Aqueous Humor Cytokines in Non-Proliferative Diabetic Retinopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Preoperative Evaluation
2.3. Surgical Procedure
2.4. Aqueous Humor Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
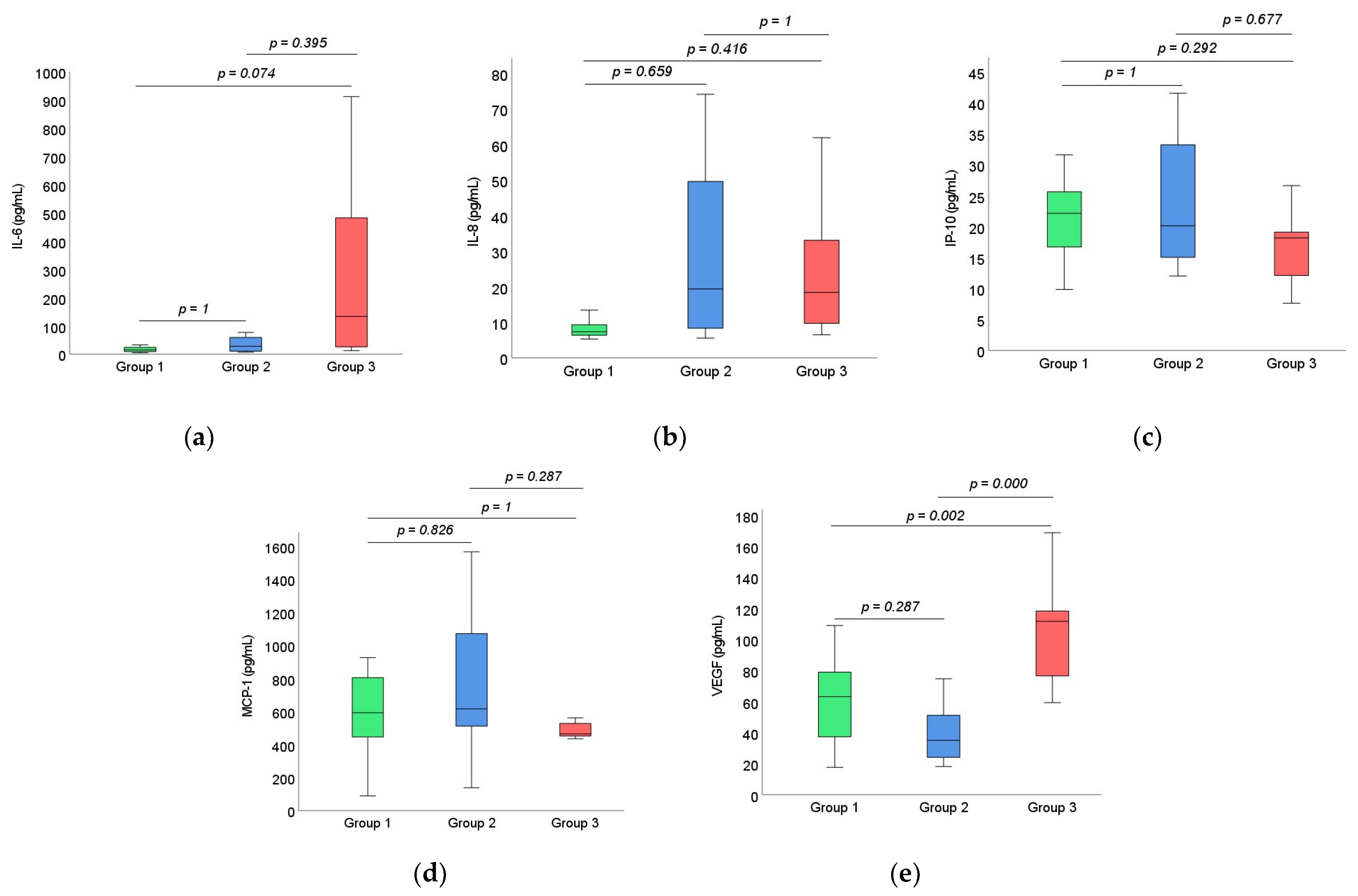
3.2. Cytokine Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinclair, S.H.; Schwartz, S.S. Diabetic Retinopathy—An Underdiagnosed and Undertreated Inflammatory, Neuro-Vascular Complication of Diabetes. Front. Endocrinol. 2019, 10, 843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García Morán, G.A.; Parra-Medina, R.; Cardona, A.G.; Quintero-Ronderos, P.; Garavito Rodríguez, E. Cytokines, chemokines, and growth factors. In Autoimmunity: From Bench to Bedside, 1st ed.; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Levy, R.A., Cervera, R., Eds.; El Rosario University Press: Bogota, Colombia, 2013; Section I; pp. 133–169. [Google Scholar]
- Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Tamhane, M.; Cabrera-Ghayouri, S.; Abelian, G.; Viswanath, V. Review of Biomarkers in Ocular Matrices: Challenges and Opportunities. Pharm. Res. 2019, 36, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damian, I.; Nicoară, S. Local Inflammatory Biomarkers and Potential Inflammation-Targeting Therapies in Diabetic Retinopathy. In Diabetic Eye Disease: From Therapeutic Pipeline to the Real World; Giudice, G.L., Ed.; IntechOpen: London, UK, 2021; Available online: https://www.intechopen.com/chapter (accessed on 22 May 2022).
- Funatsu, H.; Yamashita, H.; Noma, H.; Mimura, T.; Nakamura, S.; Sakata, K.; Hori, S. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 3–8. [Google Scholar] [CrossRef]
- Midena, E.; Frizziero, L.; Midena, G.; Pilotto, E. Intraocular fluid biomarkers (liquid biopsy) in human diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3549–3560. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. The Eye Sees Eye to Eye with the Immune System: The 2019 Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4489–4495. [Google Scholar] [CrossRef] [Green Version]
- Katamay, R.; Nussenblatt, R.B. Blood–retinal barrier, immune privilege, and autoimmunity. In Retina, 5th ed.; Ryan, S.J., Sadda, S.R., Hinton, D.R., Schachat, A.P., Sadda, S.R., Wilkinson, C.P., Eds.; W.B. Saunders: London, UK, 2013; Volume 1, pp. 579–589. [Google Scholar]
- Vujosevic, S.; Simó, R. Local and Systemic Inflammatory Biomarkers of Diabetic Retinopathy: An Integrative Approach. Investig. Ophthalmol. Vis. Sci. 2017, 58, bio68–bio75. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef]
- Ergul, A. Endothelin-1 and diabetic complications: Focus on the vasculature. Pharmacol. Res. 2011, 63, 477–482. [Google Scholar] [CrossRef]
- Khuu, L.A.; Tayyari, F.; Sivak, J.M.; Flanagan, J.G.; Singer, S.; Brent, M.H.; Huang, D.; Tan, O.; Hudson, C. Aqueous humor endothelin-1 and total retinal blood flow in patients with non-proliferative diabetic retinopathy. Eye 2017, 31, 1443–1450. [Google Scholar] [CrossRef]
- Taurone, S.; Ralli, M.; Nebbioso, M.; Greco, A.; Artico, M.; Attanasio, G.; Gharbiya, M.; Plateroti, A.M.; Zamai, L.; Micera, A. The role of inflammation in diabetic retinopathy: A review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10319–10329. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Toma, C. Diabetic retinopathy: An inflammatory disease. Ann. Eye Sci. 2018, 3, 52–62. [Google Scholar] [CrossRef]
- Nentwich, M.M.; Ulbig, M.W. Diabetic retinopathy—Ocular complications of diabetes mellitus. World J. Diabetes 2015, 6, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hwang, D.K.; Song, X.; Tao, Y. Association between Aqueous Cytokines and Diabetic Retinopathy Stage. J. Ophthalmol. 2017, 9402198. [Google Scholar] [CrossRef]
- Chen, R.; Lowe, L.; Wilson, J.D.; Crowther, E.; Tzeggai, K.; Bishop, J.E.; Varro, R. Simultaneous Quantification of Six Human Cytokines in a Single Sample Using Microparticle-based Flow Cytometric Technology. Clin. Chem. 1999, 45, 1693–1694. [Google Scholar] [CrossRef] [Green Version]
- Cvitkovic, K.; Sesar, A.; Sesar, I.; Pusic-Sesar, A.; Pejic, R.; Kelava, T.; Sucur, A.; Cavar, A. Concentrations of Selected Cytokines and Vascular Endothelial Growth Factor in Aqueous Humor and Serum of Diabetic Patients. Semin. Ophthalmol. 2020, 35, 126–133. [Google Scholar] [CrossRef]
- Antunica, A.G.; Karaman, K.; Znaor, L.; Sapunar, A.; Buško, V.; Puzović, V. IL-12 concentrations in the aqueous humor and serum of diabetic retinopathy patients. Graefes. Arch. Clin. Exp. Ophthalmol. 2012, 250, 815–821. [Google Scholar] [CrossRef]
- Sheemar, A.; Soni, D.; Takkar, B.; Basu, S.; Venkatesh, P. Inflammatory mediators in diabetic retinopathy: Deriving clinicopathological correlations for potential targeted therapy. Indian J. Ophthalmol. 2021, 69, 3035–3049. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, L.; Huang, R.; Wu, P.; He, L. Comparison of inflammatory cytokines levels in the aqueous humor with diabetic retinopathy. Int. Ophthalmol. 2020, 40, 2763–2769. [Google Scholar] [CrossRef]
- Cheung, C.M.; Vania, M.; Ang, M.; Chee, S.P.; Li, J. Comparison of aqueous humor cytokine and chemokine levels in diabetic patients with and without retinopathy. Mol. Vis. 2012, 18, 83–837. [Google Scholar]
- Chen, H.; Zhang, X.; Liao, N.; Wen, F. Assessment of biomarkers using multiplex assays in aqueous humor of patients with diabetic retinopathy. BMC Ophthalmol. 2017, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, H.; Yamashita, H.; Shimizu, E.; Kojima, R.; Hori, S. Relationship between vascular endothelial growth factor and interleukin-6 in diabetic retinopathy. Retina 2001, 21, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.; Liao, N.; Wen, F. Increased levels of IL-6, sIL-6R, and sgp130 in the aqueous humor and serum of patients with diabetic retinopathy. Mol. Vis. 2016, 22, 1005–1014. [Google Scholar]
- Owen, L.A.; Hartnett, M.E. Soluble Mediators of Diabetic Macular Edema: The Diagnostic Role of Aqueous VEGF and Cytokine Levels in Diabetic Macular Edema. Curr. Diabetes Rep. 2013, 13, 476–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Yu, H.; Yu, Y.; Geng, Y.; Li, D.; Yang, C.; Lv, Q.; Lu, L.; Liu, T.; Li, G.; et al. Levels of Inflammatory Cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in Aqueous Humour of Patients with Diabetic Retinopathy. J. Diabetes Res. 2018, 8546423. [Google Scholar] [CrossRef] [Green Version]
- Boulday, G.; Haskova, Z.; Reinders, M.E.; Pal, S.; Briscoe, D.M. Vascular Endothelial Growth Factor-Induced Signaling Pathways in Endothelial Cells That Mediate Overexpression of the Chemokine IFN-γ-Inducible Protein of 10 kDa In Vitro and In Vivo. J. Immunol. 2006, 176, 3098–3107. [Google Scholar] [CrossRef] [Green Version]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.; Amutha, A.; Rajalakshmi, R.; Bhaskaran, R.; Monickaraj, F.; Rangasamy, S.; Anjana, R.M.; Abhijit, S.; Gokulakrishnan, K.; Das, A.; et al. Association of increased levels of MCP-1 and cathepsin-D in young onset type 2 diabetes patients (T2DM-Y) with severity of diabetic retinopathy. J. Diabetes Complicat. 2017, 31, 804–809. [Google Scholar] [CrossRef]
- Tashimo, A.; Mitamura, Y.; Nagai, S.; Nakamura, Y.; Ohtsuka, K.; Mizue, Y.; Nishihira, J. Aqueous levels of macrophage migration inhibitory factor and monocyte chemotactic protein-1 in patients with diabetic retinopathy. Diabetes Med. 2004, 21, 1292–1297. [Google Scholar] [CrossRef]
- Yao, Y.; Li, R.; Du, J.; Li, X.; Zhao, L.; Long, L.; Li, D.; Lu, S. Tumor necrosis factor-α and diabetic retinopathy: Review and meta-analysis. Clin. Chim. Acta 2018, 485, 210–217. [Google Scholar] [CrossRef]
- Loukovaara, S.; Piippo, N.; Kinnunen, K.; Hytti, M.; Kaarniranta, K.; Kauppinen, A. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol. 2017, 95, 803–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, T.S. Contributions of Inflammatory Processes to the Development of the Early Stages of Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 95103. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Wykoff, C.C.; Boyer, D.; Heier, J.S.; Clark, W.L.; Emanuelli, A.; Higgins, P.M.; Singer, M.; Weinreich, D.M.; Yancopoulos, G.D.; et al. Evaluation of Intravitreal Aflibercept for the Treatment of Severe Nonproliferative Diabetic Retinopathy: Results from the PANORAMA Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Arabi, A.; Tadayoni, R.; Ahmadieh, H.; Shahraki, T.; Nikkhah, H. Update on Management of Non-proliferative Diabetic Retinopathy without Diabetic Macular Edema; Is There a Paradigm Shift? J. Ophthalmic. Vis. Res. 2022, 17, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Maturi, R.K.; Glassman, A.R.; Josic, K.; Antoszyk, A.N.; Blodi, B.A.; Jampol, L.M.; Marcus, D.M.; Martin, D.F.; Melia, M.; Salehi-Had, H.; et al. DRCR Retina Network. Effect of Intravitreous Anti-Vascular Endothelial Growth Factor vs. Sham Treatment for Prevention of Vision-Threatening Complications of Diabetic Retinopathy: The Protocol W Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Chatziralli, I.; Loewenstein, A. Intravitreal Anti-Vascular Endothelial Growth Factor Agents for the Treatment of Diabetic Retinopathy: A Review of the Literature. Pharmaceutics 2021, 13, 1137. [Google Scholar] [CrossRef]
- Ahuja, S.; Saxena, S.; Akduman, L.; Meyer, C.H.; Kruzliak, P.; Khanna, V.K. Serum vascular endothelial growth factor is a biomolecular biomarker of severity of diabetic retinopathy. Int. J. Retin. Vitr. 2019, 5, 29. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M.; Forrester, J.V. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009, 28, 348–368. [Google Scholar] [CrossRef]
- Bianchetti, G.; Viti, L.; Scupola, A.; Di Leo, M.; Tartaglione, L.; Flex, A.; De Spirito, M.; Pitocco, D. Erythrocyte membrane fluidity as a marker of diabetic retinopathy in type 1 diabetes mellitus. Eur. J. Clin. Investig. 2020, 51, e13455. [Google Scholar] [CrossRef]
- Bianchetti, G.; Clementi, M.E.; Sampaolese, B.; Serantoni, C.; Abeltino, A.; De Spirito, M.; Sasson, S.; Maulucci, G. Investigation of DHA-Induced Regulation of Redox Homeostasis in Retinal Pigment Epithelium Cells through the Combination of Metabolic Imaging and Molecular Biology. Antioxidants 2022, 11, 1072. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, B.; Zhou, Z.; Guo, Y.; Wang, D. The value of glycosylated hemoglobin in the diagnosis of diabetic retinopathy: A systematic review and Meta-analysis. BMC Endocr. Disord. 2021, 21, 82. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Group 1 (n = 37) | Group 2 (n = 13) | Group 3 (n = 12) | p1 |
|---|---|---|---|---|
| Age (y) | 72.78 ± 9.92 | 72.08 ± 6.87 | 66.42 ± 5.14 | 0.042 |
| Sex (n, male:female) | 17:20 | 5:8 | 4:8 | 0.714 |
| BCVA (decimal scale) | 0.23 ± 0.24 | 0.10 ± 0.19 | 0.20 ± 0.19 | 0.222 |
| IOP (mmHg) | 12.84 ± 2.66 | 13.77 ± 2.48 | 15.5 ± 3.03 | 0.016 |
| Duration of DM (y) | – | 6.83 ± 5.13 | 10 ± 7.9 | 0.406 |
| Fasting blood sugar (mmol/L) | 5.49 ± 0.67 | 7.39 ± 1.55 | 10.26 ± 3.54 | 0.001 |
| HbA1c (%) | 5.5 ± 0.41 | 6.78 ± 0.84 | 8.49 ± 2.63 | 0.001 |
| CRP (mg/L) | 2.56 ± 2.33 | 8.85 ± 6.33 | 6.95 ± 8.55 | 0.001 |
| DM treatment (n, OADs:Insulin) | – | 10:3 | 3:9 | – |
| Cytokine | <LOD (n, %) | <LLOQ (n, %) | Valid Cases (n) | Observed Concentrations (OC) (pg/mL) | Concentration in Range (CR) (pg/mL) | Mean of CR (pg/mL) | Median of CR (pg/mL) |
|---|---|---|---|---|---|---|---|
| IL-1β | 36 (97.29) | 1 (2.7) | 0 | 14.27 | – | – | – |
| IL-6 | 1 (2.7) | 18 (48.64) | 18 | 0.31–272.15 | 5.27–272.15 | 50.30 | 18.11 |
| IL-8 | 0 (0) | 7 (18.91) | 30 | 2.79–334.51 | 5.35–334.51 | 31.86 | 9.36 |
| IL-10 | 0 (0) | 33 (89.18) | 4 | 0.64–5.15 | 3.90–5.15 | 4.44 | 4.37 |
| IL-12 | 2 (5,4) | 30 (81.08) | 5 | 0.05–332.54 | 153.17–332.54 | 234.44 | 229.35 |
| IP-10 | 0 (0) | 1 (2.7) | 36 | 3.13–79.49 | 9.87–79.49 | 28.76 | 23.75 |
| MCP-1 | 0 (0) | 0 (0) | 37 | 150.57–4825.60 | 150.57–4825.60 | 812.06 | 603.29 |
| TNF-α | 26 (70.27) | 11 (29.72) | 0 | 0.04–6.71 | – | – | – |
| VEGF | 0 (0) | 1 (2.7) | 36 | 6.83–141.09 | 17.73–141.09 | 63.49 | 63.39 |
| Cytokine | <LOD (n, %) | <LLOQ (n, %) | Valid Cases (n) | Observed Concentrations (OC) (pg/mL) | Concentration in Range (CR) (pg/mL) | Mean of CR (pg/mL) | Median of CR (pg/mL) |
|---|---|---|---|---|---|---|---|
| IL-1β | 13 (100) | 0 (0) | 0 | – | – | – | – |
| IL-6 | 0 (0) | 9 (69.23) | 4 | 0.49–77.19 | 7.32–77.19 | 35.21 | 28.16 |
| IL-8 | 0 (0) | 3 (23.07) | 10 | 1.86–74.11 | 5.62–74.11 | 28.35 | 19.42 |
| IL-10 | 0 (0) | 13 (100) | 0 | 1.00–3.81 | – | – | – |
| IL-12 | 1 (7.69) | 11 (84.61) | 1 | 0.05–153.17 | – | – | – |
| IP-10 | 0 (0) | 0 (0) | 13 | 12.04–85.89 | 12.04–85.89 | 28.71 | 20.55 |
| MCP-1 | 0 (0) | 0 (0) | 13 | 89.83–1391.52 | 89.83–1391.52 | 652.69 | 589.60 |
| TNF-α | 9 (69.23) | 4 (30.76) | 0 | 0.63–1.63 | – | – | – |
| VEGF | 0 (0) | 0 (0) | 13 | 18.28–1190.09 | 18.28–1190.09 | 130.55 | 45.21 |
| Cytokine | <LOD (n, %) | <LLOQ (n, %) | Valid Cases (n) | Observed Concentrations (OC) (pg/mL) | Concentration in Range (CR) (pg/mL) | Mean of CR (pg/mL) | Median of CR (pg/mL) |
|---|---|---|---|---|---|---|---|
| IL-1β | 12 (100) | 0 (0) | 0 | – | – | – | – |
| IL-6 | 0 (0) | 4 (33.33) | 8 | 0.68–911.81 | 12.96–911.81 | 276.36 | 134.08 |
| IL-8 | 0 (0) | 2 (16.66) | 10 | 3.69–109.65 | 6.53–109.65 | 31.52 | 19.91 |
| IL-10 | 0 (0) | 11 (91.66) | 1 | 0.76–10.72 | – | – | – |
| IL-12 | 0 (0) | 8 (66.66) | 4 | 0.05–264.90 | 132.50–264.90 | 185.71 | 172.72 |
| IP-10 | 0 (0) | 1 (8.33) | 11 | 2.32–101.04 | 7.65–101.04 | 25.42 | 18.27 |
| MCP-1 | 0 (0) | 0 (0) | 12 | 139.24–2040.32 | 139.24–2040.32 | 829.13 | 550.03 |
| TNF-α | 7 (58.33) | 5 (41.66) | 0 | 0.08–5.10 | – | – | – |
| VEGF | 0 (0) | 0 (0) | 12 | 59.51–506.60 | 59.51–506.60 | 149.01 | 115.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obadă, O.; Pantalon, A.D.; Rusu-Zota, G.; Hăisan, A.; Lupuşoru, S.I.; Constantinescu, D.; Chiseliţă, D. Aqueous Humor Cytokines in Non-Proliferative Diabetic Retinopathy. Medicina 2022, 58, 909. https://doi.org/10.3390/medicina58070909
Obadă O, Pantalon AD, Rusu-Zota G, Hăisan A, Lupuşoru SI, Constantinescu D, Chiseliţă D. Aqueous Humor Cytokines in Non-Proliferative Diabetic Retinopathy. Medicina. 2022; 58(7):909. https://doi.org/10.3390/medicina58070909
Chicago/Turabian StyleObadă, Otilia, Anca Delia Pantalon, Gabriela Rusu-Zota, Anca Hăisan, Smaranda Ioana Lupuşoru, Daniela Constantinescu, and Dorin Chiseliţă. 2022. "Aqueous Humor Cytokines in Non-Proliferative Diabetic Retinopathy" Medicina 58, no. 7: 909. https://doi.org/10.3390/medicina58070909







