The Angiogenic Balance and Its Implications in Cancer and Cardiovascular Diseases: An Overview
Abstract
1. Introduction
2. Formation and Remodeling of Blood Vessels
3. The Angiogenic Balance: Synthetic and Endogenous Regulators
3.1. Synthetic Modulators of Angiogenesis
3.2. Endogenous Regulators of Angiogenesis
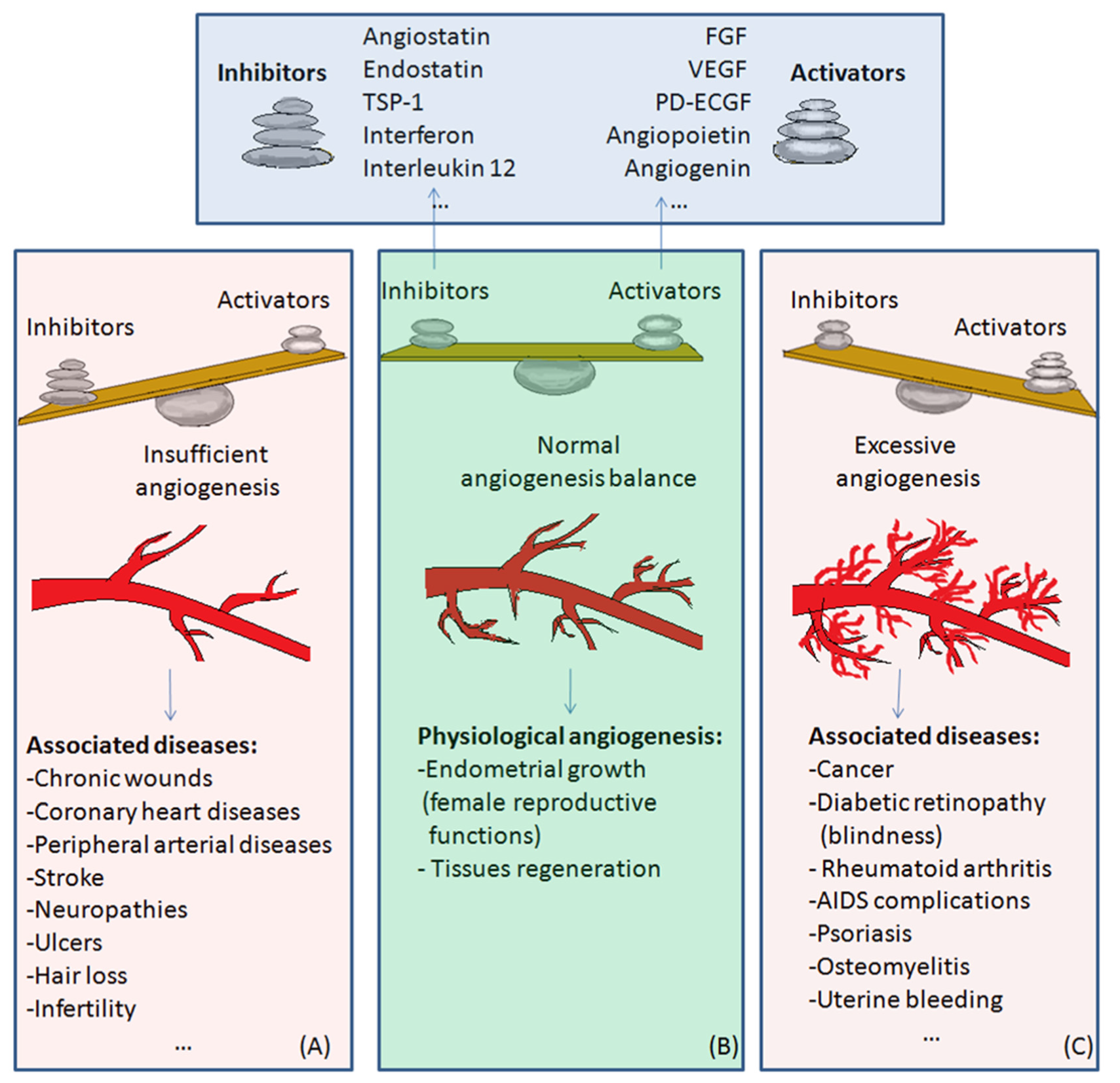
4. Physiological and Pathological Angiogenesis
5. Angiogenesis Inhibition (Anti-Angiogenic Therapy) and Current Implications in Cancer Treatment
6. Angiogenesis Activation (Therapeutic Angiogenesis) and Current Implications in Cardiovascular Diseases
- Protein therapy: consists of the repeated administration of angiogenic factors in order to promote angiogenesis. This strategy has the advantage that angiogenic protein production and purification is known, and proteins may now be stored after lyophilization and reconditioned in a buffer upon need. Most of the angiogenic proteins are now commercialized and available for research. Besides VEGF and FGF (the most extensively studied proteins in therapeutic angiogenesis), other factors such as the PDGF family and Angiopoietin-1 have also been investigated. Systemic delivery of proteins has as a drawback the low concentration of the angiogenic factor in the desired tissue, due to both low targeting and rapid protein clearance by the mononuclear phagocyte system. Because of the rapid clearance of proteins in blood, their local delivery, either directly, or using adequate biomaterials with slow-delivery of active principles, is better suited in this case. Local administration (intracoronary, intramyocardial or intracerebral) is possible, but involves the usage of special devices or invasive surgery. Biomaterials of natural or synthetic origin such as hydrogels (e.g., alginate hydrogel and peptide nanofibers); micro- and nano-particles (e.g., poly (lactic acid-co-glycolic acid) microspheres and liposomes); porous scaffolds (e.g., poly (ε-caprolactone) scaffolds); coacervate (e.g., (polycation–heparin) coacervates), have been studied as delivery vehicles of angiogenic proteins [135].
- Gene therapy consists of administrating genes whose expression would lead to proteins that will induce angiogenesis activation [136]. Gene therapy has as an advantage the fact that the protein continues to be secreted a long time after drug administration, as well as the fact that genes might be targeted to specific tissues [137]. Employed vector systems are plasmids and viral vectors. Adeno-associated viruses have been investigated as promising new vectors for gene therapy. The obstacles that have to be overcome are related to the low concentration of gene product at the target site as well as the activation of inflammatory and immune responses. Additional aspects, such as the incomplete understanding of angiogenesis mechanisms at molecular level and the difference between animal models and humans, represent the main obstacles encountered when angiogenesis gene therapy has been applied to humans. Although not yet implemented in clinical practice, data gathered in more than 20 years of preclinical and clinical studies has brought great insights and advancement in this field [138,139]. Several clinical trials investigating the effect of different gene therapies on cardiac regeneration are currently ongoing [140].
- Cellular therapy [141,142] induces angiogenesis using cells known to produce angiogenic factors, such as monocytes and endothelial progenitor cells. Recent research indicates that stem cells-derived extracellular vesicles promote angiogenesis in cellular experiments and animal models. Extracellular vesicles transport informational molecules, including proteins, mRNA, microRNAs, DNA fragments, and lipids [143,144,145,146].
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Heart Association. New Statistics Show One of Every Three US Deaths Caused by Cardiovascular Disease. ScienceDaily. 16 December 2015. Available online: www.sciencedaily.com/releases/2015/12/151216144511.htm (accessed on 2 March 2021).
- Al Sabti, H. Therapeutic angiogenesis in cardiovascular disease. J. Cardiothorac. Surg. 2007, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Pandya, N.M.; Dhalla, N.S.; Santani, D.D. Angiogenesis—A new target for future therapy. Vascul. Pharmacol. 2006, 44, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bouıs, D.; Kusumanto, Y.; Meijer, C.; Mulder, N.H.; Hospers, G.A. A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol. Res. 2006, 53, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Al Kawas, H.; Saaid, I.; Jank, P.; Westhoff, C.C.; Denkert, C.; Pross, T.; Weiler, K.B.S.; Karsten, M.M. How VEGF-A and its splice variants affect breast cancer development—clinical implications. Cell Oncol. 2022, 45, 227–239. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Jacinta Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Diana, C.; Alcobia, D.C.; Stephen, J.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Cui, H.; Shi, J.; Yuan, G.; Shi, S.; Hu, Y. The Role of the VEGF Family in Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 738325. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Rosca, E.V.; Koskimaki, J.E.; Rivera, C.G.; Pandey, N.B.; Tamiz, A.P.; Popel, A.S. Anti-angiogenic peptides for cancer therapeutics. Curr. Pharm. Biotechnol. 2011, 12, 1101–1116. [Google Scholar] [CrossRef]
- Fan, T.P.; Jaggar, R.; Bicknell, R. Controlling the vasculature: Angiogenesis, anti-angiogenesis and vascular targeting of gene therapy. Trends Pharmacol. Sci. 1995, 16, 57–66. [Google Scholar] [CrossRef]
- Quesada, A.R.; Munoz-Chapuli, R.; Medina, M.A. Anti-Angiogenic Drugs: From Bench to Clinical Trials. Med. Res. Rev. 2006, 26, 483–530. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, M.; Masuda, H.; Asahara, T. Endothelial progenitor cells for postnatal vasculogenesis. Clin. Exp. Nephrol. 2007, 11, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Murasawa, S.; Asahara, T. Endothelial Progenitor Cells for Vasculogenesis. Physiology 2005, 20, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Djonov, V.; Baum, O.; Burri, P.H. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003, 314, 107–117. [Google Scholar] [CrossRef]
- Djonov, V.; Schmid, M.; Tschanz, S.A.; Burri, P.H. Intussusceptive Angiogenesis: Its Role in Embryonic Vascular Network Formation. Circ. Res. 2000, 86, 286–292. [Google Scholar] [CrossRef]
- Tomanek, R.J.; Schatteman, G.C. Angiogenesis: New insights and therapeutic potential. Anat. Rec. 2000, 261, 126–135. [Google Scholar] [CrossRef]
- Couffinhal, T.; Dufourcq, P.; Daret, D.; Duplaa, C. The mechanisms of angiogenesis. Medical and therapeutic applications. Rev. Méd. Interne 2001, 22, 1064–1082. [Google Scholar] [CrossRef]
- Bussolino, F.; Mantovani, A.; Persico, G. Molecular mechanisms of blood vessel formation. Trends Biochem. Sci. 1997, 22, 251–256. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. “Sprouting angiogenesis”, a reappraisal. Dev. Biol. 2012, 372, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, N.; Amaya, H.; Matsumura, M.; Shimomatsuya, T.; Horiuchi, T.; Muraoka, R.; Iki, M. Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res. 1996, 56, 2671–2676. [Google Scholar] [PubMed]
- Scholz, D.; Cai, W.J.; Schaper, W. Arteriogenesis, a new concept of vascular adaptation in occlusive disease. Angiogenesis 2001, 4, 247–257. [Google Scholar] [CrossRef]
- Heil, M.; Wagner, S.; Schaper, W. Arterial regeneration by collateral artery growth (arteriogenesis). Drug Discov. Today Dis. Models 2004, 1, 265–271. [Google Scholar] [CrossRef]
- Stephan, D.; Weltin, D.; Zaric, V.; Chapelon, D.; Da Silva, A.; Lugnier, C. Angiogenèse: De la physiologie à la thérapeutique. Réanim. Urgences 2000, 9, 534–544. [Google Scholar] [CrossRef]
- Levy, A.P.; Levy, N.S.; Goldberg, N.A. Post-transcriptional Regulation of Vascular Endothelial Growth Factor by Hypoxia. J. Biol. Chem. 1996, 271, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Baccarini, S.; Raz, A. Carbohydrate-recognition and angiogenesis. Cancer Metastasis Rev. 2000, 19, 51–57. [Google Scholar] [CrossRef]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014, 24, 886–891. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L.; Bresalier, R.; Raz, A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer. Inst. 2002, 94, 1854–1862. [Google Scholar] [CrossRef]
- Johnstone, K.D.; Karoli, T.; Liu, L.; Dredge, K.; Copeman, E.; Li, C.P.; Davis, K.; Hammond, E.; Bytheway, I.; Kostewicz, E.; et al. Synthesis and biological evaluation of polysulfated oligosaccharide glycosides as inhibitors of angiogenesis and tumor growth. J. Med. Chem. 2010, 53, 1686–1699. [Google Scholar] [CrossRef]
- Barragan-Montero, V.; Awwad, A.; Combemale, S.; de Santa Barbara, P.; Jover, B.; Molès, J.P.; Montero, J.L. Synthesis of Mannose-6-Phosphate Analogues and their Utility as Angiogenesis Regulators. ChemMedChem 2011, 6, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, C.; Sippelli, S.; Toupet, L.; Barragan-Montero, V. New mannose derivatives: The tetrazole analogue of mannose-6-phosphate as angiogenesis inhibitor. Bioorg. Med. Chem. Lett. 2016, 26, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Combemale, S.; Assam-Evoung, J.N.; Houaidji, S.; Bibi, R.; Barragan-Montero, V. Gold Nanoparticles Decorated with Mannose-6-phosphate Analogues. Molecules 2014, 19, 1120–1149. [Google Scholar] [CrossRef]
- Volpert, O.; Jackson, D.; Bouck, N.; Linzer, D.I. The insulin-like growth factor II/Mannose 6 -phosphate receptor is required for proliferin-induced angiogenesis. Endocrinology 1996, 137, 3871–3876. [Google Scholar] [CrossRef] [PubMed]
- Doyagüez, G.E.; Carrero, P.; Madrona, A.; Rodriguez-Salamanca, P.; Martínez-Gualda, B.; Camarasa, M.J.; Jimeno, M.L.; Bennallack, P.R.; Finnell, J.G.; Tsang, T.M.; et al. Galloyl Carbohydrates with Antiangiogenic Activity Mediated by Capillary Morphogenesis Gene 2 (CMG2) Protein Binding. J. Med. Chem. 2019, 62, 3958–3970. [Google Scholar] [CrossRef] [PubMed]
- Cryan, L.M.; Bazinet, L.; Habeshian, K.A.; Cao, S.; Clardy, J.; Christensen, K.A.; Rogers, M.S. 1,2,3,4,6-Penta-O-galloyl-β-D-glucopyranose inhibits angiogenesis via inhibition of capillary morphogenesis gene 2. J. Med. Chem. 2013, 56, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Lee, E.O.; Kim, M.S.; Kang, K.S.; Kim, C.H.; Cha, B.C.; Surh, Y.J.; Kim, S.H. Penta-O-galloyl-beta-D-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: Roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis 2005, 26, 1436–1445. [Google Scholar] [CrossRef]
- Nyberg, P.; Xie, L.; Kalluri, R. Endogenous Inhibitors of Angiogenesis. Cancer Res. 2005, 65, 3967–3979. [Google Scholar] [CrossRef]
- Iruela-Arispe, M.L.; Dvorak, H.F. Angiogenesis: A dynamic balance of stimulators and inhibitors. Thromb. Haemost. 1997, 78, 672–677. [Google Scholar] [CrossRef]
- Gospodarowicz, D. Purification of a fibroblast growth factor from bovine pituitary. J. Biol. Chem. 1975, 250, 2515–2520. [Google Scholar] [CrossRef]
- Esch, F.; Baird, A.; Ling, N.; Ueno, N.; Hill, F.; Denoroy, L.; Klepper, R.; Gospodarowicz, D.; Böhlen, P.; Guillemin, R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc. Natl. Acad. Sci. USA 1985, 82, 6507–6511. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–9855. [Google Scholar] [CrossRef]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef]
- Guo, N.; Krutzsch, H.C.; Inman, J.K.; Roberts, D.D. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997, 57, 1735–1742. [Google Scholar]
- Good, D.J.; Polverini, P.J.; Rastinejad, F.; Le Beau, M.M.; Lemons, R.S.; Frazier, W.A.; Bouck, N.P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc. Natl. Acad. Sci. USA 1990, 87, 6624–6628. [Google Scholar] [CrossRef]
- Mateescu, G.O.; Comanescu, M.; Mehedinţi, R.; Niculescu, Z.; Bold, A.; Panduru, L.; Cernea, D. Immunohistochemical expression of growth factors in the exocrine pancreas of patients with chronic liver diseases. Rom. J. Morphol. Embryol. 2010, 51, 303–307. [Google Scholar]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Luca Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in Cardiomyocytes and Heart Diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef]
- Manetti, F.; Corelli, F.; Botta, M. Fibroblast growth factors and their inhibitors. Curr. Pharm. Des. 2000, 6, 1897–1924. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, M.; Marcinkowska, E.; Wiedlocha, A. FGF-1: From biology through engineering to potential medical applications. Crit. Rev. Clin. Lab. Sci. 2008, 45, 91–135. [Google Scholar] [CrossRef] [PubMed]
- Yayon, A.; Klagsbrun, M.; Esko, J.D.; Leder, P.; Omitz, D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 1991, 64, 841–848. [Google Scholar] [CrossRef]
- Roghani, M.; Mansukhani, A.; Dell’Era, P.; Bellosta, P.; Basilico, C.; Rifkin, D.B.; Moscatelli, D. Heparin increases the affinity of basic fibroblast growth factor for its receptor but is not required for binding. J. Biol. Chem. 1994, 269, 3976–3984. [Google Scholar] [CrossRef]
- Friesel, R.E.; Maciag, T. Molecular mechanisms of angiogenesis: Fibroblast growth factor signal transduction. FASEB J. 1995, 9, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; Bikfalvi, A. Target molecules for anti-angiogenic therapy: From basic research to clinical trials. Crit. Rev. Oncol. Hematol. 2000, 34, 89–110. [Google Scholar] [CrossRef]
- Meyer, M.; Clauss, M.; Lepple-Wienhues, A.; Waltenberger, J.; Augustin, H.G.; Ziche, M.; Lanz, C.; Büttner, M.; Rziha, H.J.; Dehio, C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Veikkola, T.; Alitalo, K. VEGFs, receptors and angiogenesis. Semin. Cancer Bio. 1999, 9, 211–220. [Google Scholar] [CrossRef]
- Koblizek, T.I.; Weiss, C.; Yancopoulos, G.D.; Deutsch, U.; Risau, W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr. Biol. 1998, 8, 529–532. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef]
- Yu, X.; Seegar, T.M.C.; Dalton, A.C.; Tzvetkova-Robev, D.; Goldgur, Y.; Rajashankar, K.R.; Nikolov, D.B.; Barton, W.A. Structural basis for angiopoietin-1–mediated signaling initiation. Proc. Natl. Acad. Sci. USA 2013, 110, 7205–7210. [Google Scholar] [CrossRef]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef]
- Cheng, N.; Brantley, D.M.; Chen, J. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 2002, 13, 75–85. [Google Scholar] [CrossRef]
- Salvucci, O.; Tosato, G. Essential roles of EphB receptors and EphrinB ligands in endothelial cell function and angiogenesis. Adv. Cancer Res. 2012, 114, 21–57. [Google Scholar] [CrossRef] [PubMed]
- Kullander, K.; Klein, R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 2002, 3, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Kukacka, J.; Krizkova, S.; Huska, D.; Adam, V.; Masarik, M.; Prusa, R.; Kizek, R. Matrix metalloproteinases. Curr. Med. Chem. 2010, 17, 3751–3768. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.L.; Dyer, R.; Hupe, D.J. Matrix metalloproteinases. Curr. Opin. Chem. Biol. 1998, 2, 466–471. [Google Scholar] [CrossRef]
- Boire, A.; Covic, L.; Agarwal, A.; Jacques, S.; Sherifi, S.; Kuliopulos, A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 2005, 120, 303–313. [Google Scholar] [CrossRef]
- Sasaki, T.; Larsson, H.; Kreuger, J.; Salmivirta, M.; Claesson-Welsh, L.; Lindahl, U.; Hohenester, E.; Timpl, R. Structural basis and potential role of heparin/heparan sulfate binding to the angiogenesis inhibitor endostatin. EMBO J. 1999, 18, 6240–6248. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jang, J.W.; Lee, O.H.; Yeon, J.; Choi, E.Y.; Kim, K.W.; Lee, S.-T.; Kwon, Y.G. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000, 60, 5410–5413. [Google Scholar]
- Sudhakar, A.; Sugimoto, H.; Yang, C.; Lively, J.; Zeisberg, M.; Kalluri, R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc. Natl. Acad. Sci. USA 2003, 100, 4766–4771. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Sage, E.; Folkman, J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994, 79, 315–328. [Google Scholar] [CrossRef]
- Geiger, J.H.; Cnudde, S.E. What the structure of angiostatin may tell us about its mechanism of action. J. Thromb. Haemost. 2004, 2, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Troyanovsky, B.; Levchenko, T.; Mansson, G.; Matvijenko, O.; Holmgren, L. Angiomotin: An angiostatin binding protein that regulates endothelial cell migration and tube formation. J. Cell Biol. 2001, 152, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Iruela-Arispe, M.L.; Lombardo, M.; Krutzsch, H.C.; Lawler, J.; Roberts, D.D. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation 1999, 100, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Lawler, J. The thrombospondin family. Curr. Biol. 1993, 3, 188–190. [Google Scholar] [CrossRef]
- Bornstein, P. Thrombospondins function as regulators of angiogenesis. J. Cell Commun. Signal 2009, 3, 189–200. [Google Scholar] [CrossRef]
- Mirochnik, Y.; Kwiatek, A.; Volpert, O.V. Thrombospondin and apoptosis: Molecular mechanisms and use for design of complementation treatments. Curr. Drug Targets 2008, 9, 851–862. [Google Scholar] [CrossRef]
- Pribluda, V.S.; Gubish, E.R., Jr.; Lavallee, T.M.; Treston, A.; Swartz, G.M.; Green, S.J. 2-Methoxyestradiol: An endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev. 2000, 19, 173–179. [Google Scholar] [CrossRef]
- Yue, T.L.; Wang, X.; Louden, C.S.; Gupta, S.; Pillarisetti, K.; Gu, J.L.; Hart, T.K.; Lysko, P.G.; Feuerstein, G.Z. 2-Methoxyestradiol, an endogenous estrogen metabolite, induces apoptosis in endothelial cells and inhibits angiogenesis: Possible role for stress-activated protein kinase signaling pathway and Fas expression. Mol. Pharmacol. 1997, 51, 951–962. [Google Scholar] [CrossRef]
- Oliver, R.C.; Tervonen, T. Diabetes-a risk factor for periodontitis in adults? J. Periodontol. 1994, 65, 530–538. [Google Scholar] [CrossRef]
- Yalda, B.; Offenbacher, S.; Collins, J.G. Diabetes as a modifier of periodontal disease expression. Periodontology 2000 1994, 6, 37–49. [Google Scholar] [CrossRef]
- Minchenko, A.; Bauer, T.; Salceda, S.; Caro, J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab. Investig. 1994, 71, 374–379. Available online: https://pubmed.ncbi.nlm.nih.gov/7933988/ (accessed on 4 July 2022). [PubMed]
- Teshima-Kondo, S.; Kondo, K.; Prado-Lourenco, L.; Gonzalez-Herrera, I.; Rokutan, K.; Bayard, F.; Arnal, J.F.; Prats, A.C. Hyperglycemia up-regulates translation of the fibroblast growth factor 2 mRNA in mouse aorta via internal ribosome entry site. FASEB J. 2004, 18, 1583–1585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Groothius, P.G. Angiogenesis and vascular remodelling in female reproductive organs. Angiogensis 2005, 8, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Rogers, P.A.W. Human endometrial angiogenesis. Reproduction 2001, 121, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Tsuzuki, T.; Shindoh, H.; Nishigaki, A.; Yasuda, K.; Kanzaki, H. Regulation of decidualization and angiogenesis in the human endometrium: Mini review. J. Obstet. Gynaecol. Res. 2014, 40, 1180–1187. [Google Scholar] [CrossRef]
- Chung, A.S.; Lee, J.; Ferrara, N. Targeting the tumour vasculature: Insights from physiological angiogenesis. Nat. Rev. Cancer 2010, 10, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Boldeanu, L.; Dijmărescu, A.L.; Radu, M.; Siloşi, C.A.; Popescu-Drigă, M.V.; Poenariu, I.S.; Siloşi, I.; Boldeanu, M.V.; Novac, M.B.; Novac, L.V. The role of mediating factors involved in angiogenesis during implantation. Rom. J. Morphol. Embryol. 2020, 61, 665–672. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Adamis, A.P.; Miller, J.W.; Bernal, M.T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 1994, 118, 445–450. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Folkman, J.; Shing, Y. Angiogenesis. J. Biol. Chem. 1992, 267, 10931–10934. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, C.Q.; Post, M.; Simard, B.; Deslandes, Y.; Hseih, T.H. Design of Nanoparticles as Drug Carriers for Cancer Therapy. Cancer Genom. Proteom. 2006, 3, 147–158. [Google Scholar]
- Heldin, C.H.; Rubin, K.; Pietras, K.; Ostman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987, 6, 559–593. [Google Scholar] [CrossRef]
- Jain, R.K. Transport of molecules in the tumor interstitium: A review. Cancer Res. 1987, 47, 3039–3051. [Google Scholar]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities, Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- National Cancer Institute. Angiogenesis Inhibitors. Available online: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/angiogenesis-inhibitors-fact-sheet (accessed on 4 July 2022).
- Drugbank Online, General Site. Available online: https://go.drugbank.com/drugs (accessed on 4 July 2022).
- Keating, G.M. Axitinib: A review in advanced renal cell carcinoma. Drugs 2015, 75, 1903–1913. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Pal, S.K.; Tangen, C.; Thompson, I.M., Jr.; Balzer-Haas, N.; George, D.J.; Heng, D.Y.C.; Shuch, B.; Stein, M.; Tretiakova, M.; Humphrey, P.; et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. Lancet 2021, 397, 695–703. [Google Scholar] [CrossRef]
- Adib, E.; Klonowska, K.; Giannikou, K.; Do, K.T.; Pruitt-Thompson, S.; Bhushan, K.; Milstein, M.I.; Hedglin, J.; Kargus, K.E.; Sholl, L.M.; et al. Phase II Clinical Trial of Everolimus in a Pan-Cancer Cohort of Patients with mTOR Pathway Alterations. Clin. Cancer Res. 2021, 27, 3845–3853. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.-V.; Hernández, M.-T.; Giraldo, P.; De La Rubia, J.; De Arriba, F. Lenalidomide plus Dexamethasone for High-Risk Smoldering Multiple Myeloma. N. Engl. J. Med. 2013, 369, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.E.; Stadler, W.M. Pazopanib in renal cell carcinoma. Clin. Cancer. Res. 2010, 16, 5923–5927. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, H.; Tabernero, J.; Macarulla, T. Ramucirumab in metastatic colorectal cancer: Evidence to date and place in therapy. Ther. Adv. Med. Oncol. 2016, 8, 230–242. [Google Scholar] [CrossRef]
- Aljubran, A.; Elshenawy, M.A.; Kandil, M.; Zahir, M.N.; Shaheen, A.; Gad, A.; Alshaer, O.; Alzahrani, A.; Eldali, A.; Bazarbashi, S. Efficacy of Regorafenib in Metastatic Colorectal Cancer: A Multi-institutional Retrospective Study. Clin. Med. Insights Oncol. 2019, 13, 1179554918825447. [Google Scholar] [CrossRef]
- Keating, G.M.; Santoro, A. Sorafenib: A review of its use in advanced hepatocellular carcinoma. Drugs 2009, 69, 223–240. [Google Scholar] [CrossRef]
- Mulet-Margalef, N.; Garcia-Del-Muro, X. Sunitinib in the treatment of gastrointestinal stromal tumor: Patient selection and perspectives. Onco. Targets Ther. 2016, 9, 7573–7582. [Google Scholar] [CrossRef]
- Breitkreutz, I.; Anderson, K.C. Thalidomide in multiple myeloma-clinical trials and aspects of drug metabolism and toxicity. Expert Opin. Drug. Metab. Toxicol. 2008, 4, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yoon, J.H.; Ahn, J.; Jeon, M.J.; Kim, H.K.; Lim, D.J.; Kang, H.C.; Kim, I.J.; Shong, Y.K.; Kim, T.Y.; et al. Vandetanib for the Management of Advanced Medullary Thyroid Cancer: A Real-World Multicenter Experience. Endocrinol. Metab. 2020, 35, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Perkins, S.L.; Cole, S.W. Ziv-aflibercept (Zaltrap) for the treatment of metastatic colorectal cancer. Ann. Pharmacother. 2014, 48, 93–98. [Google Scholar] [CrossRef]
- Kamba, T.; McDonald, D.M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer 2007, 96, 1788–1795. [Google Scholar] [CrossRef]
- Yu, I.; Chen, L.; Ruan, J.Y.; Chang, J.T.; Cheung, W.Y. Risk and management of venous thromboembolisms in bevacizumab-treated metastatic colorectal cancer patients. Support Care Cancer 2016, 24, 1199–1208. [Google Scholar] [CrossRef]
- Yamamizu, K.; Hamada, Y.; Narita, M. Opioid receptor ligands regulate angiogenesis in development and in tumours. Br. J. Pharmaco. 2015, 172, 268–276. [Google Scholar] [CrossRef]
- Norden, A.D.; Drappatz, J.; Muzikansky, A.; David, K.; Gerard, M.; McNamara, M.B.; Phan, P.; Ross, A.; Kesari, S.; Wen, P.Y. An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J. Neurooncol. 2009, 92, 149–155. [Google Scholar] [CrossRef]
- Lu, K.V.; Bergers, G. Mechanisms of evasive resistance to anti-VEGF therapy in glioblastoma. CNS Oncol. 2013, 2, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, G.; Li, J.; Wang, Y. Recent advance in molecular angiogenesis in glioblastoma: The challenge and hope for anti-angiogenic therapy. Brain Tumor Pathol. 2015, 32, 229–236. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Moserle, L.; Jiménez-Valerio, G.; Casanovas, O. Antiangiogenic Therapies: Going beyond Their Limits. Cancer Discov. 2014, 4, 31–41. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M. Antiangiogenesis drug promising for metastatic colorectal cancer. Lancet 2003, 361, 1959. [Google Scholar] [CrossRef]
- Michieli, P. Hypoxia, angiogenesis and cancer therapy: To breathe or not to breathe? Cell Cycle 2009, 8, 3291–3296. [Google Scholar] [CrossRef] [PubMed]
- Hayden, E.C. Cutting off cancer’s supply lines. Nature 2009, 458, 686–687. [Google Scholar] [CrossRef]
- You, W.K.; McDonald, D.M. The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep. 2008, 41, 833–839. [Google Scholar] [CrossRef]
- Hidalgo, M.; Martinez-Garcia, M.; Le Tourneau, C.; Massard, C.; Garralda, E.; Boni, V.; Taus, A.; Albanell, J.; Sablin, M.P.; Alt, M.; et al. First-in-Human Phase i Study of Single-Agent Vanucizumab, a First-in-Class Bispecific Anti-Angiopoietin-2/Anti-Vegf-a Antibody, in Adult Patients with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1536–1545. [Google Scholar] [CrossRef]
- Kajiyama, H.; Suzuki, S.; Yoshihara, M.; Nishino, K.; Yoshikawa, N.; Utsumi, F.; Niimi, K.; Mizuno, M.; Kawai, M.; Oguchi, H.; et al. The Possible Existence of Occult Metastasis in Patients with Ovarian Clear-Cell Carcinoma Who Underwent Complete Resection without Any Residual Tumours. Oncotarget 2018, 9, 6298–6630. [Google Scholar] [CrossRef][Green Version]
- Deveza, L.; Choi, J.; Yang, F. Therapeutic Angiogenesis for Treating Cardiovascular Diseases. Theranostics 2012, 2, 801–814. [Google Scholar] [CrossRef]
- Kawasuji, M. Therapeutic Angiogenesis for Ischemic Heart Disease. Ann. Thorac. Cardiovasc. Surg. 2002, 8, 59–61. [Google Scholar]
- Tabibiazar, R.; Rockson, S.G. Angiogenesis and the ischaemic heart. Eur. Heart J. 2001, 22, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wang, Y. Therapeutic angiogenesis: Controlled delivery of angiogenic factors. Ther. Deliv. 2012, 3, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Nordlie, M.A.; Wold, L.E.; Simkhovich, B.Z.; Sesti, C.; Kloner, R.A. Molecular Aspects of Ischemic Heart Disease: Ischemia/Reperfusion–Induced Genetic Changes and Potential Applications of Gene and RNA Interference Therapy. J. Cardiovasc. Pharmacol. Therapeut. 2006, 11, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.S.; Flugelman, M.Y.; Weisz, A.; Keren-Tal, I.; Schaper, W. Angiogenesis by gene therapy: A new horizon for myocardial revascularization? Cardiovasc. Res. 1997, 35, 490–497. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; Morishita, R. Gene-Therapeutic Strategies Targeting Angiogenesis in Peripheral Artery Disease. Medicines 2018, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S.; Bridges, C.; Katz, M.G.; Korpisalo, P. Angiogenic gene therapy in cardiovascular diseases: Dream or vision? Eur. Heart. J. 2017, 38, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zharkinbekov, Z.; Sarsenova, M.; Yeltay, G.; Saparov, A. Recent Advances in Gene Therapy for Cardiac Tissue Regeneration. Int. J. Mol. Sci. 2021, 22, 9206. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Kay, G.L.; Kloner, R.A. The Therapeutic Effect of Cell Transplantation Versus Noncellular Biomaterial Implantation on Cardiac Structure and Function Following Myocardial Infarction. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 350–357. [Google Scholar] [CrossRef]
- Tse, H.F.; Lau, C.P. Therapeutic Angiogenesis With Bone Marrow—Derived Stem Cells. J. Cardiovasc. Pharmacol. Ther. 2007, 12, 89–97. [Google Scholar] [CrossRef]
- Bian, X.; Ma, K.; Zhang, C.; Fu, X. Therapeutic angiogenesis using stem cell-derived extracellular vesicles: An emerging approach for treatment of ischemic diseases. Stem Cell Res. Ther. 2019, 10, 158. [Google Scholar] [CrossRef]
- Merino-González, C.; Zuñiga, F.A.; Escudero, C.; Ormazabal, V.; Reyes, C.; Nova-Lamperti, E.; Salomón, C.; Aguayo, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front. Physiol. 2016, 7, 24. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rhim, W.K.; Yoo, Y.I.; Kim, D.S.; Ko, K.W.; Heo, Y.; Park, C.G.; Han, D.K. Defined MSC exosome with high yield and purity to improve regenerative activity. J. Tissue Eng. 2021, 12, 20417314211008626. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Jo, D.H.; Kim, J.H. Toward the clinical application of therapeutic angiogenesis against pediatric ischemic retinopathy. J. Lipid Atheroscler. 2020, 9, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Antognazza, M.R.; Lodola, F. Towards Novel Geneless Approaches for Therapeutic Angiogenesis. Front. Physiol. 2021, 11, 616189. [Google Scholar] [CrossRef] [PubMed]
- Qadura, M.; Terenzi, D.C.; Verma, S.; Al-Omran, M.; Hess, D.A. Concise review: Cell therapy for critical limb ischemia: An integrated review of preclinical and clinical studies: Stem cell therapy for critical limb ischemia. Stem Cells 2018, 36, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Corban, M.T.; Henry, T.D.; Dietz, A.B.; Lerman, L.O.; Lerman, A. Promise of autologous CD34+ stem/progenitor cell therapy for treatment of cardiovascular disease. Cardiovasc. Res. 2020, 116, 1424–1433. [Google Scholar] [CrossRef]
- Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.; Yamaguchi, T.; et al. Endothelial progenitors: A consensus statement on nomenclature: Endothelial progenitors nomenclature. Stem Cells Transl. Med. 2017, 6, 1316–1320. [Google Scholar] [CrossRef]
- Basile, D.P.; Yoder, M.C. Chapter 9—Regeneration and replacement of endothelial cells and renal vascular repair. In Regenerative Nephrology, 2nd ed.; Goligorsky, M.S., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 129–144. ISBN 9780128233184. [Google Scholar] [CrossRef]
- Shen, J.; Xie, Y.; Liu, Z.; Zhang, S.; Wang, Y.; Jia, L.; Wang, Y.; Cai, Z.; Ma, H.; Xiang, M. Increased myocardial stiffness activates cardiac microvascular endothelial cell via VEGF paracrine signaling in cardiac hypertrophy. J. Mol. Cell. Cardiol. 2018, 122, 140–151. [Google Scholar] [CrossRef]
- Eibel, B.; Rodrigues, C.G.; Giusti, I.I.; Nesralla, I.A.; Prates, P.R.; Sant’Anna, R.T.; Nardi, N.B.; Kalil, R.A. Gene therapy for ischemic heart disease: Review of clinical trials. Rev. Bras. Cir. Cardiovasc. 2011, 26, 635–646. [Google Scholar] [CrossRef]
- Hollon, T. Researchers and regulators reflect on first gene therapy death. Nat. Med. 2000, 6, 6. [Google Scholar] [CrossRef]
- Annex, B.H.; Cooke, J.P. New Directions in Therapeutic Angiogenesis and Arteriogenesis in Peripheral Arterial Disease. Circ Res. 2021, 128, 1944–1957. [Google Scholar] [CrossRef]
- Iyer, S.R.; Annex, B.H. Therapeutic angiogenesis for peripheral artery disease: Lessons learned in translational science. JACC Basic Transl. Sci. 2017, 2, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P.; Meng, S. Vascular regeneration in peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, D.L.; Flugelman, M.Y. Cell and gene therapies in cardiovascular disease with special focus on the no Option patient. Curr. Gene Ther. 2006, 6, 609–623. [Google Scholar] [CrossRef] [PubMed]

| Activator | Description/Structure | Receptor(s)/Cellular Targets | Mechanism of Action | References |
|---|---|---|---|---|
| FGF family |
|
|
| [4,49,50,51,52,53] |
| VEGF family |
|
|
| [3,4,54,55,56] |
| Ang 1 |
|
|
| [4,57,58,59,60] |
| Ephrins |
|
|
| [4,61,62,63] |
| MMPs |
|
|
| [4,64,65,66] |
| Inhibitor | Description/Structure | Receptor(s)/Cellular Targets | Mechanism of Action | References |
|---|---|---|---|---|
| Endostatin |
|
|
| [39,67,68,69] |
| Tumstatin |
|
|
| [39,70] |
| Angiostatin |
|
|
| [39,71,72,73] |
| TSPs |
|
|
| [39,45,46,74,75,76,77] |
| 2-ME |
|
|
| [39,78,79] |
| Angiogenesis Inhibitor Generic Name (Trade Name) | Description/Chemical Taxonomy | Mechanism of Action [102] | Approved to Treat (Alone or with Other Drugs) [101] |
|---|---|---|---|
| Axitinib (Inlyta®) https://go.drugbank.com/drugs/DB06626, accessed on 4 July 2022 | 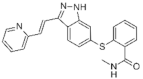 | Axitinib selectively blocks the tyrosine kinase receptors VEGFR-1, VEGFR-2, and VEGFR-3. | Renal cell carcinoma [103] |
| Bevacizumab (Avastin®, Mvasi®, Zirabev®) https://go.drugbank.com/drugs/DB00112 accessed on 4 July 2022 | Recombinant humanized monoclonal antibody | VEGF-A inhibitor | Cervical and colorectal cancer, glioblastoma, hepatocellular carcinoma, Non-squamous non-small cell lung cancer, Ovarian epithelial, fallopian tube or primary peritoneal cancer, Renal cell carcinoma [104] |
| Cabozantinib (Cometriq®) https://go.drugbank.com/drugs/DB08875 accessed on 4 July 2022 | 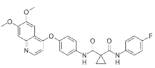 | Non-specific receptor tyrosine kinase inhibitor | Hepatocellular carcinoma, Medullary thyroid cancer, Renal cell carcinoma [105] |
| Everolimus (Afinitor®) https://go.drugbank.com/drugs/DB01590 accessed on 4 July 2022 | 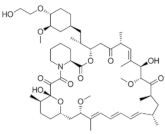 | Everolimus works similarly to Rapamycin, being a derivative of Rapamycin (sirolimus). After binding to FKBP-12, Everolimus inhibits the activation of mTOR, a key regulatory kinase. | Breast, pancreatic, gastrointestinal and lung cancer, renal cell carcinoma, subependymal giant cell astrocytoma [106] |
| Lenalidomide (Revlimid®) https://go.drugbank.com/drugs/DB00480 accessed on 4 July 2022 | 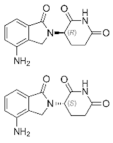 | Being an analogue of thalidomide, Lenalidomide works through various mechanisms of action, promoting malignant cell death and enhancing host immunity. | Anemia, Follicular lymphoma, mantle cell lymphoma, marginal zone lymphoma, multiple myeloma [107] |
| Lenvatinib mesylate (Lenvima®) https://go.drugbank.com/drugs/DB09078 accessed on 4 July 2022 | 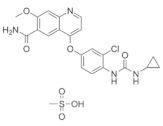 | Receptor tyrosine kinase inhibitor | Endometrial carcinoma, hepatocellular carcinoma, renal cell carcinoma, thyroid cancer [108] |
| Pazopanib (Votrient®) https://go.drugbank.com/drugs/DB06589 accessed on 4 July 2022 | 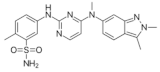 | Second-generation multitargeted tyrosine kinase inhibitor | Renal cell carcinoma; Soft tissue sarcoma [109] |
| Ramucirumab (Cyramza®) https://go.drugbank.com/drugs/DB05578 accessed on 4 July 2022 | Human monoclonal antibody (IgG1) against vascular endothelial growth factor receptor 2 (VEGFR2) | Ramucirumab is a direct VEGFR-2 antagonist, that blocks the binding of natural VEGF ligands. | Colorectal cancer, Hepatocellular carcinoma, Non-small cell lung cancer, Stomach adenocarcinoma or gastroesophageal junction adenocarcinoma [110] |
| Regorafenib (Stivarga®) https://go.drugbank.com/drugs/DB08896 accessed on 4 July 2022 | 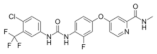 | Multiple kinases inhibitor | Colo-rectal cancer, Gastrointestinal stromal tumor, Hepato-cellular carcinoma [111] |
| Sorafenib (Nexavar®) https://go.drugbank.com/drugs/DB00398 accessed on 4 July 2022 | 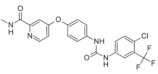 | Kinase inhibitor | Hepatocellular carcinoma, Renal cell carcinoma, Thyroid cancer [112] |
| Sunitinib (Sutent®) https://go.drugbank.com/drugs/DB01268 accessed on 4 July 2022 |  | Receptor tyrosine kinase inhibitor | Gastrointestinal stromal tumor; Pancreatic cancer; Renal cell carcinoma [113] |
| Thalidomide (Synovir, Thalomid®) https://go.drugbank.com/drugs/DB01041 accessed on 4 July 2022 | 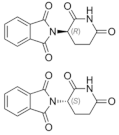 | As a cancer treatment, thalidomide may act as a VEGF inhibitor. | Multiple myeloma [114] |
| Vandetanib (Caprelsa®) https://go.drugbank.com/drugs/DB05294 accessed on 4 July 2022 | 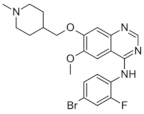 | Tyrosine kinases inhibitor | Medullary thyroid cancer [115] |
| Ziv-aflibercept (Zaltrap®) https://go.drugbank.com/drugs/DB08885 accessed on 4 July 2022 | Recombinant protein composed of the binding domains of two human VEGFRs fused with the Fc region of human IgG1. | VEGF inhibitor | Metastasized colorectal cancer [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, C.; Oprea, B.; Ciobanu, G.; Georgescu, M.; Bică, R.; Mateescu, G.-O.; Huseynova, F.; Barragan-Montero, V. The Angiogenic Balance and Its Implications in Cancer and Cardiovascular Diseases: An Overview. Medicina 2022, 58, 903. https://doi.org/10.3390/medicina58070903
Ionescu C, Oprea B, Ciobanu G, Georgescu M, Bică R, Mateescu G-O, Huseynova F, Barragan-Montero V. The Angiogenic Balance and Its Implications in Cancer and Cardiovascular Diseases: An Overview. Medicina. 2022; 58(7):903. https://doi.org/10.3390/medicina58070903
Chicago/Turabian StyleIonescu, Cătălina, Bogdan Oprea, Georgeta Ciobanu, Milena Georgescu, Ramona Bică, Garofiţa-Olivia Mateescu, Fidan Huseynova, and Veronique Barragan-Montero. 2022. "The Angiogenic Balance and Its Implications in Cancer and Cardiovascular Diseases: An Overview" Medicina 58, no. 7: 903. https://doi.org/10.3390/medicina58070903
APA StyleIonescu, C., Oprea, B., Ciobanu, G., Georgescu, M., Bică, R., Mateescu, G.-O., Huseynova, F., & Barragan-Montero, V. (2022). The Angiogenic Balance and Its Implications in Cancer and Cardiovascular Diseases: An Overview. Medicina, 58(7), 903. https://doi.org/10.3390/medicina58070903






