The Usefulness of the Athens Insomnia Scale for Evaluating Sleep Disturbance in Patients with Chronic Liver Disease Comparing with Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Sleep Quality Questionnaires
2.3. Statistical Analysis
3. Results
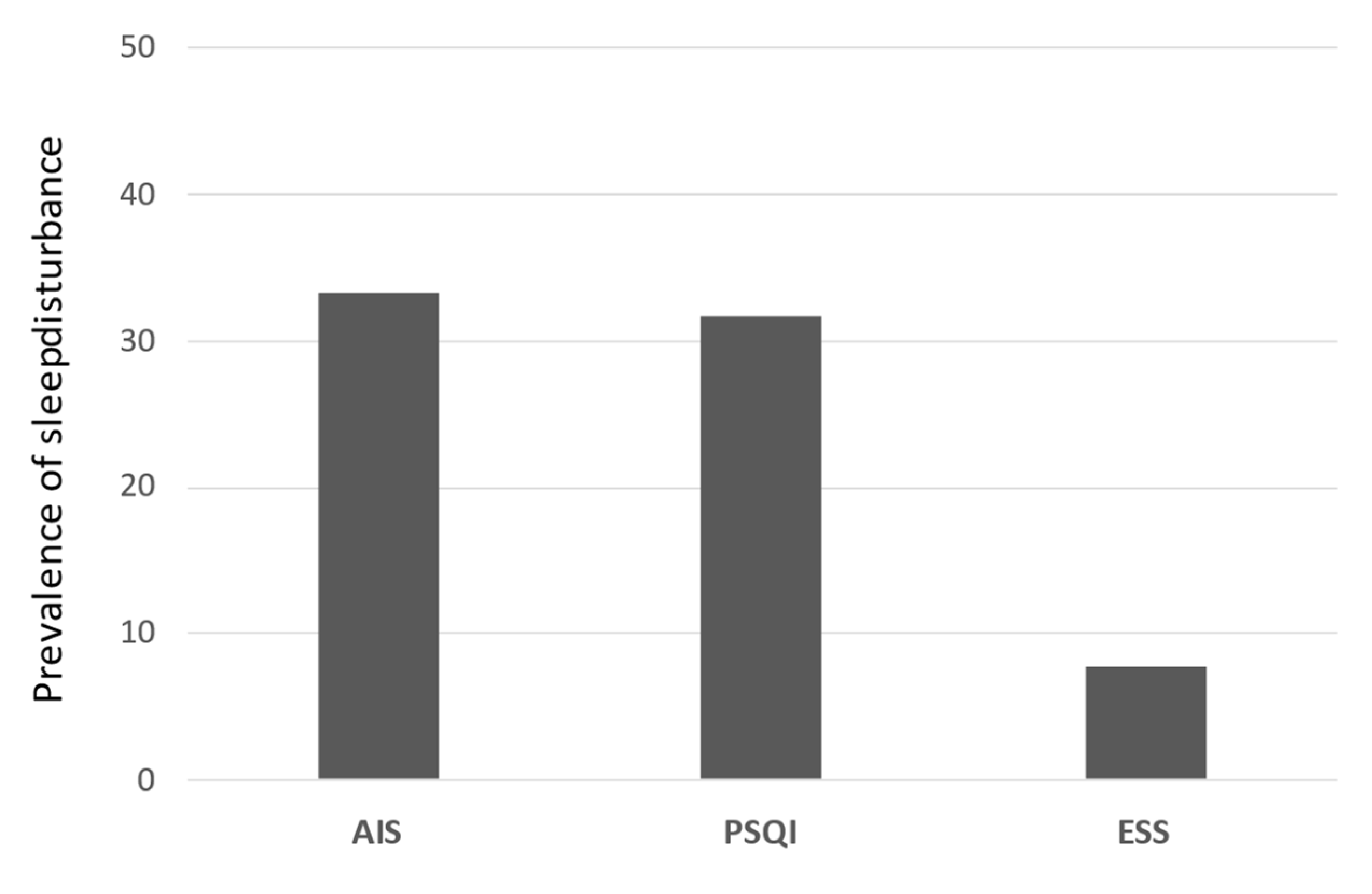
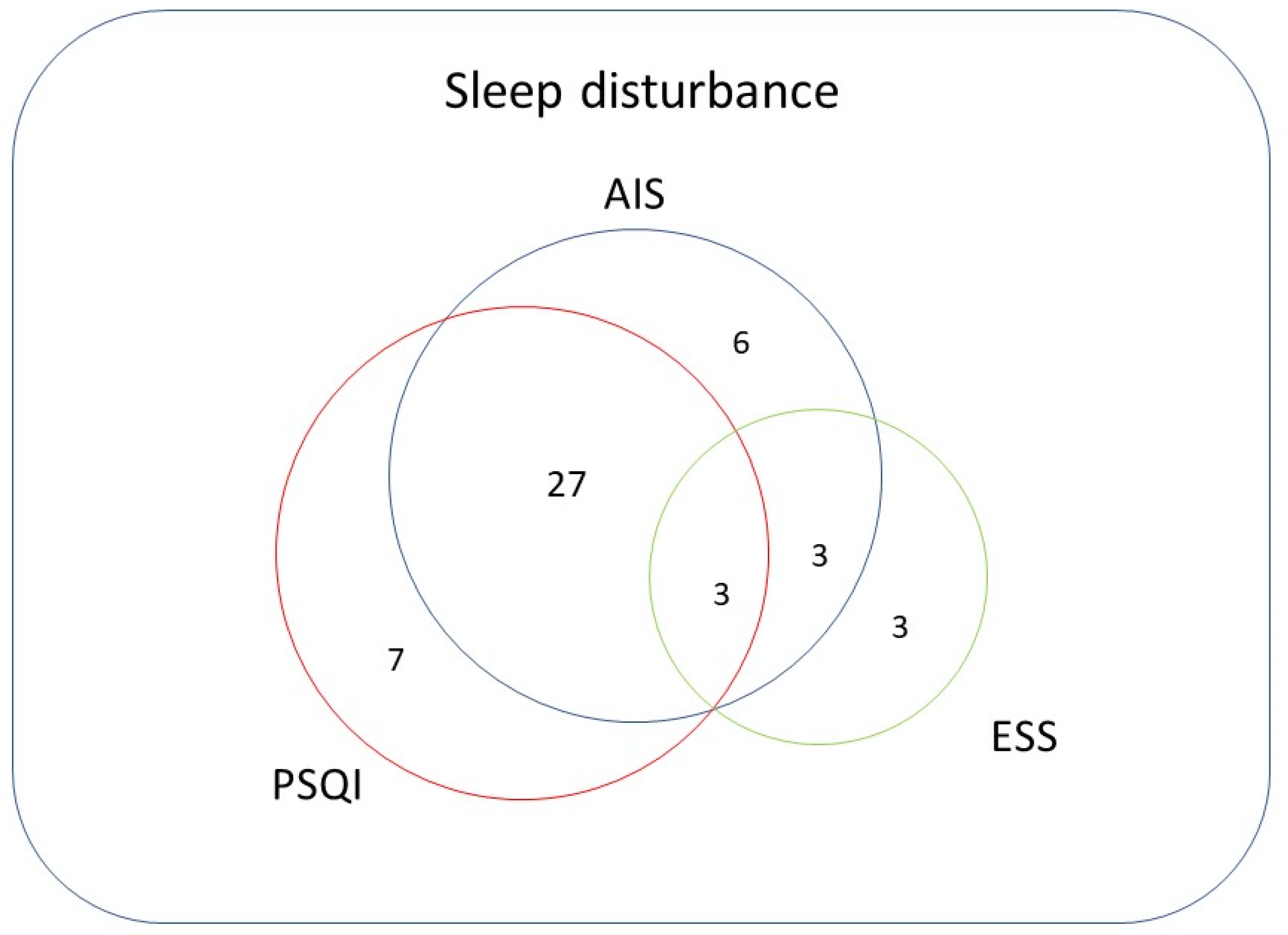
3.1. Prevalence of Sleep Disturbance
3.2. Baseline Characteristics of PSQI and AIS Scores
3.3. Relationships among Questionnaires
3.4. Comparison between the AIS and PSQI
3.5. Comparison of the ESS and PSQI or AIS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bianchi, G.; Amodio, P.; Salerno, F.; Merli, M.; Panella, C.; Loguercio, C.; Apolone, G.; Niero, M.; Abbiati, R. Italian Study Group for quality of life in cirrhosis. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology 2001, 120, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Ridola, L.; Nardelli, S.; Gioia, S.; Riggio, O. Quality of life in patients with minimal hepatic encephalopathy. World J. Gastroenterol. 2018, 24, 5446–5453. [Google Scholar] [CrossRef] [PubMed]
- Ghabril, M.; Jackson, M.; Gotur, R.; Weber, R.; Orman, E.; Vuppalanchi, R.; Chalasani, N. Most individuals with advanced cirrhosis have sleep disturbances, which are associated with poor quality of life. Clin. Gastroenterol. Hepatol. 2017, 15, 1271–1278.e6. [Google Scholar] [CrossRef] [Green Version]
- Bruyneel, M.; Sersté, T. Sleep disturbances in patients with liver cirrhosis: Prevalence, impact, and management challenges. Nat. Sci. Sleep 2018, 10, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Iwasa, M.; Karino, Y.; Kawaguchi, T.; Nakanishi, H.; Miyaaki, H.; Shiraki, M.; Nakajima, T.; Sawada, Y.; Yoshiji, H.; Okita, K.; et al. Relationship of muscle cramps to quality of life and sleep disturbance in patients with chronic liver diseases: A nationwide study. Liver Int. 2018, 38, 2309–2316. [Google Scholar] [CrossRef]
- Blei, A.T.; Córdoba, J.; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am. J. Gastroenterol. 2001, 96, 1968–1976. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Thacker, L.R.; Leszczyszyn, D.; Taylor, S.A.; Heuman, D.M.; Raman, S.; Sterling, R.K.; Siddiqui, M.S.; Stravitz, R.T.; Sanyal, A.J.; et al. Effects of obstructive sleep apnea on sleep quality, cognition, and driving performance in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2015, 13, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Dasarathy, S.; Mullen, K.D. Benzodiazepines in hepatic encephalopathy: Sleeping with the enemy. Gut 1998, 42, 764–765. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Labenz, C.; Baron, J.S.; Toenges, G.; Schattenberg, J.M.; Nagel, M.; Sprinzl, M.F.; Nguyen-Tat, M.; Zimmermann, T.; Huber, Y.; Marquardt, J.U.; et al. Prospective evaluation of the impact of covert hepatic encephalopathy on quality of life and sleep in cirrhotic patients. Aliment. Pharmacol. Ther. 2018, 48, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okajima, I.; Nakajima, S.; Kobayashi, M.; Inoue, Y. Development and validation of the Japanese version of the Athens Insomnia Scale. Psychiatry Clin. Neurosci. 2013, 67, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. The diagnostic validity of the Athens Insomnia Scale. J. Psychosom. Res. 2003, 55, 263–267. [Google Scholar] [CrossRef]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Walker, N.A.; Sunderram, J.; Zhang, P.; Lu, S.E.; Scharf, M.T. Clinical utility of the Epworth sleepiness scale. Sleep Breath 2020, 24, 1759–1765. [Google Scholar] [CrossRef]
- Mostacci, B.; Ferlisi, M.; Baldi Antognini, A.; Sama, C.; Morelli, C.; Mondini, S.; Cirignotta, F. Sleep disturbance and daytime sleepiness in patients with cirrhosis: A case control study. Neurol. Sci. 2008, 29, 237–240. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Hoch, C.C.; Yeager, A.L.; Kupfer, D.J. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 1991, 14, 331–338. [Google Scholar]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Iwata, Y.; Nishimura, T.; Nishiguchi, S.; Iijima, H. Frailty and sleep disorder in chronic liver diseases. Life 2020, 10, 137. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Uchiyama, M.; Okawa, M.; Liu, X.; Ogihara, R. An epidemiological study of insomnia among the Japanese general population. Sleep 2000, 23, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagnese, S.; Middleton, B.; Skene, D.J.; Morgan, M.Y. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int. 2009, 29, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Yun, K.E.; Jung, H.S.; Chang, Y.; Choi, E.S.; Kwon, M.J.; Lee, E.H.; Woo, E.J.; Kim, N.H.; Shin, H.; et al. Sleep duration and quality in relation to non-alcoholic fatty liver disease in middle-aged workers and their spouses. J. Hepatol. 2013, 59, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Jeon, Y.; Ma, J.; Choi, Y.; Ban, S.; Lee, S.; Lee, B.; Im, J.J.; Yoon, S.; Kim, J.E.; et al. Validation of the Athens Insomnia Scale for screening insomnia in South Korean firefighters and rescue workers. Qual. Life Res. 2015, 24, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, B.C.; Puri, V.; Sachdeva, S.; Srivastava, S. Sleep disturbances in patients of liver cirrhosis with minimal hepatic encephalopathy before and after lactulose therapy. Metab. Brain Dis. 2017, 32, 595–605. [Google Scholar] [CrossRef]
- Miyaaki, H.; Hiraoka, A.; Haraguchi, M.; Uojima, H.; Kawaratani, H.; Hiramatsu, A.; Hanai, T.; Hiasa, Y.; Yoshiji, H.; Okita, K.; et al. Proposal for new sleep disorder criteria in patients with chronic liver disease: Influence of liver-related complications. Hepatol. Res. 2022, 52, 364–370. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Effect of sarcopenia on sleep disturbance in patients with chronic liver diseases. J. Clin. Med. 2018, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, T.; Naota, T.; Miyaaki, H.; Miuma, S.; Isomoto, H.; Takeshima, F.; Nakao, K. Effect of an oral branched chain amino acid-enriched snack in cirrhotic patients with sleep disturbance. Hepatol. Res. 2010, 40, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Shimada, M.; Hirashima, N.; Iwase, H.; Saito, M.; Kondo, H.; Urata, N.; Unita, S.; Kondo, T.; Tanaka, D.; Tsunekawa, T.; et al. Evaluation of muscle cramp associated with liver cirrhosis with a focus on the liver function and nutritional status. Intern. Med. 2021, 60, 1343–1348. [Google Scholar] [CrossRef]
- Reves, J.G.; Fragen, R.J.; Vinik, H.R.; Greenblatt, D.J. Midazolam: Pharmacology and uses. Anesthesiology 1985, 62, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Wahab, E.A.; Hamed, E.F.; Ahmad, H.S.; Abdel Monem, S.M.; Fathy, T. Conscious sedation using propofol versus midazolam in cirrhotic patients during upper GI endoscopy: A comparative study. JGH Open 2019, 3, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Abnormal PSQI (n = 37) | Normal PSQI (n = 80) | p-Value | |
|---|---|---|---|
| Age (years) | 66.9 ± 9.5 | 70.1 ± 12.2 | 0.133 |
| Sex (male:female) | 24:13 | 54:26 | 0.834 |
| Cause (B:C:NAFLD:ALD:AIH/PBC:others) | 6: 3:11:11:4:2 | 9:18:22:19:6:6 | 0.477 |
| Cirrhosis (yes:no) | 36:1 | 68:12 | 0.060 |
| HCC (yes:no) | 22:15 | 51:29 | 0.685 |
| Alb | 3.6 ± 0.7 | 3.7 ± 0.6 | 0.422 |
| T-Bil | 1.6 ± 1.9 | 1.6 ± 3.6 | 0.876 |
| ALBI score | −2.22 ± 0.71 | −2.31 ± 0.52 | 0.461 |
| mALBI grade (1:2a:2b:3) | 12:8:16:2 | 26:19:29:4 | 0.967 |
| Child–Pugh score (5:6:7:8:9:10 or more) | 11:8:15:3:0:0 | 27:19:30:3:0:0 | 0.745 |
| BUN | 19.4 ± 11.4 | 17.4 ± 9.3 | 0.359 |
| Cre | 1.0 ± 0.6 | 0.9 ± 0.3 | 0.106 |
| ALT | 29.2 ± 19.7 | 57.4 ± 165.6 | 0.139 |
| NH3 | 72.3 ± 51.9 | 51.5 ± 30.2 | 0.049 * |
| Hb | 12.1 ± 1.5 | 12.6 ± 2.0 | 0.089 |
| PT% | 83.1 ± 21.1 | 81.2 ± 17.5 | 0.649 |
| PLT | 11.0 ± 6.6 | 12.5 ± 6.9 | 0.250 |
| Sleep medication (yes:no) | 14:23 | 7:73 | <0.001 ** |
| Shakuyakukanzoto (yes:no) | 5:32 | 7:73 | 0.515 |
| BCAA (yes:no) | 19:18 | 24:56 | 0.039 * |
| Carnitine (yes:no) | 3:34 | 0:80 | 0.030 * |
| Abnormal AIS Score (n = 39) | Normal AIS Score (n = 78) | p-Value | |
|---|---|---|---|
| Age (years) | 66.4 ± 9.1 | 70.5 ± 12.3 | 0.044 * |
| Sex (male:female) | 24:15 | 54:24 | 0.414 |
| Cause (B:C:NAFLD:ALD:AIH/PBC:others) | 4:2:13:13:5:2 | 11:19:20:17:5:6 | 0.082 |
| Cirrhosis (yes:no) | 38:1 | 66:12 | 0.058 |
| HCC (yes:no) | 21:18 | 52:26 | 0.225 |
| Alb | 3.7 ± 0.7 | 3.7 ± 0.6 | 0.912 |
| T-Bil | 1.5 ± 1.8 | 1.7 ± 3.7 | 0.783 |
| ALBI score | −2.27 ± 0.67 | −2.29 ± 0.54 | 0.919 |
| mALBI grade (1:2a:2b:3) | 12:8:16:2 | 26:19:29:4 | 0.967 |
| Child–Pugh score (5:6:7:8:9:10 or more) | 20:8:3:3:2:2 | 34:24:8:5:5:2 | 0.748 |
| BUN | 18.6 ± 11.0 | 17.7 ± 9.5 | 0.656 |
| Cre | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.999 |
| ALT | 31.9 ± 20.3 | 56.8 ± 167.8 | 0.201 |
| NH3 | 64.8 ± 37.5 | 55.0 ± 40.6 | 0.255 |
| Hb | 12.2 ± 1.4 | 12.6 ± 2.0 | 0.327 |
| PT% | 85.9 ± 20.3 | 79.7 ± 17.6 | 0.118 |
| PLT | 11.3 ± 7.9 | 12.4 ± 6.5 | 0.453 |
| Sleep medication (yes:no) | 11:28 | 10:68 | 0.071 |
| Shakuyakukanzoto (yes:no) | 9:30 | 3:75 | 0.002 ** |
| BCAA (yes:no) | 19:20 | 24:54 | 0.069 |
| Carnitine (yes:no) | 2:37 | 1:77 | 0.257 |
| Abnormal AIS and PSQI (n = 30) | Normal AIS and PSQI (n = 71) | p-Value | |
|---|---|---|---|
| Age (years) | 66.1 ± 8.2 | 70.5 ± 12.2 | 0.041 * |
| Sex (male:female) | 19:11 | 49:22 | 0.136 |
| Cause (B:C:NAFLD:ALD:AIH/PBC:others) | 3:2:9:11:4:1 | 8:18:18:17:5:5 | 0.024 * |
| Cirrhosis (yes:no) | 30:0 | 60:11 | 0.468 |
| HCC (yes:no) | 18:12 | 48:23 | 0.681 |
| Alb | 3.6 ± 0.7 | 3.7 ± 0.6 | 0.893 |
| T-Bil | 1.6 ± 2.1 | 1.7 ± 3.8 | 0.703 |
| ALBI score | −2.21 ± 0.71 | −2.31 ± 0.52 | 0.931 |
| mALBI grade (1:2a:2b:3) | 9:7:12:2 | 24:18:26:3 | 0.729 |
| Child–Pugh score (5:6:7:8:9:10 or more) | 15:6:3:3:2:1 | 32:22:7:5:3:2 | 0.729 |
| BUN | 19.5 ± 12.3 | 17.6 ± 9.7 | 0.457 |
| Cre | 1.0 ± 0.5 | 0.9 ± 0.3 | 0.366 |
| ALT | 31.5 ± 21.2 | 60.4 ± 175.6 | 0.176 |
| NH3 | 69.1 ± 40.7 | 51.3 ± 31.1 | 0.071 |
| Hb | 12.3 ± 1.5 | 12.7 ± 2.0 | 0.251 |
| PT% | 85.6 ± 20.4 | 80.4 ± 17.0 | 0.247 |
| PLT | 11.4 ± 7.1 | 12.7 ± 6.7 | 0.406 |
| Sleep medication (yes:no) | 11:19 | 7:64 | 0.003 ** |
| Shakuyakukanzoto (yes:no) | 5:25 | 3:68 | 0.048 * |
| BCAA (yes:no) | 16:14 | 21:50 | 0.041 * |
| Carnitine (yes:no) | 2:28 | 0:71 | 0.086 |
| Abnormal ESS Score | Normal ESS Score | Sum | |
|---|---|---|---|
| Abnormal AIS score | 6 | 33 | 39 |
| Normal AIS score | 3 | 75 | 78 |
| Sum | 9 | 108 | 117 |
| (A) | |||
|---|---|---|---|
| Abnormal AIS | Normal AIS | Sum | |
| Abnormal PSQI | 30 | 7 | 37 |
| Normal PSQI | 9 | 71 | 80 |
| Sum | 39 | 78 | 117 |
| (B) | |||
| Abnormal ESS Score | Normal ESS Score | Sum | |
| Abnormal PSQI score | 3 | 34 | 37 |
| Normal PSQI score | 6 | 74 | 80 |
| Sum | 9 | 108 | 117 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaratani, H.; Miyaaki, H.; Hiraoka, A.; Nakao, K.; Hiasa, Y.; Yoshiji, H.; Okita, K.; Koike, K. The Usefulness of the Athens Insomnia Scale for Evaluating Sleep Disturbance in Patients with Chronic Liver Disease Comparing with Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale. Medicina 2022, 58, 741. https://doi.org/10.3390/medicina58060741
Kawaratani H, Miyaaki H, Hiraoka A, Nakao K, Hiasa Y, Yoshiji H, Okita K, Koike K. The Usefulness of the Athens Insomnia Scale for Evaluating Sleep Disturbance in Patients with Chronic Liver Disease Comparing with Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale. Medicina. 2022; 58(6):741. https://doi.org/10.3390/medicina58060741
Chicago/Turabian StyleKawaratani, Hideto, Hisamitsu Miyaaki, Atsushi Hiraoka, Kazuhiko Nakao, Yoichi Hiasa, Hitoshi Yoshiji, Kiwamu Okita, and Kazuhiko Koike. 2022. "The Usefulness of the Athens Insomnia Scale for Evaluating Sleep Disturbance in Patients with Chronic Liver Disease Comparing with Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale" Medicina 58, no. 6: 741. https://doi.org/10.3390/medicina58060741
APA StyleKawaratani, H., Miyaaki, H., Hiraoka, A., Nakao, K., Hiasa, Y., Yoshiji, H., Okita, K., & Koike, K. (2022). The Usefulness of the Athens Insomnia Scale for Evaluating Sleep Disturbance in Patients with Chronic Liver Disease Comparing with Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale. Medicina, 58(6), 741. https://doi.org/10.3390/medicina58060741






