Impact of Sarcopenia and Bone Mineral Density on Implant Failure after Dorsal Instrumentation in Patients with Osteoporotic Vertebral Fractures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Complications and Implant Failure
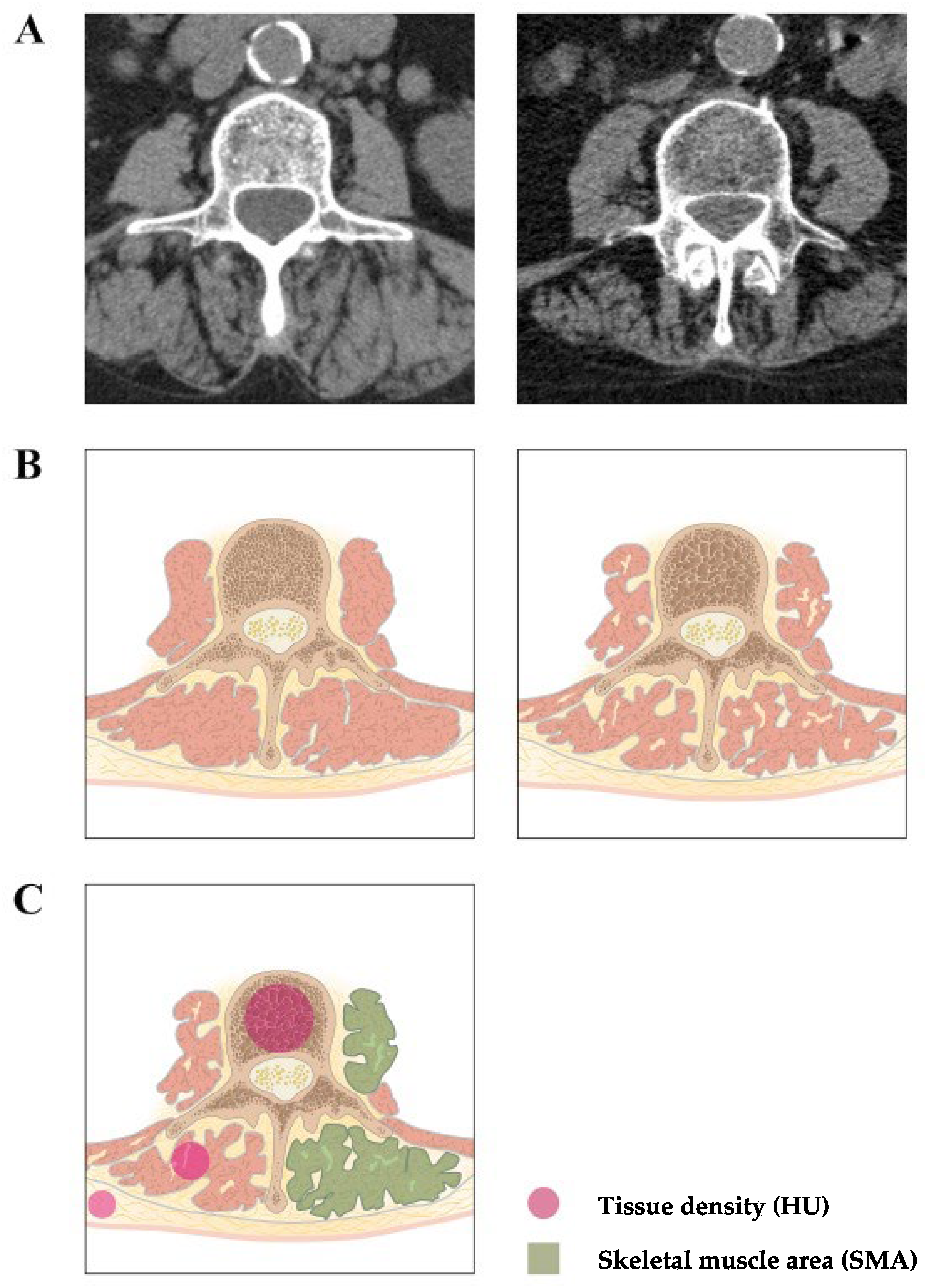
2.3. Measurement of Sarcopenia and Osteoporosis
2.4. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Postoperative Complications
3.3. Sarcopenia, BMI, and Osteoporosis
3.4. Sarcopenia, BMD, and Implant Failure
3.5. Sarcopenia, Chronological Age, and Frailty
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mijnarends, D.M.; Luiking, Y.C.; Halfens, R.J.G.; Evers, S.; Lenaerts, E.L.A.; Verlaan, S.; Wallace, M.; Schols, J.; Meijers, J.M.M. Muscle, Health and Costs: A Glance at their Relationship. J. Nutr. Health Aging 2018, 22, 766–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, Y.J.; Chang, M.C. Prevalence of Sarcopenia among the Elderly in Korea: A Meta-Analysis. J. Prev. Med. Public Health 2021, 54, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Hoshino, M.; Ohyama, S.; Terai, H.; Suzuki, A.; Yamada, K.; Takahashi, S.; Hayashi, K.; Tamai, K.; Hori, Y.; et al. Impact of Sarcopenia on Clinical Outcomes of Minimally Invasive Lumbar Decompression Surgery. Sci. Rep. 2019, 9, 16619. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Kats, A.M.; Schousboe, J.T.; Taylor, B.C.; Vo, T.N.; Cawthon, P.M.; Hoffman, A.R.; Langsetmo, L.; Osteoporotic Fractures in Men, S. Frailty Phenotype and Healthcare Costs and Utilization in Older Men. J. Am. Geriatr. Soc. 2020, 68, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [Green Version]
- Masanes, F.; Rojano, I.L.X.; Salva, A.; Serra-Rexach, J.A.; Artaza, I.; Formiga, F.; Cuesta, F.; Lopez Soto, A.; Ruiz, D.; Cruz-Jentoft, A.J. Cut-off Points for Muscle Mass-Not Grip Strength or Gait Speed-Determine Variations in Sarcopenia Prevalence. J. Nutr. Health Aging 2017, 21, 825–829. [Google Scholar] [CrossRef]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Healthspan. 2014, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Bahat, G.; Erdogan, T.; Ilhan, B. SARC-F and other screening tests for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 37–42. [Google Scholar] [CrossRef]
- Locquet, M.; Beaudart, C.; Reginster, J.Y.; Petermans, J.; Bruyere, O. Comparison of the performance of five screening methods for sarcopenia. Clin. Epidemiol. 2018, 10, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Rossi, A.P.; Fantin, F.; Micciolo, R.; Bertocchi, M.; Bertassello, P.; Zanandrea, V.; Zivelonghi, A.; Bissoli, L.; Zamboni, M. Identifying sarcopenia in acute care setting patients. J. Am. Med. Dir. Assoc. 2014, 15, 303.e7–303.e12. [Google Scholar] [CrossRef]
- Beaudart, C.; McCloskey, E.; Bruyere, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo de Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertiere, M.C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Optimal body size adjustment of L3 CT skeletal muscle area for sarcopenia assessment. Sci. Rep. 2021, 11, 279. [Google Scholar] [CrossRef]
- Reinders, I.; Murphy, R.A.; Brouwer, I.A.; Visser, M.; Launer, L.; Siggeirsdottir, K.; Eiriksdottir, G.; Gudnason, V.; Jonsson, P.V.; Lang, T.F.; et al. Muscle Quality and Myosteatosis: Novel Associations with Mortality Risk: The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. Am. J. Epidemiol. 2016, 183, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Goodpaster, B.H.; Thaete, F.L.; Kelley, D.E. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Ewaschuk, J.B.; Almasud, A.; Mazurak, V.C. Role of n-3 fatty acids in muscle loss and myosteatosis. Appl. Physiol. Nutr. Metab. 2014, 39, 654–662. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y.; Scientific Advisory Board of the European Society for Clinical; Economic Aspects of Osteoporosis; Osteoarthritis; Committees of Scientific Advisors; National Societies of the International Osteoporosis Foundation. Executive summary of the European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Calcif. Tissue Int. 2019, 104, 235–238. [Google Scholar] [CrossRef]
- Shevroja, E.; Lamy, O.; Kohlmeier, L.; Koromani, F.; Rivadeneira, F.; Hans, D. Use of Trabecular Bone Score (TBS) as a Complementary Approach to Dual-energy X-ray Absorptiometry (DXA) for Fracture Risk Assessment in Clinical Practice. J. Clin. Densitom. 2017, 20, 334–345. [Google Scholar] [CrossRef]
- Ruaro, B.; Casabella, A.; Paolino, S.; Pizzorni, C.; Alessandri, E.; Seriolo, C.; Botticella, G.; Molfetta, L.; Odetti, P.; Smith, V.; et al. Correlation between bone quality and microvascular damage in systemic sclerosis patients. Rheumatology 2018, 57, 1548–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girgis, C.M.; Mokbel, N.; Digirolamo, D.J. Therapies for musculoskeletal disease: Can we treat two birds with one stone? Curr. Osteoporos. Rep. 2014, 12, 142–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef]
- Cederholm, T.; Cruz-Jentoft, A.J.; Maggi, S. Sarcopenia and fragility fractures. Eur. J. Phys. Rehabil. Med. 2013, 49, 111–117. [Google Scholar] [PubMed]
- Schreiber, J.J.; Anderson, P.A.; Rosas, H.G.; Buchholz, A.L.; Au, A.G. Hounsfield units for assessing bone mineral density and strength: A tool for osteoporosis management. J. Bone Joint Surg. Am. 2011, 93, 1057–1063. [Google Scholar] [CrossRef]
- Schreiber, J.J.; Anderson, P.A.; Hsu, W.K. Use of computed tomography for assessing bone mineral density. Neurosurg. Focus 2014, 37, E4. [Google Scholar] [CrossRef]
- Buckens, C.F.; Dijkhuis, G.; de Keizer, B.; Verhaar, H.J.; de Jong, P.A. Opportunistic screening for osteoporosis on routine computed tomography? An external validation study. Eur. Radiol. 2015, 25, 2074–2079. [Google Scholar] [CrossRef] [Green Version]
- Yaprak, G.; Gemici, C.; Seseogullari, O.O.; Karabag, I.S.; Cini, N. CT Derived Hounsfield Unit: An Easy Way to Determine Osteoporosis and Radiation Related Fracture Risk in Irradiated Patients. Front. Oncol. 2020, 10, 742. [Google Scholar] [CrossRef]
- Emohare, O.; Cagan, A.; Morgan, R.; Davis, R.; Asis, M.; Switzer, J.; Polly, D.W., Jr. The use of computed tomography attenuation to evaluate osteoporosis following acute fractures of the thoracic and lumbar vertebra. Geriatr. Orthop. Surg. Rehabil. 2014, 5, 50–55. [Google Scholar] [CrossRef]
- Hou, J.; He, C.; He, W.; Yang, M.; Luo, X.; Li, C. Obesity and Bone Health: A Complex Link. Front. Cell. Dev. Biol. 2020, 8, 600181. [Google Scholar] [CrossRef]
- Wahlen, B.M.; Mekkodathil, A.; Al-Thani, H.; El-Menyar, A. Impact of sarcopenia in trauma and surgical patient population: A literature review. Asian J. Surg. 2020, 43, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Prado, C.M.; Ilich, J.Z.; Purcell, S.; Siervo, M.; Folsom, A.; Panton, L. Osteosarcopenic obesity: The role of bone, muscle, and fat on health. J. Cachexia Sarcopenia Muscle 2014, 5, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, R. Obesity, sarcopenia and postmenopausal osteoporosis: An interlinked triad! J. Midlife Health 2014, 5, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Shimokata, H.; Sakai, Y.; Ito, S.; Matsui, Y.; Takemura, M.; Kasai, T.; Ishiguro, N.; Harada, A. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur. Spine J. 2016, 25, 3424–3431. [Google Scholar] [CrossRef]
- Ohyama, S.; Hoshino, M.; Terai, H.; Toyoda, H.; Suzuki, A.; Takahashi, S.; Hayashi, K.; Tamai, K.; Hori, Y.; Nakamura, H. Sarcopenia is related to spinal sagittal imbalance in patients with spinopelvic mismatch. Eur. Spine J. 2019, 28, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Isogai, N.; Hosogane, N.; Funao, H.; Nojiri, K.; Suzuki, S.; Okada, E.; Ueda, S.; Hikata, T.; Shiono, Y.; Watanabe, K.; et al. The Surgical Outcomes of Spinal Fusion for Osteoporotic Vertebral Fractures in the Lower Lumbar Spine with a Neurological Deficit. Spine Surg. Relat. Res. 2020, 4, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Rometsch, E.; Spruit, M.; Zigler, J.E.; Menon, V.K.; Ouellet, J.A.; Mazel, C.; Hartl, R.; Espinoza, K.; Kandziora, F. Screw-Related Complications after Instrumentation of the Osteoporotic Spine: A Systematic Literature Review with Meta-Analysis. Glob. Spine J. 2020, 10, 69–88. [Google Scholar] [CrossRef] [Green Version]
- Roffman, C.E.; Buchanan, J.; Allison, G.T. Charlson Comorbidities Index. J. Physiother. 2016, 62, 171. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Poh, A.W.Y.; Teo, S.P. Utility of Frailty Screening Tools in Older Surgical Patients. Ann. Geriatr. Med. Res. 2020, 24, 75–82. [Google Scholar] [CrossRef]
- Wu, X.; Shi, J.; Wu, J.; Cheng, Y.; Peng, K.; Chen, J.; Jiang, H. Pedicle screw loosening: The value of radiological imagings and the identification of risk factors assessed by extraction torque during screw removal surgery. J. Orthop. Surg. Res. 2019, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Camino Willhuber, G.; Elizondo, C.; Slullitel, P. Analysis of Postoperative Complications in Spinal Surgery, Hospital Length of Stay, and Unplanned Readmission: Application of Dindo-Clavien Classification to Spine Surgery. Glob. Spine J. 2019, 9, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Ji, G.; Bao, T.; Fu, H.; Yang, L.; Yang, M. Diagnosing sarcopenia and myosteatosis based on chest computed tomography images in healthy Chinese adults. Insights Imaging 2021, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Syddall, H.E.; Gilbody, H.J.; Dennison, E.M.; Cooper, C. Does sarcopenia originate in early life? Findings from the Hertfordshire cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, M930–M934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Argiles, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Manas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Barbat-Artigas, S.; Pion, C.H.; Leduc-Gaudet, J.P.; Rolland, Y.; Aubertin-Leheudre, M. Exploring the role of muscle mass, obesity, and age in the relationship between muscle quality and physical function. J. Am. Med. Dir. Assoc. 2014, 15, 303.e13–303.e20. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Beaudart, C.; Buckinx, F.; Bruyere, O. Osteoporosis and sarcopenia: Two diseases or one? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Isaacson, J.; Brotto, M. Physiology of Mechanotransduction: How Do Muscle and Bone “Talk” to One Another? Clin. Rev. Bone Miner. Metab. 2014, 12, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.M.; Cruz-Jentoft, A.J.; Fielding, R.A.; Kanis, J.A.; Reginster, J.Y.; Bruyere, O.; Cesari, M.; Chapurlat, R.; Al-Daghri, N.; Dennison, E.; et al. Correction to: Is There Enough Evidence for Osteosarcopenic Obesity as a Distinct Entity? A Critical Literature Review. Calcif. Tissue Int. 2019, 105, 125–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahat, G.; Tufan, A.; Kilic, C.; Karan, M.A.; Cruz-Jentoft, A.J. Prevalence of sarcopenia and its components in community-dwelling outpatient older adults and their relation with functionality. Aging Male 2020, 23, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Reginster, J.Y.; Slomian, J.; Buckinx, F.; Dardenne, N.; Quabron, A.; Slangen, C.; Gillain, S.; Petermans, J.; Bruyere, O. Estimation of sarcopenia prevalence using various assessment tools. Exp. Gerontol. 2015, 61, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Lee, J.K.; Choi, D.S.; Han, S.H. Prevalence and Associated Risk Factors of Sarcopenia in Female Patients with Osteoporotic Fracture. J. Bone Metab. 2018, 25, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Wagenaar, C.A.; Dekker, L.H.; Navis, G.J. Prevalence of sarcopenic obesity and sarcopenic overweight in the general population: The lifelines cohort study. Clin. Nutr. 2021, 40, 4422–4429. [Google Scholar] [CrossRef]
- Schaap, L.A.; van Schoor, N.M.; Lips, P.; Visser, M. Associations of Sarcopenia Definitions, and Their Components, with the Incidence of Recurrent Falling and Fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1199–1204. [Google Scholar] [CrossRef]
- Gao, K.; Cao, L.F.; Ma, W.Z.; Gao, Y.J.; Luo, M.S.; Zhu, J.; Li, T.; Zhou, D. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: Findings from the China health and retirement longitudinal study. eClinicalMedicine 2022, 44, 101264. [Google Scholar] [CrossRef]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164.e7–1164.e15. [Google Scholar] [CrossRef]
- Bone, A.E.; Hepgul, N.; Kon, S.; Maddocks, M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir. Dis. 2017, 14, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Mallet, R.; Modzelewski, R.; Lequesne, J.; Mihailescu, S.; Decazes, P.; Auvray, H.; Benyoucef, A.; Di Fiore, F.; Vera, P.; Dubray, B.; et al. Prognostic value of sarcopenia in patients treated by Radiochemotherapy for locally advanced oesophageal cancer. Radiat. Oncol. 2020, 15, 116. [Google Scholar] [CrossRef]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: A meta-analysis. J. Cachexia Sarcopenia Muscle 2021, 12, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Noble, S.; Chester, J.; Coles, B.; Byrne, A. The assessment and impact of sarcopenia in lung cancer: A systematic literature review. BMJ Open 2014, 4, e003697. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Ruan, J.; Chen, T.; Lin, E.; Shi, L. CT-assessed sarcopenia is a predictive factor for both long-term and short-term outcomes in gastrointestinal oncology patients: A systematic review and meta-analysis. Cancer Imaging 2019, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P.; Panikkar, R. Sarcopenia associated with chemotherapy and targeted agents for cancer therapy. Ann. Palliat. Med. 2019, 8, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Matsui, H.; Ito, S.; Hida, T.; Ito, K.; Koshimizu, H.; Harada, A. Sarcopenia in elderly patients with chronic low back pain. Osteoporos. Sarcopenia 2017, 3, 195–200. [Google Scholar] [CrossRef]
- Matsuo, S.; Kawakami, M.; Minetama, M.; Nakagawa, M.; Teraguchi, M.; Kagotani, R.; Mera, Y.; Yamamoto, Y.; Sakon, N.; Nakatani, T.; et al. Clinical Features of Sarcopenia in Patients With Lumbar Spinal Stenosis. Spine 2020, 45, E1105–E1110. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, S.Y.; Park, S.J.; Lee, Y.S. Relationships between Spinal Sarcopenia and Spinal Sagittal Balance in Older Women. Ann. Geriatr. Med. Res. 2019, 23, 141–148. [Google Scholar] [CrossRef]
- Inose, H.; Yamada, T.; Hirai, T.; Yoshii, T.; Abe, Y.; Okawa, A. The impact of sarcopenia on the results of lumbar spinal surgery. Osteoporos. Sarcopenia 2018, 4, 33–36. [Google Scholar] [CrossRef]
- Simonsen, C.; de Heer, P.; Bjerre, E.D.; Suetta, C.; Hojman, P.; Pedersen, B.K.; Svendsen, L.B.; Christensen, J.F. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann. Surg. 2018, 268, 58–69. [Google Scholar] [CrossRef]
- Tamura, T.; Sakurai, K.; Nambara, M.; Miki, Y.; Toyokawa, T.; Kubo, N.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Ohira, M. Adverse Effects of Preoperative Sarcopenia on Postoperative Complications of Patients with Gastric Cancer. Anticancer Res. 2019, 39, 987–992. [Google Scholar] [CrossRef]
- Galbusera, F.; Volkheimer, D.; Reitmaier, S.; Berger-Roscher, N.; Kienle, A.; Wilke, H.J. Pedicle screw loosening: A clinically relevant complication? Eur. Spine J. 2015, 24, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.H.; Yiu, T.; Ong, M.T.; Lee, W.Y. Sarcopenia: Current treatments and new regenerative therapeutic approaches. J. Orthop. Translat. 2020, 23, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Chung, C.K. Strategies of spinal fusion on osteoporotic spine. J. Korean Neurosurg. Soc. 2011, 49, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, A.; Burton, D.C.; Diebo, B.G.; Smith, J.S.; Hostin, R.; Shaffrey, C.I.; Boachie-Adjei, O.; Mundis, G.M., Jr.; Ames, C.; Errico, T.J.; et al. Impact of obesity on complications, infection, and patient-reported outcomes in adult spinal deformity surgery. J. Neurosurg. Spine 2015, 23, 656–664. [Google Scholar] [CrossRef] [Green Version]
- Lingutla, K.K.; Pollock, R.; Benomran, E.; Purushothaman, B.; Kasis, A.; Bhatia, C.K.; Krishna, M.; Friesem, T. Outcome of lumbar spinal fusion surgery in obese patients: A systematic review and meta-analysis. Bone Joint J. 2015, 97-B, 1395–1404. [Google Scholar] [CrossRef] [PubMed]

| Total | No Implant Failure | Implant Failure | |
|---|---|---|---|
| Age, mean (range) | 73.7 (59–89) | 73.4 (59–88) | 72.5 (59–87) |
| Sex (%) | |||
| Female | 41 (60.3) | 29 (59.2) | 12 (63.2) |
| Male | 27 (39.7) | 20 (40.8) | 7 (36.8) |
| Comorbidity and Frailty, median (range) | |||
| CCI | 4 (2–6) | 4 (2–6) | 4 (2–6) |
| GFI | 4 (2–6) | 4 (2–6) | 4 (2–6) |
| Weight (kg), mean (SD) | 76.1 (±20.9) | 77.3 (±22.6) | 73.1 (±15.9) |
| BMI (kg/m2), mean (SD) | 25.1 (±6.9) | 27.8 (±6.6) | 27.2 (±5.8) |
| Localization instrumentation | |||
| Thoracic | 11 (16.7) | 8 (16) | 3 (18.8) |
| Thoracolumbar | 39 (59.1) | 31 (62) | 8 (50) |
| Lumbar/lumbosacral | 11 (16.7) | 8 (16) | 3 (18.8) |
| Lumbosacral | 5 (7.6) | 3 (6) | 2 (12.5) |
| Instrumented levels | |||
| 1 level | 0 | 0 | 0 |
| 2 levels | 13 (19) | 8 (16) | 5 (31.3) |
| 3 levels | 3 (4.5) | 2 (4) | 1 (6.3) |
| 4 levels | 41 (62.1) | 33 (66) | 8 (50) |
| 5 or more levels | 9 (13.6) | 7 (14) | 2 (12.5) |
| Total | No Implant Failure | Implant Failure | |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 5 (7.6) | 2 (4) | 3 (18.8) |
| 3 | 7 (10.6) | 6 (12) | 1 (6.3) |
| 4 | 48 (72.7) | 37 (74) | 11 (68.8) |
| 5 | 6 (9.1) | 5 (10) | 1 (6.3) |
| Total | No Implant Failure | Implant Failure | |
|---|---|---|---|
| SMA, mean (SD | 105.9 (±31) | 115.2 (±26.7) *** | 85.8 (±30.7) *** |
| zSMA(HT), mean (SD) | −0.6 (±0.3) | 0.1 (±0.3) *** | −1.8 (±0.4) *** |
| BMD | 77.5 (±4.9) | 81 (±6.5) * | 65 (±4.3) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krenzlin, H.; Schmidt, L.; Jankovic, D.; Schulze, C.; Brockmann, M.A.; Ringel, F.; Keric, N. Impact of Sarcopenia and Bone Mineral Density on Implant Failure after Dorsal Instrumentation in Patients with Osteoporotic Vertebral Fractures. Medicina 2022, 58, 748. https://doi.org/10.3390/medicina58060748
Krenzlin H, Schmidt L, Jankovic D, Schulze C, Brockmann MA, Ringel F, Keric N. Impact of Sarcopenia and Bone Mineral Density on Implant Failure after Dorsal Instrumentation in Patients with Osteoporotic Vertebral Fractures. Medicina. 2022; 58(6):748. https://doi.org/10.3390/medicina58060748
Chicago/Turabian StyleKrenzlin, Harald, Leon Schmidt, Dragan Jankovic, Carina Schulze, Marc A. Brockmann, Florian Ringel, and Naureen Keric. 2022. "Impact of Sarcopenia and Bone Mineral Density on Implant Failure after Dorsal Instrumentation in Patients with Osteoporotic Vertebral Fractures" Medicina 58, no. 6: 748. https://doi.org/10.3390/medicina58060748
APA StyleKrenzlin, H., Schmidt, L., Jankovic, D., Schulze, C., Brockmann, M. A., Ringel, F., & Keric, N. (2022). Impact of Sarcopenia and Bone Mineral Density on Implant Failure after Dorsal Instrumentation in Patients with Osteoporotic Vertebral Fractures. Medicina, 58(6), 748. https://doi.org/10.3390/medicina58060748





