Relationship between Acute-Phase Symptoms and Immunoglobulin G Seropositivity up to Eight Months after COVID-19
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Laboratory Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, B.; Carius, B.M.; Chavez, S.; Liang, S.Y.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Clinical update on COVID-19 for the emergency clinician: Presentation and evaluation. Am. J. Emerg. Med. 2022, 54, 46–57. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, H.; Piralla, A.; Zuo, F.; Du, L.; Cassaniti, I.; Wan, H.; Kumagai-Braesh, M.; Andréll, J.; Percivalle, E.; Sammartino, J.C.; et al. Immunity to SARS-CoV-2 up to 15 months after infection. iScience 2022, 25, 103743. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, A.; Thomas, R.E.; Krishnan, R.A.; Thomas, T.; Thomas, R.; Vijayan, D.K.; Paul, J.K.; Vasudevan, D.M. Sequential Profiling of Anti-SARS CoV-2 IgG Antibody in Post COVID-19 Patients. Indian J. Clin. Biochem. 2021, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Štěpánek, L.; Janošíková, M.; Štěpánek, L.; Nakládalová, M.; Boriková, A. The kinetics and predictors of anti-SARS-CoV-2 antibodies up to eight months after symptomatic COVID-19: A Czech cross-sectional study. J. Med. Virol. 2022, 94, 1–8. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19) Panel. Symptoms. Available online: https://www.who.int/health-topics/coronavirus#tab=tab_3 (accessed on 12 April 2022).
- Centers for Disease Control and Prevention. COVID-19 Panel. Symptoms of COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 12 April 2022).
- Institute of Health Information and Statistics of the Czech Republic. COVID-19 Panel. Dokumenty a Pokyny, Zejména Pro Lékaře a Odbornou Veřejnost. Available online: https://www.uzis.cz/index.php?pg=covid-19 (accessed on 12 April 2022). (In Czech).
- DiaSorin. LIAISON® SARS-CoV-2 S1/S2 IgG. The Fully Automated Serology Test for the Detection of SARS-CoV-2 IgG Antibodies. Available online: https://www.diasorin.com/sites/default/files/allegati/liaisonr_sars-cov-2_s1s2_igg_brochure.pdf.pdf (accessed on 12 April 2022).
- Boonyaratanakornkit, J.; Morishima, C.; Selke, S.; Zamora, D.; McGuffin, S.; Shapiro, A.E.; Campbell, V.L.; McClurkan, C.L.; Jing, L.; Gross, R.; et al. Clinical, laboratory, and temporal predictors of neutralizing antibodies against SARS-CoV-2 among COVID-19 convalescent plasma donor candidates. J. Clin. Investig. 2021, 131, e144930. [Google Scholar] [CrossRef]
- Wardhani, S.O.; Fajar, J.K.; Nurarifah, N.; Hermanto, D.H.; Fatonah, S.; Djajalaksana, S.; Fatoni, A.Z.; Arsana, P.M.; Wulandari, L.; Soegiarto, G.; et al. The predictors of high titer of anti-SARS-CoV-2 antibody of convalescent plasma donors. Clin. Epidemiol. Glob. Health 2021, 11, 100763. [Google Scholar] [CrossRef]
- Balou, H.A.; Yaghubi Kalurazi, T.; Joukar, F.; Hassanipour, S.; Shenagari, M.; Khoshsorour, M.; Mansour-Ghanaei, F. High Seroprevalence of SARS-CoV-2 (COVID-19)-Specific Antibodies among Healthcare Workers: A Cross-Sectional Study in Guilan, Iran. J. Environ. Public Health 2021, 2021, 9081491. [Google Scholar] [CrossRef]
- Siller, A.; Wachter, G.A.; Neururer, S.; Pfeifer, B.; Astl, M.; Borena, W.; Kimpel, J.; Elmer, S.; Spöck, F.; Vales, A.; et al. Prevalence of SARS-CoV-2 antibodies in healthy blood donors from the state of Tyrol, Austria, in summer 2020. Wien Klin. Wochenschr. 2021, 133, 1272–1280. [Google Scholar] [CrossRef]
- Levi, R.; Ubaldi, L.; Pozzi, C.; Angelotti, G.; Sandri, M.T.; Azzolini, E.; Salvatici, M.; Savevski, V.; Mantovani, A.; Rescigno, M. The antibody response to SARS-CoV-2 infection persists over at least 8 months in symptomatic patients. Commun. Med. 2021, 1, 32. [Google Scholar] [CrossRef]
- Johnson, A.; Vincent, B.; Carson, P.; Skoy, E. Prevalence of SARS-CoV-2 antibodies among North Dakota community pharmacy personnel: A seroprevalence survey. J. Am. Pharm. Assoc. 2021, 61, e127–e132. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi Mehrabani, M.; Karvandi, M.S.; Maafi, P.; Doroudian, M. Neurological complications associated with COVID-19; molecular mechanisms and therapeutic approaches. Rev. Med. Virol. 2022, 32, e2334. [Google Scholar] [CrossRef]
- Veleri, S. Neurotropism of SARS-CoV-2 and neurological diseases of the central nervous system in COVID-19 patients. Exp. Brain Res. 2022, 240, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.M.; Faustini, S.E.; Perez-Toledo, M.; Jossi, S.; Allen, J.D.; Al-Taei, S.; Backhouse, C.; Dunbar, L.A.; Ebanks, D.; Emmanuel, B.; et al. Serological responses to SARS-CoV-2 following non-hospitalised infection: Clinical and ethnodemographic features associated with the magnitude of the antibody response. BMJ Open Respir. Res. 2021, 8, e000872. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.B.; Jarlhelt, I.; Pérez-Alós, L.; Hummelshøj Landsy, L.; Loftager, M.; Rosbjerg, A.; Helgstrand, C.; Bjelke, J.R.; Egebjerg, T.; Jardine, J.G.; et al. SARS-CoV-2 Antibody Responses Are Correlated to Disease Severity in COVID-19 Convalescent Individuals. J. Immunol. 2021, 206, 109–117. [Google Scholar] [CrossRef]
- Chia, W.N.; Zhu, F.; Ong, S.; Young, B.E.; Fong, S.W.; Le Bert, N.; Tan, C.W.; Tiu, C.; Zhang, J.; Tan, S.Y.; et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: A longitudinal study. Lancet Microbe 2021, 2, e240–e249. [Google Scholar] [CrossRef]
- Štěpánek, L.; Nakládalová, M.; Štěpánek, L.; Janošíková, M.; Boriková, A.; Vidlová, H. COVID-19 symptom duration predicts immunoglobulin G seropositivity. Bratisl. Lek. Listy 2021, 122, 861–865. [Google Scholar] [CrossRef]
- Masiá, M.; Telenti, G.; Fernández, M.; García, J.A.; Agulló, V.; Padilla, S.; García-Abellán, J.; Guillén, L.; Mascarell, P.; Asenjo, J.C.; et al. SARS-CoV-2 Seroconversion and Viral Clearance in Patients Hospitalized with COVID-19: Viral Load Predicts Antibody Response. Open Forum Infect. Dis. 2021, 8, ofab005. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Dadras, O.; Afsahi, A.M.; Pashaei, Z.; Mojdeganlou, H.; Karimi, A.; Habibi, P.; Barzegary, A.; Fakhfouri, A.; Mirzapour, P.; Janfaza, N.; et al. The relationship between COVID-19 viral load and disease severity: A systematic review. Immun. Inflamm. Dis. 2022, 10, e580. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Pandey, A.K.; Kaur, J.; Kumar, L.; Singh, M.; Das, S.; Purohit, S. Is there a correlation between viral load and olfactory & taste dysfunction in COVID-19 patients? Am. J. Otolaryngol. 2021, 42, 102911. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Deiana, G.; Lechien, J.R.; De Vito, A.; Cossu, A.; Dettori, M.; Del Rio, A.; Saussez, S.; Madeddu, G.; Babudieri, S.; et al. Correlations Between Olfactory Psychophysical Scores and SARS-CoV-2 Viral Load in COVID-19 Patients. Laryngoscope 2021, 131, 2312–2318. [Google Scholar] [CrossRef]
- Cho, R.; To, Z.; Yeung, Z.; Tso, E.; Fung, K.; Chau, S.; Leung, E.; Hui, T.; Tsang, S.; Kung, K.N.; et al. COVID-19 Viral Load in the Severity of and Recovery from Olfactory and Gustatory Dysfunction. Laryngoscope 2020, 130, 2680–2685. [Google Scholar] [CrossRef]
- National Institutes of Health. COVID-19 Treatment Guidelines. Clinical Spectrum of SARS-CoV-2 Infection. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 22 May 2022).
- Mankowski, N.; Al-Qurayshi, Z.; Souza, S.; Campbell, B.; Beighley, A.; Denson, J.; Mauldin, B.; Bojanowski, C.; Friedlander, P.; Zifodya, J. The effect of race, socioeconomic status, and comorbidity on patients afflicted with COVID 19: A Local Perspective. Ann. Epidemiol. 2021, 64, 83–87. [Google Scholar] [CrossRef]
- Mustanski, B.; Saber, R.; Ryan, D.T.; Benbow, N.; Madkins, K.; Hayford, C.; Newcomb, M.E.; Schrock, J.M.; Vaught, L.A.; Reiser, N.L.; et al. Geographic disparities in COVID-19 case rates are not reflected in seropositivity rates using a neighborhood survey in Chicago. Ann. Epidemiol. 2021, 66, 44–51. [Google Scholar] [CrossRef] [PubMed]
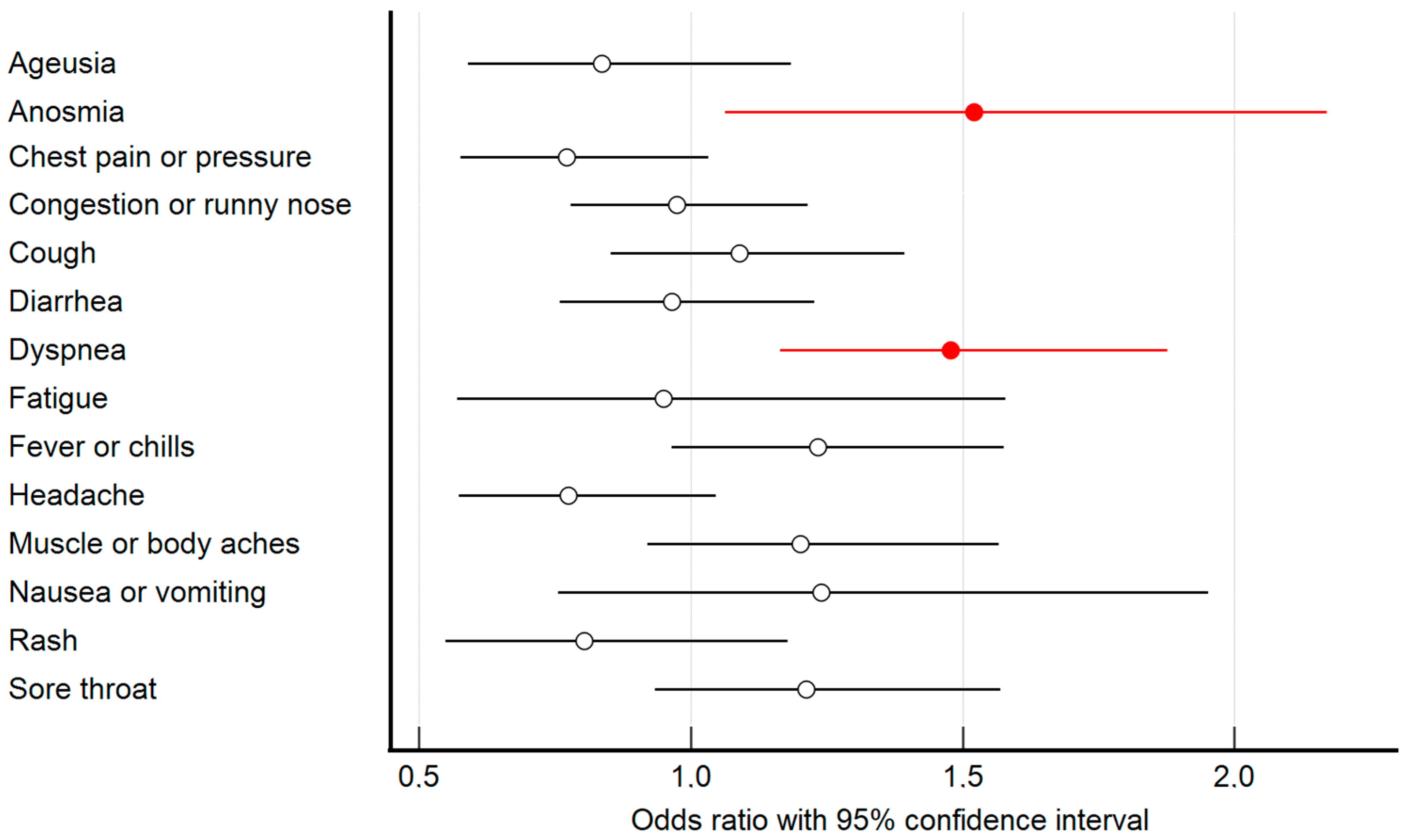
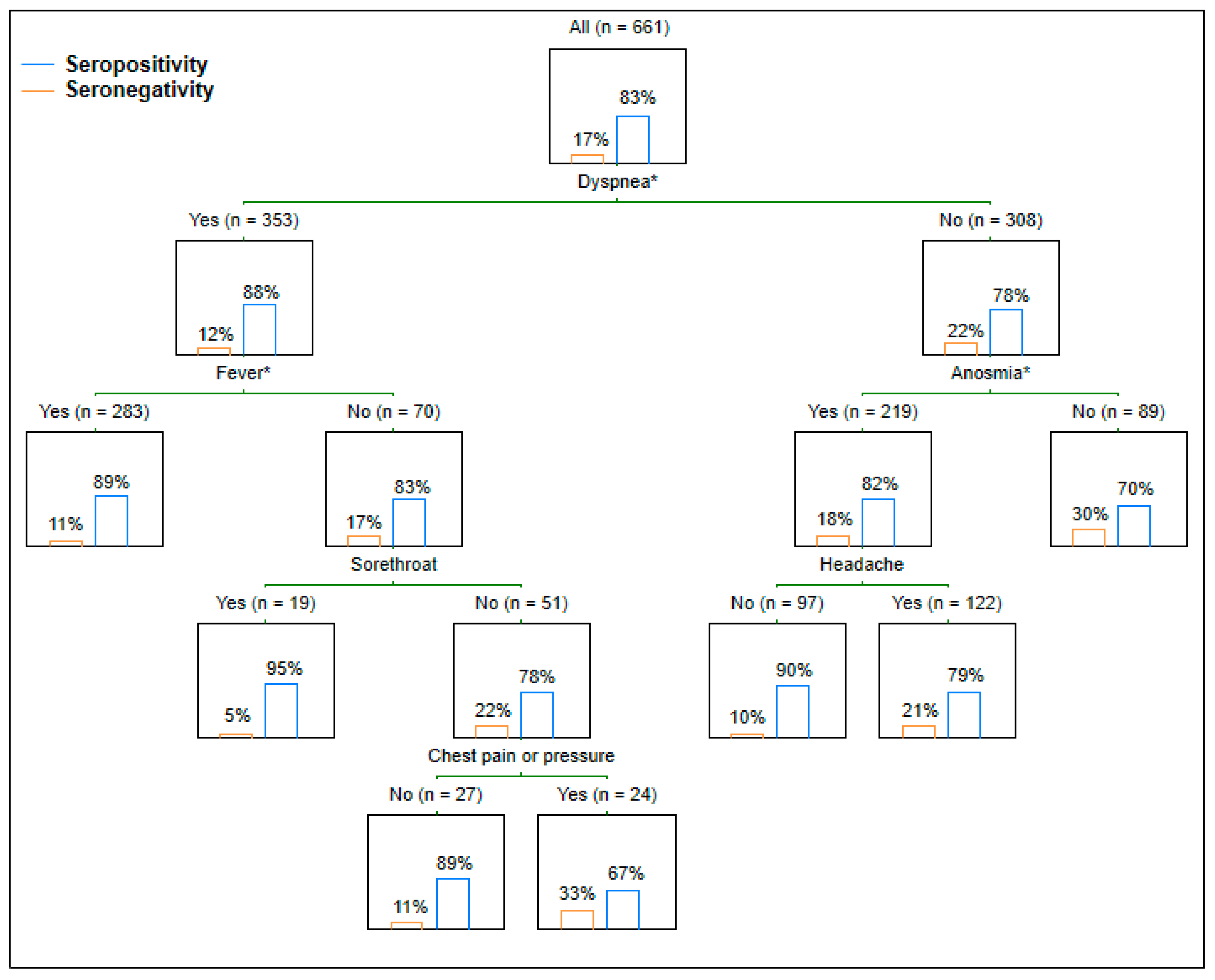
| Symptoms | Symptom Occurrence | Regression Analysis | ||||
|---|---|---|---|---|---|---|
| Entire Sample | Serology Status | Odds Ratio of Seropositivity | 95% Confidence Interval (Lower; Upper Value) | p-Value | ||
| Seropositivity (N, %) | Seronegativity (N, %) | |||||
| Ageusia/dysgeusia | 444 (67.2%) | 376 (68.2%) | 68 (61.8%) | 0.836 | 0.591; 1.184 | 0.314 |
| Anosmia/dysosmia | 479 (72.5%) | 409 (74.2%) | 70 (63.6%) | 1.520 | 1.064; 2.170 | 0.021 |
| Chest pain or pressure | 245 (37.1%) | 202 (36.7%) | 43 (39.1%) | 0.772 | 0.577; 1.032 | 0.223 |
| Congestion or runny nose | 335 (50.7%) | 281 (51%) | 54 (49.1%) | 0.974 | 0.78; 1.216 | 0.817 |
| Cough | 469 (71%) | 400 (72.6%) | 69 (62.7%) | 1.090 | 0.853; 1.394 | 0.49 |
| Diarrhea | 202 (30.6%) | 169 (30.7%) | 33 (30%) | 0.965 | 0.759; 1.228 | 0.773 |
| Dyspnea | 353 (53.4%) | 310 (56.3%) | 43 (39.1%) | 1.478 | 1.164; 1.877 | <0.001 |
| Fatigue | 631 (95.5%) | 527 (95.6%) | 104 (94.5%) | 0.950 | 0.571; 1.579 | 0.842 |
| Fever or chills | 483 (73.1%) | 412 (74.8%) | 71 (64.5%) | 1.233 | 0.965; 1.576 | 0.094 |
| Headache | 540 (81.7%) | 448 (81.3%) | 92 (83.6%) | 0.775 | 0.574; 1.047 | 0.096 |
| Muscle or body aches | 526 (79.6%) | 446 (80.9%) | 80 (72.7%) | 1.201 | 0.920; 1.567 | 0.178 |
| Nausea or vomiting | 54 (8.2%) | 48 (8.7%) | 6 (5.5%) | 1.240 | 0.787; 1.952 | 0.354 |
| Rash on skin | 50 (7.6%) | 40 (7.3%) | 10 (9.1%) | 0.804 | 0.549; 1.178 | 0.263 |
| Sore throat | 185 (28%) | 161 (29.2%) | 24 (21.8%) | 1.212 | 0.935; 1.570 | 0.146 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štěpánek, L.; Janošíková, M.; Nakládalová, M.; Štěpánek, L.; Tihelka, A.; Boriková, A.; Večeřová, R.; Sauer, P. Relationship between Acute-Phase Symptoms and Immunoglobulin G Seropositivity up to Eight Months after COVID-19. Medicina 2022, 58, 708. https://doi.org/10.3390/medicina58060708
Štěpánek L, Janošíková M, Nakládalová M, Štěpánek L, Tihelka A, Boriková A, Večeřová R, Sauer P. Relationship between Acute-Phase Symptoms and Immunoglobulin G Seropositivity up to Eight Months after COVID-19. Medicina. 2022; 58(6):708. https://doi.org/10.3390/medicina58060708
Chicago/Turabian StyleŠtěpánek, Ladislav, Magdaléna Janošíková, Marie Nakládalová, Lubomír Štěpánek, Antonín Tihelka, Alena Boriková, Renata Večeřová, and Pavel Sauer. 2022. "Relationship between Acute-Phase Symptoms and Immunoglobulin G Seropositivity up to Eight Months after COVID-19" Medicina 58, no. 6: 708. https://doi.org/10.3390/medicina58060708
APA StyleŠtěpánek, L., Janošíková, M., Nakládalová, M., Štěpánek, L., Tihelka, A., Boriková, A., Večeřová, R., & Sauer, P. (2022). Relationship between Acute-Phase Symptoms and Immunoglobulin G Seropositivity up to Eight Months after COVID-19. Medicina, 58(6), 708. https://doi.org/10.3390/medicina58060708






