Factors Associated with Prolonged RT-PCR SARS-CoV-2 Positive Testing in Patients with Mild and Moderate Forms of COVID-19: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
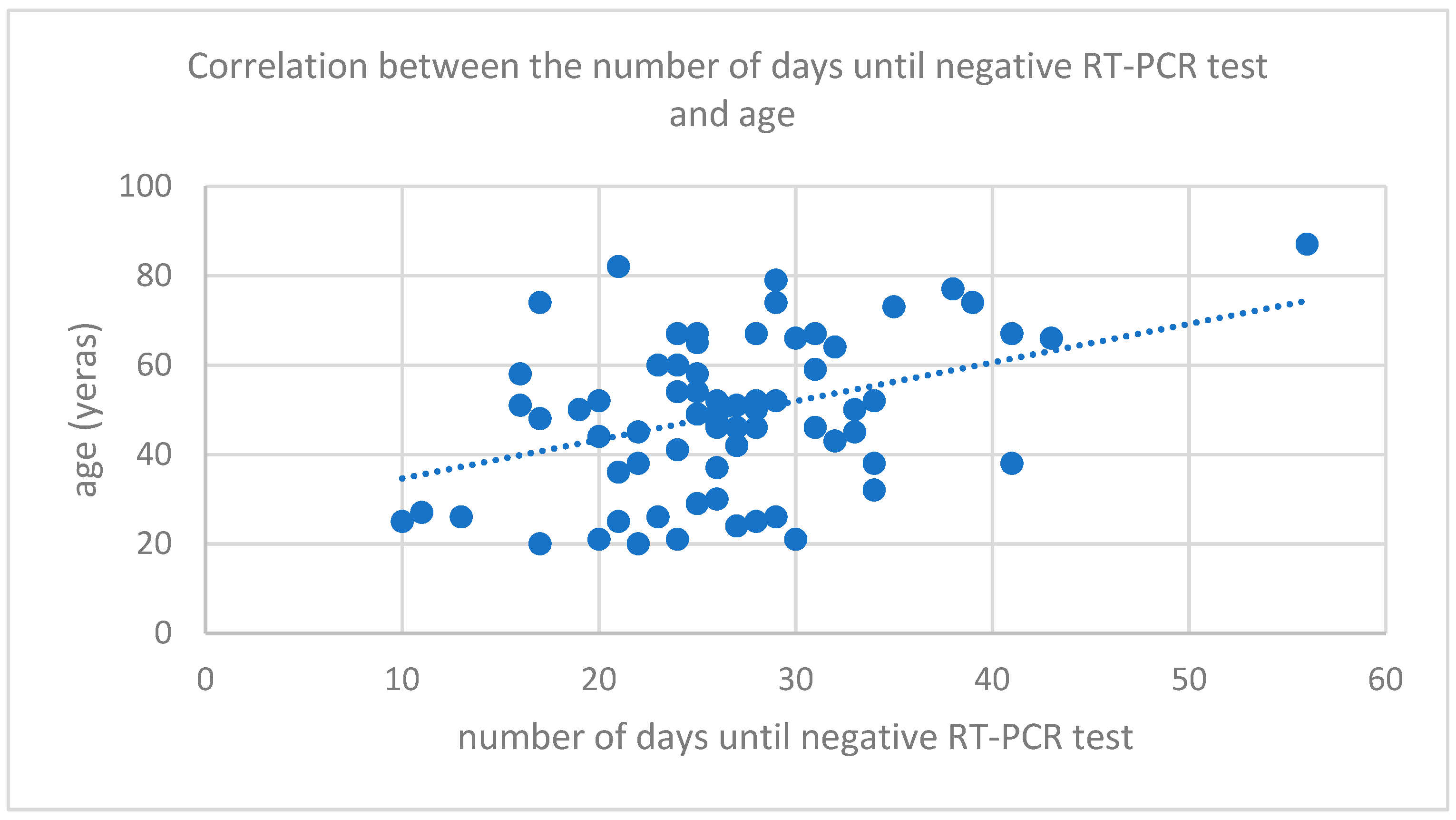
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romanian National Institute of Public Health. COVID-19 Surveillance Methodology Update 27 January 2020. Available online: https://www.cnscbt.ro/index.php/metodologii/infectia-2019-cu-ncov/1334-metodologia-de-supraveghere-a-infectiei-cu-2019-ncov-27-01-2020 (accessed on 26 February 2022).
- Man, M.A.; Rajnoveanu, R.-M.; Motoc, N.S.; Bondor, C.I.; Chis, A.F.; Lesan, A.; Puiu, R.; Lucaciu, S.-R.; Dantes, E.; Gergely-Domokos, B.; et al. Neutrophil-to-lymphocyte ratio, platelets-to-lymphocyte ratio, and eosinophils correlation with high-resolution computer tomography severity score in COVID-19 patients. PLoS ONE 2021, 16, e0252599. [Google Scholar] [CrossRef]
- Romanian National Institute of Public Health. COVID-19 Surveillance Methodology Update 21 February 2022. Available online: https://insp.gov.ro/centrul-national-de-supraveghere-si-control-al-bolilor-transmisibile-cnscbt/infectia-cu-noul-coronavirus-sars-cov-2/informatii-pentru-personalul-medico-sanitar/ (accessed on 26 February 2022).
- Morone, G.; Palomba, A.; Iosa, M.; Caporaso, T.; De Angelis, D.; Venturiero, V.; Savo, A.; Coiro, P.; Carbone, D.; Gimigliano, F.; et al. Incidence and Persistence of Viral Shedding in COVID-19 Post-acute Patients with Negativized Pharyngeal Swab: A Systematic Review. Front. Med. 2020, 7, 562. [Google Scholar] [CrossRef]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [Green Version]
- Long, H.; Zhao, J.; Zeng, H.-L.; Lu, Q.-B.; Fang, L.-Q.; Wang, Q.; Wu, Q.-M.; Liu, W. Prolonged viral shedding of SARS-CoV-2 and related factors in symptomatic COVID-19 patients: A prospective study. BMC Infect. Dis. 2021, 21, 1282. [Google Scholar] [CrossRef]
- Walsh, K.A.; Jordan, K.; Clyne, B.; Rohde, D.; Drummond, L.; Byrne, P.; Ahern, S.; Carty, P.G.; O'Brien, K.K.; O'Murchu, E.; et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 2020, 81, 357–371. [Google Scholar] [CrossRef]
- Zheng, S.; Fan, J.; Yu, F.; Feng, B.; Lou, B.; Zou, Q.; Xie, G.; Lin, S.; Wang, R.; Yang, X.; et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: Retrospective cohort study. BMJ 2020, 369, m1443. [Google Scholar] [CrossRef] [Green Version]
- Tarabay, J.; Babiker, A.; Adelman, M.W.; Stittleburg, V.D.; Varkey, J.; Pouch, S.M.; Waggoner, J.; Piantadosi, A. 278. Immunocompromised Patients with Prolonged Viral Shedding of SARS-CoV-2. Open Forum Infect. Dis. 2021, 8, S244–S245. [Google Scholar] [CrossRef]
- Nakajima, Y.; Ogai, A.; Furukawa, K.; Arai, R.; Anan, R.; Nakano, Y.; Kurihara, Y.; Shimizu, H.; Misaki, T.; Okabe, N. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J. Infect. Chemother. 2020, 27, 387–389. [Google Scholar] [CrossRef]
- Karataş, A.; Inkaya, A.; Demiroğlu, H.; Aksu, S.; Haziyev, T.; Çınar, O.E.; Alp, A.; Uzun, Ö.; Sayınalp, N.; Göker, H. Prolonged viral shedding in a lymphoma patient with COVID-19 infection receiving convalescent plasma. Transfus. Apher. Sci. 2020, 59, 102871. [Google Scholar] [CrossRef]
- Bennasrallah, C.; Bannour, R.; Jlassi, O.; Kacem, M.; Ben Fredj, M.; Abroug, H.; Zemni, I.; Garrach, B.; Bahri, R.; Charfeddine, N.; et al. Three COVID-19 cases with a long-term viral shedding period in Tunisia. Pan Afr. Med. J. 2020, 35, 117. [Google Scholar] [CrossRef]
- Li, H.; Gu, X.; Li, H.; Gong, F.; Xu, J.; Wang, Y.; Li, H.; Ruan, S.; Yang, Q.; Cao, B. Risk Factors of Viral RNAaemia and Its Association With Clinical Prognosis Among Patients With Severe COVID-19. Chest 2021, 159, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Veyer, D.; Kernéis, S.; Poulet, G.; Wack, M.; Robillard, N.; Taly, V.; L’Honneur, A.-S.; Rozenberg, F.; Laurent-Puig, P.; Bélec, L.; et al. Highly Sensitive Quantification of Plasma Severe Acute Respiratory Syndrome Coronavirus 2 RNA Sheds Light on its Potential Clinical Value. Clin. Infect. Dis. 2020, 73, e2890–e2897. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- ORDER No. 555 of 3 April 2020 Approving the Plan of Action for Hospital Preparedness in the Context of the COVID-19 Coronavirus Outbreak, the List of Hospitals Providing Positive Testing for SARS-CoV-2 Virus in Phase I and Phase II and the List of Hospitals Support for Patients Tested Positive or Suspected of Having the SARS-CoV-2 Virus. Available online: https://legislatie.just.ro/Public/DetaliiDocument/224705 (accessed on 26 February 2022).
- Carmo, A.; Pereira-Vaz, J.; Mota, V.; Mendes, A.; Morais, C.; Da Silva, A.C.; Camilo, E.; Pinto, C.S.; Cunha, E.; Pereira, J.; et al. Clearance and persistence of SARS-CoV-2 RNA in patients with COVID-19. J. Med. Virol. 2020, 92, 2227–2231. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- To, K.K.W.; Tsang, O.T.Y.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.Y.; Cai, J.P.; Chan, J.M.C.; Chik, T.S.H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Lau, E.H.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672. [Google Scholar] [CrossRef] [Green Version]
- COVID-19 Investigation Team. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat. Med. 2020, 26, 861. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.C.; Biele, G.; Mühlemann, B.; Veith, T.; Schneider, J.; Beheim-Schwarzbach, J.; Bleicker, T.; Tesch, J.; Schmidt, M.L.; Sander, L.E.; et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science 2021, 373, eabi5273. [Google Scholar] [CrossRef]
- Ge, Y.; Martinez, L.; Sun, S.; Chen, Z.; Zhang, F.; Li, F.; Sun, W.; Chen, E.; Pan, J.; Li, C.; et al. COVID-19 Transmission Dynamics Among Close Contacts of Index Patients With COVID-19: A Population-Based Cohort Study in Zhejiang Province, China. JAMA Intern. Med. 2021, 181, 1343. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-Y.; Jian, S.-W.; Liu, D.-P.; Ng, T.-C.; Huang, W.-T.; Lin, H.-H.; The Taiwan COVID-19 Outbreak Investigation Team. Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Intern. Med. 2020, 180, 1156–1163. [Google Scholar] [CrossRef]
- Piubelli, C.; Deiana, M.; Pomari, E.; Silva, R.; Bisoffi, Z.; Formenti, F.; Perandin, F.; Gobbi, F.; Buonfrate, D. Overall decrease in SARS-CoV-2 viral load and reduction in clinical burden: The experience of a hospital in northern Italy. Clin. Microbiol. Infect. 2020, 27, 131.e1–131.e3. [Google Scholar] [CrossRef]
- Overview of Testing for SARS-CoV-2, the Virus That Causes COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html (accessed on 25 April 2022).
- Chang, D.; Lin, M.; Wei, L.; Xie, L.; Zhu, G.; Cruz, C.S.D.; Sharma, L. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA 2020, 323, 1092. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, J.; Chong, D.S.; Lai, W.Y. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 2004, 159, 229–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghamdi, I.G.; Hussain, I.I.; Almalki, S.S.; Alghamdi, M.S.; Alghamdi, M.M.; El-Sheemy, M.A. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: A descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int. J. Gen. Med. 2014, 7, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Channappanavar, R.; Fett, C.; Mack, M.; Ten Eyck, P.P.; Meyerholz, D.K.; Perlman, S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J. Immunol. 2017, 198, 4046–4053. [Google Scholar] [CrossRef]
- Mihaltan, F.D.; Rajnoveanu, A.-G.; Rajnoveanu, R.-M. Impact of Smoking on Women During the COVID-19 Pandemic. Front. Med. 2021, 8, 584061. [Google Scholar] [CrossRef]
- Rajnoveanu, R.-M.; Rajnoveanu, A.-G.; Ardelean, A.-B.; Todea, D.; Pop, C.-M.; Antoniu, S.; Motoc, N.; Chis, A.; Fildan, A.; Man, M. Pulmonologists Adherence to the Chronic Obstructive Pulmonary Disease GOLD Guidelines: A Goal to Improve. Medicina 2020, 56, 422. [Google Scholar] [CrossRef]

| Parameters | Mild (n = 27) | Moderate (n = 43) | p Value | |
|---|---|---|---|---|
| Age | 35.78 (±14.23) | 57.23 (±13.89) | 0.001 | |
| Gender | Male | 10 | 21 | 0.33 |
| Female | 17 | 22 | ||
| Symptoms | Absent | 9 | 20 | 0.03 |
| Present | 3 | 38 | ||
| BMI | 25.49 (±3.47) | 27.74 (±7.30) | 0.13 | |
| No days with traceable viral load | 25.93 (±6.02) | 26.97 (±8.30) | 0.72 | |
| Smoking | Never | 26 | 37 | 0.25 |
| Former | 1 | 2 | ||
| Current | 0 | 4 | ||
| Chronic cardiac disease | Yes | 1 | 23 | 0.001 |
| No | 26 | 20 | ||
| Chronic pulmonary disease | Yes | 0 | 3 | 0.06 |
| No | 27 | 40 | ||
| Neurological diseases | Yes | 1 | 2 | 0.62 |
| No | 26 | 41 | ||
| Chronic renal diseases | Yes | 2 | 6 | 0.47 |
| No | 25 | 37 | ||
| Chronic hepatic diseases | Yes | 0 | 5 | 0.04 |
| No | 27 | 38 | ||
| Neoplastic diseases | Yes | 1 | 5 | 0.11 |
| No | 26 | 38 | ||
| Eosinophils | 0.05 (0.005, 0.12) | 0.04 (0.004, 0.09) | 0.57 | |
| Lymphocyte | 1.85 (1.4, 2.42) | 1.52 (1, 1.89) | 0.05 | |
| Ferritin | 122.95 (21.3, 513.5) | 323 (140, 707) | 0.02 | |
| D-dimers | 0.39 (0.28, 0.63) | 0.76 (0.53, 7.71) | 0.01 | |
| C reactive protein | 0.48 (0.19, 2.08) | 2.72 (0.83, 9.25) | 0.002 | |
| Parameters | Days Until Negative RT-PCR Mean ± SD | p-Value | |
|---|---|---|---|
| Chronic cardiac disease | Present | 28.67 ± 9.375 | 0.001 |
| Absent | 25.39 ± 6.143 | ||
| Chronic pulmonary disease | present | 22.33 ± 6.110 | 0.33 |
| absent | 26.7 ± 7.699 | ||
| Neurological diseases | present | 37.33 ± 16.197 | 0.01 |
| absent | 26.03 ± 6.908 | ||
| Chronic renal diseases | Present | 35.20 ± 14.096 | 0.004 |
| absent | 25.85 ± 6.662 | ||
| Chronic liver diseases | present | 31.50 ± 11.402 | 0.04 |
| absent | 25.87 ± 6.903 | ||
| Neoplastic diseases | present | 28.83 ± 6.706 | 0.44 |
| Absent | 26.30 ± 7.747 | ||
| Diabetes | present | 26.46 ± 5.897 | 0.97 |
| absent | 26.53 ± 8.045 | ||
| Gender | Number | ||
| Female Male | 39 | 28.15 ± 7.375 | 0.04 |
| 31 | 24.45 ± 7.611 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motoc, N.S.; Ruta, V.-M.; Man, M.A.; Ungur, R.A.; Ciortea, V.M.; Irsay, L.; Nicola, A.; Valean, D.; Usatiuc, L.O.; Matei, I.R.; et al. Factors Associated with Prolonged RT-PCR SARS-CoV-2 Positive Testing in Patients with Mild and Moderate Forms of COVID-19: A Retrospective Study. Medicina 2022, 58, 707. https://doi.org/10.3390/medicina58060707
Motoc NS, Ruta V-M, Man MA, Ungur RA, Ciortea VM, Irsay L, Nicola A, Valean D, Usatiuc LO, Matei IR, et al. Factors Associated with Prolonged RT-PCR SARS-CoV-2 Positive Testing in Patients with Mild and Moderate Forms of COVID-19: A Retrospective Study. Medicina. 2022; 58(6):707. https://doi.org/10.3390/medicina58060707
Chicago/Turabian StyleMotoc, Nicoleta Stefania, Victoria-Maria Ruta, Milena Adina Man, Rodica Ana Ungur, Viorela Mihaela Ciortea, Laszlo Irsay, Andrea Nicola, Dan Valean, Lia Oxana Usatiuc, Ileana Rodica Matei, and et al. 2022. "Factors Associated with Prolonged RT-PCR SARS-CoV-2 Positive Testing in Patients with Mild and Moderate Forms of COVID-19: A Retrospective Study" Medicina 58, no. 6: 707. https://doi.org/10.3390/medicina58060707
APA StyleMotoc, N. S., Ruta, V.-M., Man, M. A., Ungur, R. A., Ciortea, V. M., Irsay, L., Nicola, A., Valean, D., Usatiuc, L. O., Matei, I. R., & Borda, I. M. (2022). Factors Associated with Prolonged RT-PCR SARS-CoV-2 Positive Testing in Patients with Mild and Moderate Forms of COVID-19: A Retrospective Study. Medicina, 58(6), 707. https://doi.org/10.3390/medicina58060707










