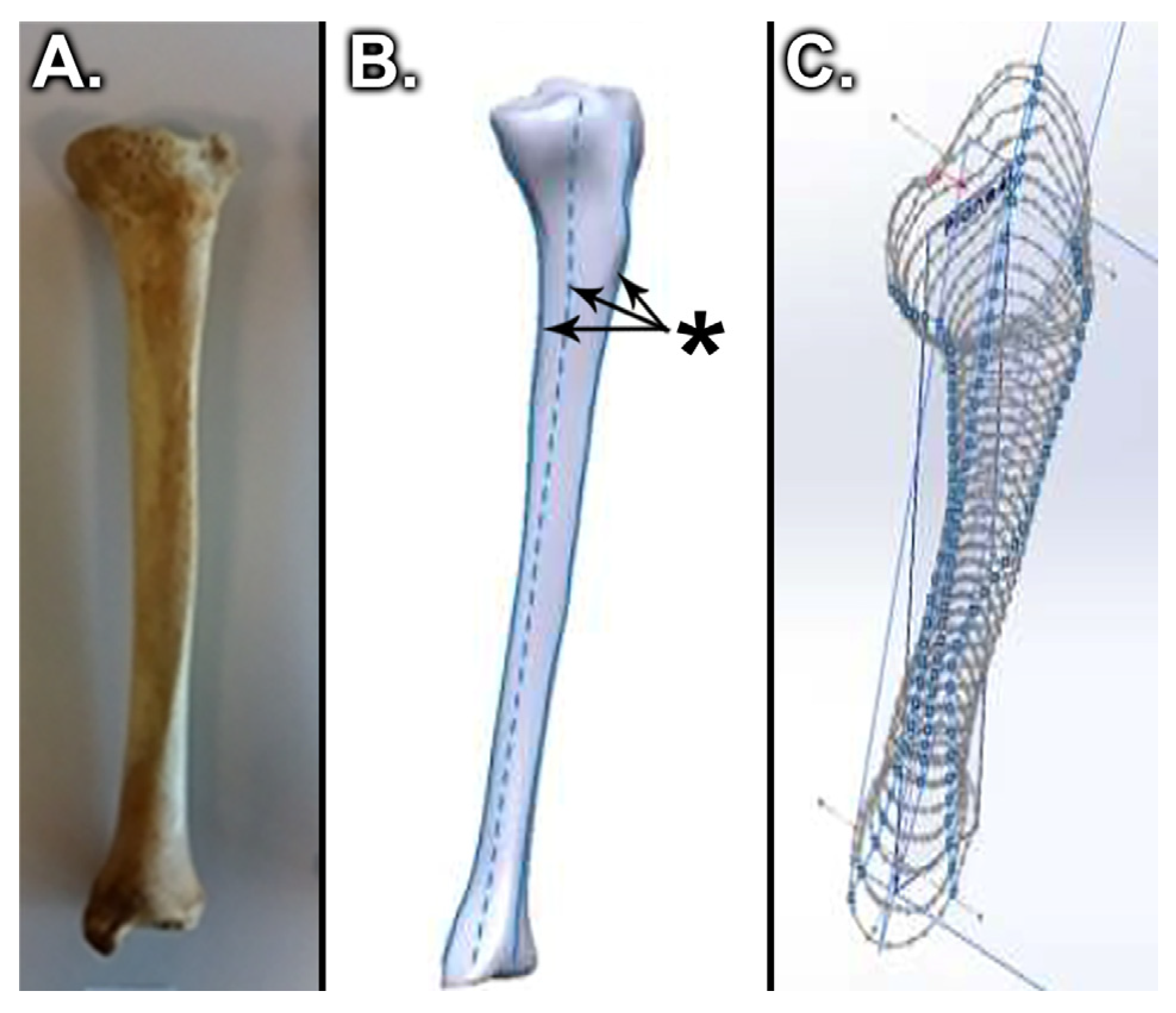
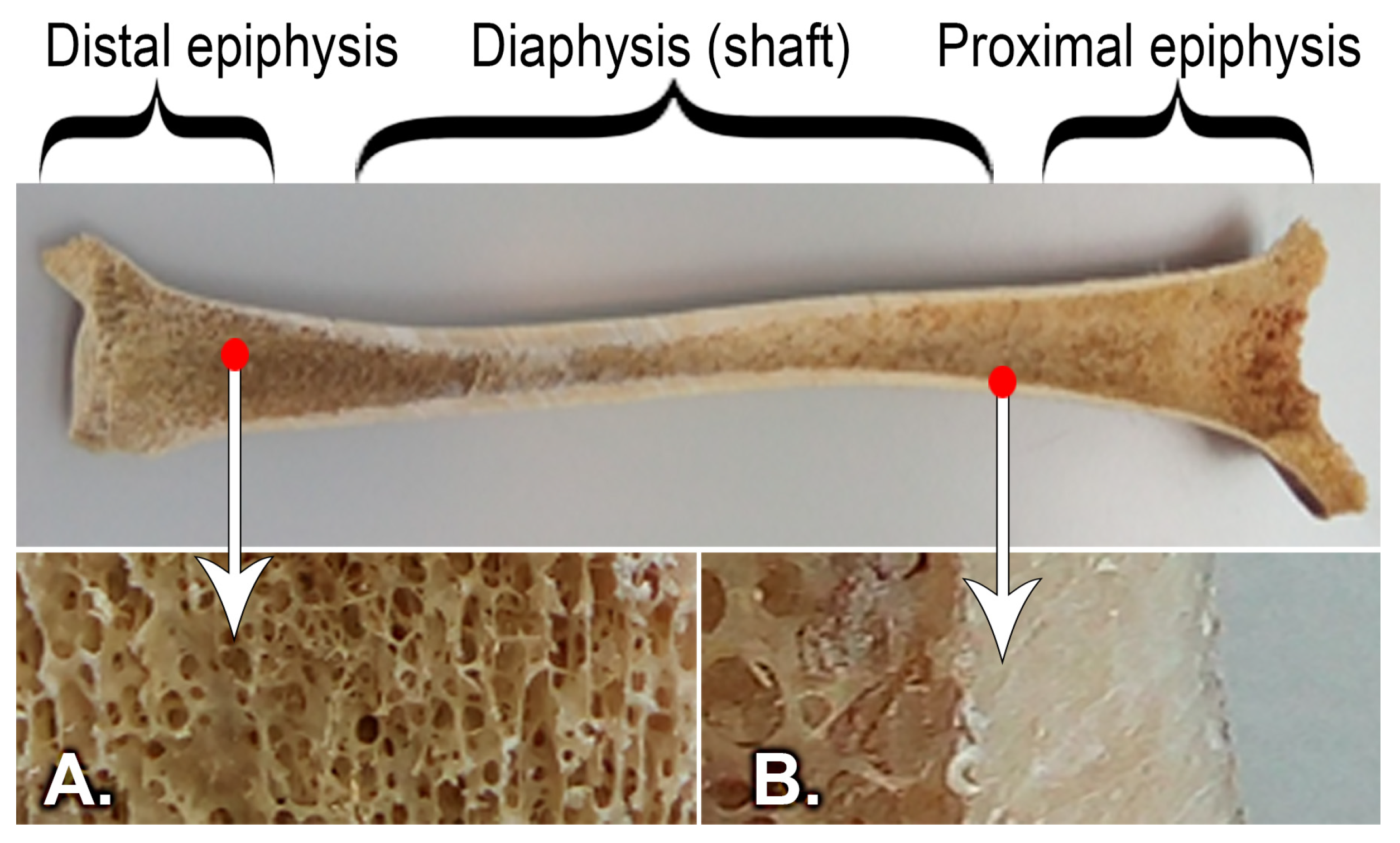
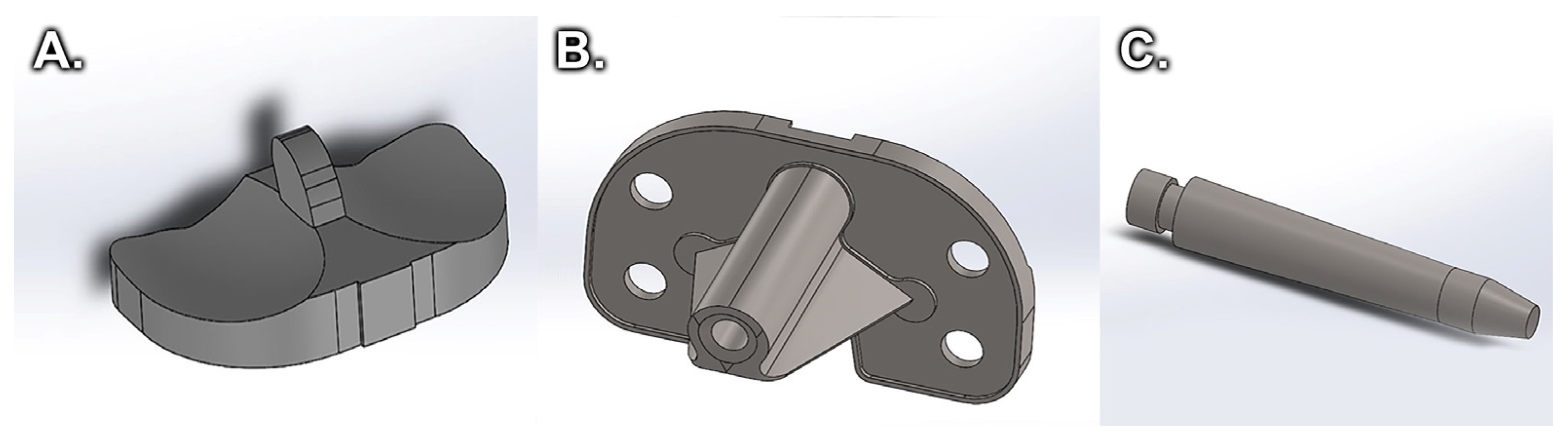
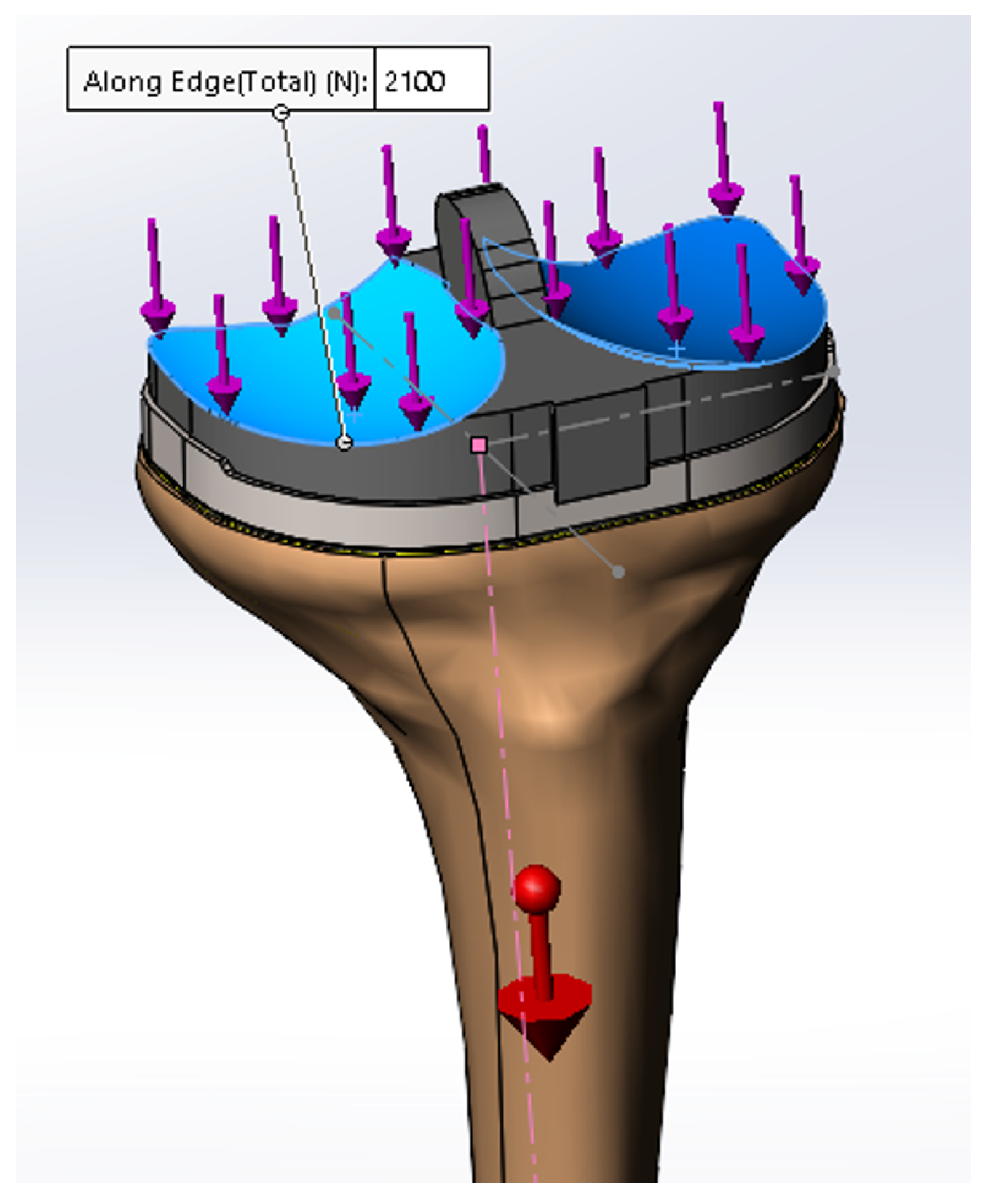
2. Materials and Methods
2.1. Static Compression Behavior
2.2. Compression Fatigue
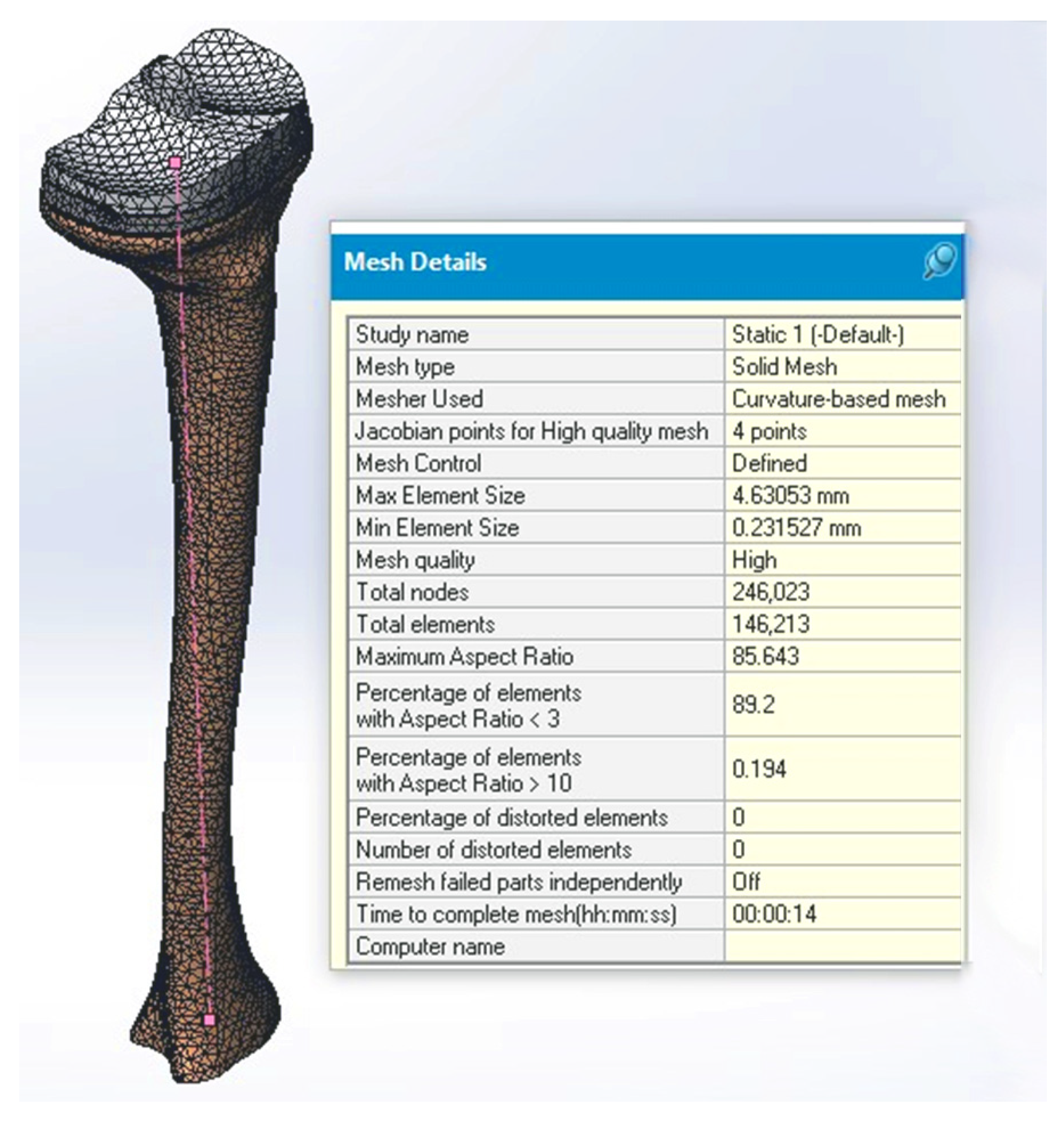
2.3. Stress in Linear Dynamic Regime
3. Results
4. Discussion
5. Conclusions
- If the bone is healthy with a good mineral density, so it has good physical properties (for example in the case of secondary arthrosis), then it is not necessary to use the tibial extension in the primary TKA surgery, because the benefits it brings are fewer than the long-term negative consequences.
- If the properties of the bone are altered, then it is necessary to use the tibial extension in the primary TKA operation, because it increases the life of the knee implant.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williams, A.; Logan, M. Understanding tibio-femoral motion. Knee 2004, 11, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bălașa, M.C.; Cuculici, S.; Panțu, C.M.; Mihai, S.; Negrea, A.D.; Zdrafcu, M.O. Using 3D scanning techniques in orthopedic systems modeling. Sci. Bull. Valahia Univ. Mater. Mech. 2017, 15, 41–47. [Google Scholar]
- Walsh, C.P.; Han, S.; Canham, C.D.; Gonzalez, J.L.; Noble, P.; Incavo, S.J. Total knee arthroplasty in the osteoporotic tibia: A biomechanical evaluation of the role of stem extensions and cementing techniques. J. Am. Acad. Orthop. Surg. 2019, 27, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, I.; Heinlein, B.; Graichen, F.; Bender, A.; Rohlmann, A.; Halder, A.M. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J. Biomech. 2010, 43, 2164–2173. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.S.; Choe, J.W.; Kim, H.J. Long-term comparison of fixed-bearing and mobile-bearing total knee replacements in patients younger than fifty-one years of age with osteoarthritis. J. Bone Jt. Surg. Am. 2012, 94, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, J.J.; Wells, C.W.; Liu, S.S.; Goetz, D.D.; Johnston, R.C. Cemented rotating platform total knee replacement: A concise follow-up, at a minimum of twenty years, of a previous report. J. Bone Jt. Surg. Am. 2010, 92, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yoon, S.H.; Kim, J.S. The long-term results of simultaneous fixed-bearing and mobile-bearing total knee replacements performed in the same patient. J. Bone Jt. Surg. Br. 2007, 89, 1317–1323. [Google Scholar] [CrossRef]
- Tang, Z.; Gong, Z.; Sun, X. LncRNA DANCR involved osteolysis after total hip arthroplasty by regulating FOXO1 expression to inhibit osteoblast differentiation. J. Biomed. Sci. 2018, 25, 4. [Google Scholar] [CrossRef] [Green Version]
- Anthony, G.; Raso, V.; Liggins, A.; Amirfazli, A. Contribution of loading conditions and material properties to stress shielding near the tibial component of total knee replacements. J. Biomech. 2007, 40, 1410–1416. [Google Scholar]
- Bălaşa, M.C.; Filip, V.; Nicolescu, C.M.; Bumbac, M.; Mihai, S.; Gheboianu, A.I. X-ray diffraction and nanoindentation characterization of bone tissue affected by severe osteoarthritis. J. Sci. Arts 2018, 1, 265–269. [Google Scholar]
- Fonseca, F.; Rebelo, E.; Completo, A. Tibial periprosthetic fracture combined with tibial stem stress fracture from total knee arthroplasty. Rev. Bras. Ortop. 2011, 46, 736–750. [Google Scholar] [CrossRef] [Green Version]
- Fournier, G.; Muller, B.; Gaillard, R.; Batailler, C.; Lustig, S.; Servien, E. Increased survival rate for primary TKA with tibial short extension stems for severe varus deformities at a minimum of 2 years follow-up. Knee Surg. Sports Traumatol Arthrosc. 2020, 28, 3780–3786. [Google Scholar] [CrossRef]
- Hegde, V.; Bracey, D.N.; Brady, A.C.; Kleeman-Forsthuber, L.T.; Dennis, D.A.; Jennings, J.M. A Prophylactic Tibial Stem Reduces Rates of Early Aseptic Loosening in Patients with Severe Preoperative Varus Deformity in Primary Total Knee Arthroplasty. J. Arthroplast. 2021, 36, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Bernakiewics, M.; Baleani, M.; Cristofolini, L. Large-sliding contact elements accurately predict levels of bone implant micromotion relevant to osseointegration. J. Biomech. 2000, 33, 1611–1618. [Google Scholar] [CrossRef]
- Stadler, C.; Luger, M.; Stevoska, S.; Gahleitner, M.; Pisecky, L.; Gotterbarm, T.; Klasan, A.; Klotz, M.C. High Reoperation Rate in Mobile-Bearing Total Ankle Arthroplasty in Young Patients. Medicina 2022, 58, 288. [Google Scholar] [CrossRef] [PubMed]
- Manzotti, A.; Confalonieri, N.; Pullen, C. Intra-operative tibial fracture during computer assisted total knee replacement: A case report. Knee Surg. Sports Traumatol Arthrosc. 2008, 16, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Tey, I.K.; Chong, K.; Singh, I. Stress fracture of the distal tibia secondary to severe knee osteoarthritis: A case report. J. Orthop. Surg. (Hong Kong) 2006, 14, 212–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullaji, A.; Shetty, G. Total knee arthroplasty for arthritic knees with tibiofibular stress fractures: Classification and treatment guidelines. J. Arthroplast. 2010, 25, 295–296. [Google Scholar] [CrossRef]
- Brouwer, R.W.; Jakma, T.S.C.; Bierma-Zeinstra, S.M.A.; Ginai, A.Z.; Verhaar, J. The whole leg radiograph standing versus supine for determining axial alignment. Acta. Orthop. Scand. 2003, 74, 565–568. [Google Scholar] [CrossRef] [Green Version]
- Haas, S.B.; Insall, J.N.; Montgomery, W.; Windsor, R.E. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J. Bone Jt. Surg. Am. 1995, 77, 1700–1707. [Google Scholar] [CrossRef]
- Cintra, F.F.; Yepéz, A.K.; Rasga, M.G.S.; Abagge, M.; Alencar, P.G.C. Tibial component in revision of total knee arthroplasty: Comparison between cement and hybrid fixation. Rev. Bras. Ortop. 2011, 46, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Shannon, B.D.; Klassen, J.F.; Rand, J.A. Revision total knee arthroplasty with cemented components and uncemented intramedullary stems. J. Arthroplast. 2003, 18, 27–32. [Google Scholar] [CrossRef]
- Lonner, J.H.; Klotz, M.; Levitz, C.; Lotke, P.A. Changes in bone density after cemented total knee arthroplasty: Influence of stem design. J. Arthroplast. 2001, 16, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Gofton, W.T.; Tsigaras, H.; Butler, R.A.; Patterson, J.J.; Barrack, R.L.; Rorabeck, C.H. Revision total knee arthroplasty: Fixation with modular stems. Clin. Orthop. Relat. Res. 2002, 404, 158–168. [Google Scholar] [CrossRef]
- Nelson, C.L.; Lonner, J.H.; Rand, J.A.; Lotke, P.A. Strategies of stem fixation and the role of supplemental bone graft in revision total knee arthroplasty. J. Bone Jt. Surg. Am. 2003, 85, S52–S57. [Google Scholar] [CrossRef]
- Whittaker, J.; Dharmarajan, R.; Toms, A.D. The management of bone loss in revision total knee replacement. J. Bone Jt. Surg. 2008, 90-B, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Mabry, T.M.; Hanssen, A.D. The role of stems and augments for bone loss in revision knee arthroplasty. J. Arthroplast. 2007, 22, 56–60. [Google Scholar] [CrossRef]
- Pasquier, G.J.M.; Huten, D.; Common, H.; Migaud, H.; Putman, S. Extraction of total knee arthroplasty intramedullary stem extensions. Orthop. Traumatol. Surg. Res. 2020, 106, S135–S147. [Google Scholar] [CrossRef]
- Mittal, A.; Bhosale, P.; Suryawanshi, A.; Purohit, S. One-stage long-stem total knee arthroplasty for arthritic knees with stress fractures. J. Orthop. Surg. 2013, 21, 199–203. [Google Scholar] [CrossRef]
- Balaşa, M.C.; Filip, V. Analysis of the static and dynamic mechanical behavior of a tibial bone-knee implant assembly without a tibial extension. In Proceedings of the International Conference of Mechatronics and Cyber-MixMechatronics-2019, Bucharest, Romania, 5–6 September 2019; pp. 194–205. [Google Scholar]
- Completo, A.; Rego, A.; Fonseca, F.; Ramos, A.; Relvas, C.; Simões, J.A. Biomechanical evaluation of proximal tibia behaviour with the use of femoral stems in revision TKA: An in vitro and finite element analysis. Clin. Biomech. 2010, 25, 159–165. [Google Scholar] [CrossRef]
- Femandes, P.R.; Folgado, J.; Jacobs, C.; Pellegrini, V. A contact model with ingrowth control for bone remodelling around cementless stems. J. Biomech. 2002, 35, 167–176. [Google Scholar] [CrossRef]
- Fernandes, P.; Rodrigues, H.; Jacobs, C. A model of bone adaptation using a global optimisation criterion based on the trajectorial theory of Wolff. Comput. Methods Biomech. Biomed. Eng. 1999, 2, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Nyman, J.F.; Hazelwood, S.J.; Bruce Martin, R.; Oscar, C.; Yeh, O.C. Long stemmed total knee arthroplasty with interlocking screws: A computational bone adaptation study. J. Orthop. Res. 2004, 22, 51–57. [Google Scholar] [CrossRef]
- Tóth, T.; Živčák, J. A comparison of the outputs of 3D scanners. Procedia. Eng. 2014, 69, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Bălaşa, M.C.; Filip, V.; Mihai, S.; Negrea, A.D.; Tomescu, G. Modeling the tibial bone using cad techniques, starting from the 3D scan Model. Int. J. Mechatron. Appl. Mech. 2018, 3, 213–219. [Google Scholar]
- Jiang, L.; Li, Y.; Xiong, C. Preparation and biological properties of a novel composite scaffold of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone tissue engineering. J. Biomed. Sci. 2009, 16, 65. [Google Scholar]
- Zhuang, W.Z.; Lin, Y.H.; Su, L.J.; Wu, M.S.; Jeng, H.Y.; Chang, H.C. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 28. [Google Scholar] [CrossRef] [PubMed]
- Mercer, C.; He, M.Y.; Wang, R.; Evans, A.G. Mechanisms governing the inelastic deformation of cortical bone and application to trabecular bone. Acta. Biomater. 2006, 2, 59–68. [Google Scholar] [CrossRef]
- Nadorf, J.; Kinkel, S.; Gantz, S.; Jakubowitz, E.; Kretzer, J.P. Tibial revision knee arthroplasty with metaphyseal sleeves: The effect of stems on implant fixation and bone flexibility. PLoS ONE 2017, 12, e0177285. [Google Scholar] [CrossRef] [Green Version]
- Bautista, A.I. A finite element analysis of tibial stem geometry for total knee replacements—A Thesis presented to the Faculty of California Polytechnic State University, Cal Poly, San Luis Obispo, California, USA. Master’s Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, 2015. [Google Scholar]
- Solidworks Web Help. Available online: https://help.solidworks.com/2017/english/SolidWorks/cworks/c_Linear_Static_versus_Linear_Dynamic_Analysis.htm (accessed on 24 September 2021).
- Xie, S.; Conlisk, N.; Hamilton, D.; Scott, C.; Burnett, R.; Pankaj, P. Metaphyseal cones in revision total knee arthroplasty: The role of stems. Bone Jt. Res. 2020, 16, 162–172. [Google Scholar] [CrossRef]
- Alipit, V.; Kirk, A.; Scholl, D.; Schmidig, G.; Springer, B.D.; Lee, G.C. Micromotion Analysis of Various Tibial Constructs in Moderate Tibial Defects in Revision Total Knee Arthroplasty. J. Arthroplast. 2021, 36, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Frehill, B.; Crocombe, A.; Cirovic, S.; Agarwal, Y.; Bradley, N. Initial stability of type-2 tibial defect treatments. Proc. Inst. Mech. Eng. H 2010, 224, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourne, R.B.; Finlay, J.B. The influence of tibial component intramedullary stems and implant-cortex contact on the strain distribution of the proximal tibia following total knee arthroplasty. An in vitro study. Clin. Orthop. Relat. Res. 1986, 208, 95–99. [Google Scholar] [CrossRef]
- Rawlinson, J.J.; Peters, L.E.; Campbell, D.A.; Windsor, R.; Wright, T.M.; Bartel, D.L. Cancellous bone strains indicate efficacy of stem augmentation in constrained condylar knees. Clin. Orthop. Relat. Res. 2005, 440, 107–116. [Google Scholar] [CrossRef]
- Soodmand, E.; Kluess, D.; Varady, P.A. Interlaboratory comparison of femur surface reconstruction from CT data compared to reference optical 3D scan. Biomed. Eng. OnLine 2018, 17, 29. [Google Scholar] [CrossRef] [Green Version]
- Quilez, M.P.; Seral, B.; Pérez, M.A. Biomechanical evaluation of tibial bone adaptation after revision total knee arthroplasty: A comparison of different implant systems. PLoS ONE 2017, 12, e0184361. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, M.; Mahbadi, H. Numerical investigation of mechanical behavior of human femoral diaphysis in normal and defective geometry: Experimental evaluation. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 637–652. [Google Scholar] [CrossRef]
- Lopes, V.M.M.; Neto, M.A.; Amaro, A.M.; Roseiro, L.M.; Paulino, M.F. FE and experimental study on how the cortex material properties of synthetic femurs affect strain levels. Med. Eng. Phys. 2017, 46, 96–109. [Google Scholar] [CrossRef]


| Density | Young’s Modulus (MPa) | Poisson’s Ratio | |
|---|---|---|---|
| (kg/m3) | υ | ||
| Cortical bone | 1800 | 18,000 | 0.3 |
| Healthy cancellous bone | 600 | 700 | 0.2 |
| Unhealthy cancellous bone | 1000 | 400 | 0.2 |
| Density | Young’s Modulus (MPa) | Poisson’s Ratio | |
|---|---|---|---|
| (kg/m3) | υ | ||
| Cement | 1100 | 2150 | 0.48 |
| Polyethylene insert | 952 | 1070 | 0.41 |
| Tibial component | 4428 | 104,800 | 0.31 |
| Tibial extension | 4428 | 104,800 | 0.31 |
| Healthy Bone | Max Stress (MPa) | Max Displacement (mm) |
| Without tibial stem | 18.04 | 0.1148 |
| With tibial stem | 21.2 | 0.1192 |
| Unhealthy Bone | Stress Max (MPa) | Displacement Max (mm) |
| Without tibial stem | 15.85 | 0.1047 |
| With tibial stem | 6.69 | 0.0645 |
| Healthy Bone | Unhealthy Bone | |||
|---|---|---|---|---|
| 1 | 2 | 1 | 2 | |
| Without tibial stem | 1,668,583 | 2,501,725 | 1,668,583 | 2,501,725 |
| With tibial stem | 6,667,433 | 7,668,583 | 4,168,008 | 5,834,292 |
| Tensile Stress | General Displacement | Z-Axis Displacement | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Max (MPa) | 1 (MPa) | 2 (MPa) | Max (mm) | 1 (mm) | 2 (mm) | Max (mm) | 1 (mm) | 2 (mm) | |
| Healthy bone | |||||||||
| Without tibial stem | 21.070 | 2.700 | 4.792 | 0.080 | 0.007 | 0.005 | 0.080 | 0.006 | 0.005 |
| With tibial stem | 15.010 | 0.470 | 2.940 | 0.081 | 0.006 | 0.003 | 0.079 | 0.004 | 0.003 |
| Unhealthy bone | |||||||||
| Without tibial stem | 9.707 | 0.893 | 0.472 | 0.080 | 0.006 | 0.004 | 0.079 | 0.005 | 0.004 |
| With tibial stem | 9.750 | 0.715 | 0.407 | 0.084 | 0.005 | 0.003 | 0.082 | 0.004 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filip, A.C.; Cuculici, S.A.; Cristea, S.; Filip, V.; Negrea, A.D.; Mihai, S.; Pantu, C.M. Tibial Stem Extension versus Standard Configuration in Total Knee Arthroplasty: A Biomechanical Assessment According to Bone Properties. Medicina 2022, 58, 634. https://doi.org/10.3390/medicina58050634
Filip AC, Cuculici SA, Cristea S, Filip V, Negrea AD, Mihai S, Pantu CM. Tibial Stem Extension versus Standard Configuration in Total Knee Arthroplasty: A Biomechanical Assessment According to Bone Properties. Medicina. 2022; 58(5):634. https://doi.org/10.3390/medicina58050634
Chicago/Turabian StyleFilip, Alexandru Cristian, Stefan Alexandru Cuculici, Stefan Cristea, Viviana Filip, Alexis Daniel Negrea, Simona Mihai, and Cosmin Marian Pantu. 2022. "Tibial Stem Extension versus Standard Configuration in Total Knee Arthroplasty: A Biomechanical Assessment According to Bone Properties" Medicina 58, no. 5: 634. https://doi.org/10.3390/medicina58050634







