Periocular Infection of Mycobacterium avium Complex in a Patient with Interferon-γ Autoantibodies: A Case Report
Abstract
:1. Introduction
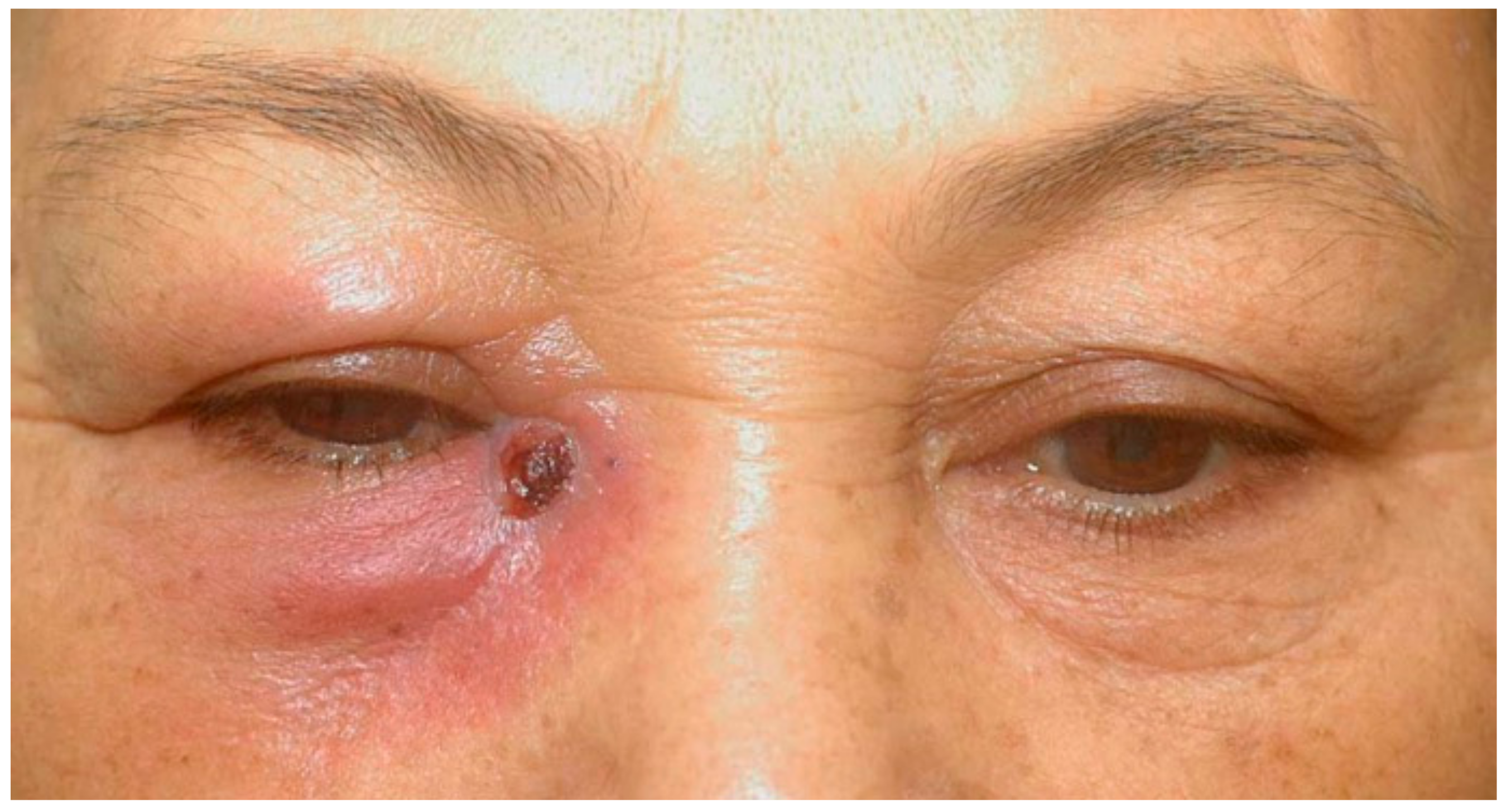
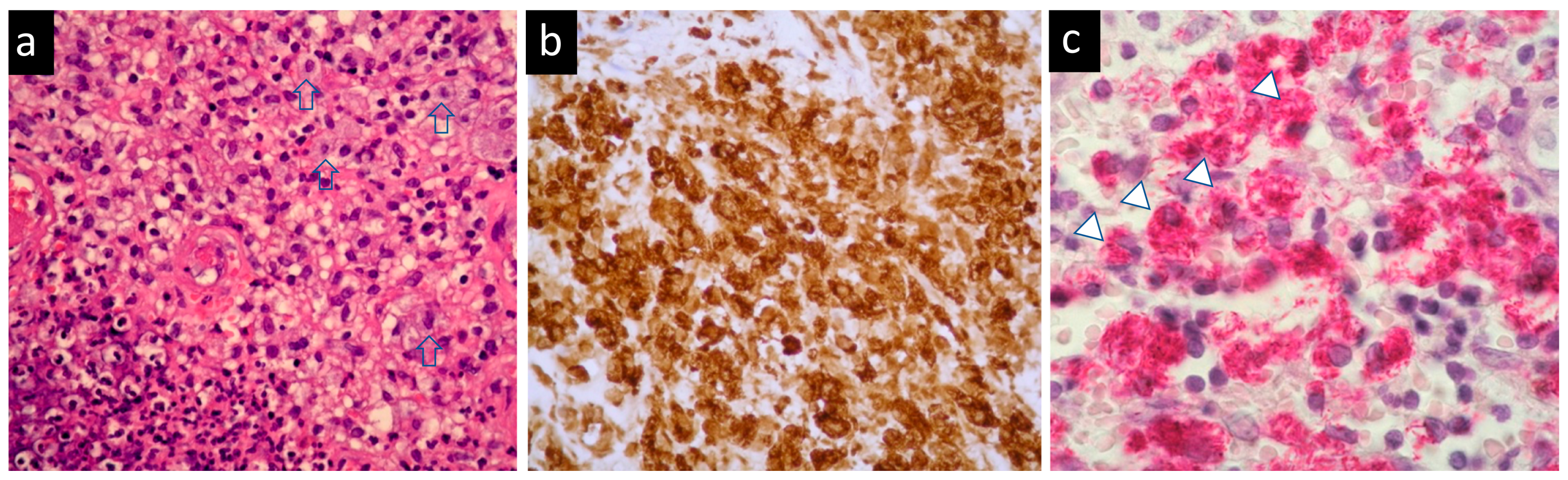
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Girgis, D.O.; Karp, C.L.; Miller, D. Ocular infections caused by non-tuberculous mycobacteria: Update on epidemiology and management. Clin. Exp. Ophthalmol. 2012, 40, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Davis, A.S.; Moreau, A.; Drevets, D.A.; Melendez, D.P. Management of nontuberculous mycobacterial infections of the eye and orbit: A retrospective case series. Am. J. Ophthalmol. Case Rep. 2020, 20, 100971. [Google Scholar] [CrossRef] [PubMed]
- Wu, U.I.; Wang, J.T.; Sheng, W.H.; Sun, H.Y.; Cheng, A.; Hsu, L.Y.; Chang, S.C.; Chen, Y.C. Incorrect diagnoses in patients with neutralizing anti-interferon-gamma-autoantibodies. Clin. Microbiol. Infect. 2020, 26, 1684.e1–1684.e6. [Google Scholar] [CrossRef] [PubMed]
- Hase, I.; Morimoto, K.; Sakagami, T.; Ishii, Y.; van Ingen, J. Patient ethnicity and causative species determine the manifestations of anti-interferon-gamma autoantibody-associated nontuberculous mycobacterial disease: A review. Diagn. Microbiol. Infect. Dis. 2017, 88, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hoflich, C.; Sabat, R.; Rosseau, S.; Temmesfeld, B.; Slevogt, H.; Docke, W.D.; Grutz, G.; Meisel, C.; Halle, E.; Gobel, U.B.; et al. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 2004, 103, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, R.S.; Valluri, S.; Rao, N.A. Nontuberculous mycobacterial ocular and adnexal infections. Surv. Ophthalmol. 2012, 57, 202–235. [Google Scholar] [CrossRef]
- Varley, C.D.; Ku, J.H.; Henkle, E.; Schafer, S.D.; Winthrop, K.L. Disseminated Nontuberculous Mycobacteria in HIV-Infected Patients, Oregon, USA, 2007–2012. Emerg. Infect. Dis. 2017, 23, 533–535. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.H.; Ortega-Villa, A.M.; Hunsberger, S.; Chetchotisakd, P.; Anunnatsiri, S.; Mootsikapun, P.; Rosen, L.B.; Zerbe, C.S.; Holland, S.M. Natural History and Evolution of Anti-Interferon-gamma Autoantibody-Associated Immunodeficiency Syndrome in Thailand and the United States. Clin. Infect. Dis. 2020, 71, 53–62. [Google Scholar] [CrossRef]
- Pepose, J.S.; Holland, G.N.; Nestor, M.S.; Cochran, A.J.; Foos, R.Y. Acquired immune deficiency syndrome. Pathogenic mechanisms of ocular disease. Ophthalmology 1985, 92, 472–484. [Google Scholar] [CrossRef]
- Fulton, S.A.; Johnsen, J.M.; Wolf, S.F.; Sieburth, D.S.; Boom, W.H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: Role of phagocytosis. Infect. Immun. 1996, 64, 2523–2531. [Google Scholar] [CrossRef] [Green Version]
- Browne, S.K.; Burbelo, P.D.; Chetchotisakd, P.; Suputtamongkol, Y.; Kiertiburanakul, S.; Shaw, P.A.; Kirk, J.L.; Jutivorakool, K.; Zaman, R.; Ding, L.; et al. Adult-onset immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 2012, 367, 725–734. [Google Scholar] [CrossRef] [Green Version]
- Chi, C.Y.; Lin, C.H.; Ho, M.W.; Ding, J.Y.; Huang, W.C.; Shih, H.P.; Yeh, C.F.; Fung, C.P.; Sun, H.Y.; Huang, C.T.; et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-gamma autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine 2016, 95, e3927. [Google Scholar] [CrossRef]
- Yeh, Y.K.; Ding, J.Y.; Ku, C.L.; Chen, W.C. Disseminated Mycobacterium avium complex infection mimicking malignancy in a patient with anti-IFN-gamma autoantibodies: A case report. BMC Infect. Dis. 2019, 19, 909. [Google Scholar] [CrossRef]
- Ikeda, H.; Nakamura, K.; Ikenori, M.; Saito, T.; Nagamine, K.; Inoue, M.; Sakagami, T.; Suzuki, H.; Usui, M.; Kanemitsu, K.; et al. Severe Disseminated Mycobacterium avium Infection in a Patient with a Positive Serum Autoantibody to Interferon-γ. Intern. Med. 2016, 55, 3053–3058. [Google Scholar] [CrossRef] [Green Version]
- Keragala, B.; Gunasekera, C.N.; Yesudian, P.D.; Guruge, C.; Dissanayaka, B.S.; Liyanagama, D.P.; Jinadasa, G.I.M.; Constantine, S.R.; Herath, H. Disseminated Mycobacterium simiae infection in a patient with adult-onset immunodeficiency due to anti-interferon-gamma antibodies—A case report. BMC Infect. Dis. 2020, 20, 258. [Google Scholar] [CrossRef] [Green Version]
- Czaja, C.A.; Merkel, P.A.; Chan, E.D.; Lenz, L.L.; Wolf, M.L.; Alam, R.; Frankel, S.K.; Fischer, A.; Gogate, S.; Perez-Velez, C.M.; et al. Rituximab as successful adjunct treatment in a patient with disseminated nontuberculous mycobacterial infection due to acquired anti-interferon-γ autoantibody. Clin. Infect. Dis. 2014, 58, e115–e118. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, Y.; Sakagami, T.; Nishiyama, N.; Hirai, J.; Hayashi, Y.; Asai, N.; Yamagishi, Y.; Kato, H.; Hagihara, M.; Sakanashi, D.; et al. Rituximab Restores IFN-γ-STAT1 Function and Ameliorates Disseminated Mycobacterium avium Infection in a Patient with Anti-Interferon-γ Autoantibody. J. Clin. Immunol. 2017, 37, 644–649. [Google Scholar] [CrossRef]
- Naik, R.; Cortes, J.A. Persistent Mycobacterium abscessus infection secondary to interferon-γ autoantibodies. Ann. Allergy Asthma Immunol. 2016, 116, 461–462. [Google Scholar] [CrossRef]
- King, E.M.; Weaver, V.K.; Kestler, M.H. Treatment Dilemmas in Disseminated Nontuberculous Mycobacterial Infections With Interferon-gamma Autoantibodies. Open Forum Infect. Dis. 2021, 8, ofab253. [Google Scholar] [CrossRef]
- Laisuan, W.; Pisitkun, P.; Ngamjanyaporn, P.; Suangtamai, T.; Rotjanapan, P. Prospective Pilot Study of Cyclophosphamide as an Adjunct Treatment in Patients With Adult-Onset Immunodeficiency Associated With Anti-interferon-γ Autoantibodies. Open Forum Infect. Dis. 2020, 7, ofaa035. [Google Scholar] [CrossRef]
- Chawansuntati, K.; Rattanathammethee, K.; Wipasa, J. Minireview: Insights into anti-interferon-gamma autoantibodies. Exp. Biol. Med. 2021, 246, 790–795. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, T.-H.; Tsai, T.-Y.; Wang, L.-S.; Huang, T.-L.; Chen, N. Periocular Infection of Mycobacterium avium Complex in a Patient with Interferon-γ Autoantibodies: A Case Report. Medicina 2022, 58, 846. https://doi.org/10.3390/medicina58070846
Lo T-H, Tsai T-Y, Wang L-S, Huang T-L, Chen N. Periocular Infection of Mycobacterium avium Complex in a Patient with Interferon-γ Autoantibodies: A Case Report. Medicina. 2022; 58(7):846. https://doi.org/10.3390/medicina58070846
Chicago/Turabian StyleLo, Tzu-Hui, Tou-Yuan Tsai, Lih-Shinn Wang, Tzu-Lun Huang, and Nancy Chen. 2022. "Periocular Infection of Mycobacterium avium Complex in a Patient with Interferon-γ Autoantibodies: A Case Report" Medicina 58, no. 7: 846. https://doi.org/10.3390/medicina58070846
APA StyleLo, T.-H., Tsai, T.-Y., Wang, L.-S., Huang, T.-L., & Chen, N. (2022). Periocular Infection of Mycobacterium avium Complex in a Patient with Interferon-γ Autoantibodies: A Case Report. Medicina, 58(7), 846. https://doi.org/10.3390/medicina58070846





