The Conundrum of Occult Cancer Screening in Venous Thromboembolism: Lessons from the REMOTEV Registry
Abstract
:1. Introduction
2. Materials and Methods
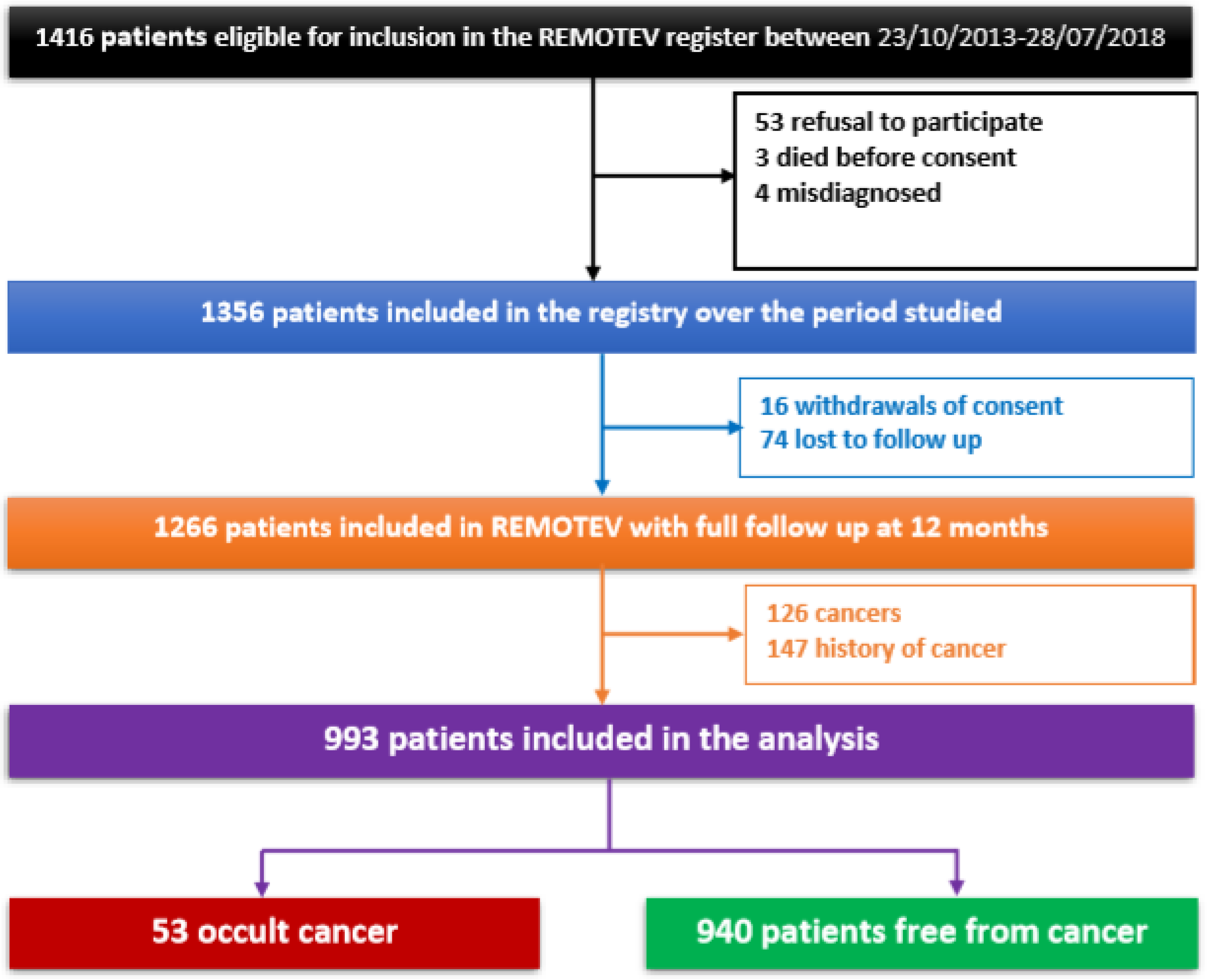
2.1. Study Design and Patient Selection
2.2. Requirements for VTE Diagnosis
2.3. Baseline Variables
2.4. Occult Cancer Screening Modalities
2.5. Occult Cancer Diagnosis
2.6. Follow-Up and Outcome Assessment
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics at Baseline
3.2. Etiological Assessment for Occult Cancer
3.3. Factors Associated with Occult Cancer
3.4. One-Year Outcomes
4. Discussion
4.1. VTE-Associated Occult Cancer Incidence
4.2. Cancer Types Associated with VTE
4.3. Accuracy of Cancer Screening Strategies in the Context of Acute VTE
4.4. Risk Factors Associated with Occult Cancer Detection
4.5. One-Year Prognosis
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, D.Y.; Khorana, A.A. Risks and Benefits of Anticoagulation in Cancer and Noncancer Patients. Semin. Thromb. Hemost. 2019, 45, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Abdol Razak, N.; Jones, G.; Bhandari, M.; Berndt, M.; Metharom, P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondon, M. Screening for Cancer in Patients with Acute Venous Thromboembolic Disease. Hämostaseologie 2021, 41, 042–047. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A. Venous thromboembolism and prognosis in cancer. Thromb. Res. 2010, 125, 490–493. [Google Scholar] [CrossRef] [Green Version]
- Delluc, A.; Antic, D.; Lecumberri, R.; Ay, C.; Meyer, G.; Carrier, M. Occult cancer screening in patients with venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2017, 15, 2076–2079. [Google Scholar] [CrossRef] [Green Version]
- Piccioli, A.; Lensing, A.W.A.; Prins, M.H.; Falanga, A.; Scannapieco, G.L.; Ieran, M.; Cigolini, M.; Ambrosio, G.B.; Monreal, M.; Girolami, A.; et al. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: A prospective randomized clinical trial. J. Thromb. Haemost. 2004, 2, 884–889. [Google Scholar] [CrossRef]
- Delluc, A.; Tromeur, C.; Le Ven, F.; Gouillou, M.; Paleiron, N.; Bressollette, L.; Nonent, M.; Salaun, P.-Y.; Lacut, K.; Leroyer, C.; et al. Current incidence of venous thromboembolism and comparison with 1998: A community-based study in Western France. Thromb. Haemost. 2016, 116, 967–974. [Google Scholar] [CrossRef]
- Robin, P.; Le Roux, P.-Y.; Planquette, B.; Accassat, S.; Roy, P.-M.; Couturaud, F.; Ghazzar, N.; Prevot-Bitot, N.; Couturier, O.; Delluc, A.; et al. Limited screening with versus without 18F-fluorodeoxyglucose PET/CT for occult malignancy in unprovoked venous thromboembolism: An open-label randomised controlled trial. Lancet Oncol. 2016, 17, 193–199. [Google Scholar] [CrossRef]
- Carrier, M.; Le Gal, G.; Wells, P.S.; Fergusson, D.; Ramsay, T.; Rodger, M.A. Systematic Review: The Trousseau Syndrome Revisited: Should We Screen Extensively for Cancer in Patients with Venous Thromboembolism? Ann. Intern. Med. 2008, 149, 323–333. [Google Scholar] [CrossRef]
- Carrier, M.; Lazo-Langner, A.; Shivakumar, S.; Tagalakis, V.; Zarychanski, R.; Solymoss, S.; Routhier, N.; Douketis, J.; Danovitch, K.; Lee, A.Y.; et al. Screening for Occult Cancer in Unprovoked Venous Thromboembolism. N. Engl. J. Med. 2015, 373, 697–704. [Google Scholar] [CrossRef]
- Robertson, L.; Broderick, C.; Yeoh, S.E.; Stansby, G. Effect of testing for cancer on cancer- or venous thromboembolism (VTE)-related mortality and morbidity in people with unprovoked VTE. Cochrane Database Syst. Rev. 2021, 10, CD010837. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.I.; Carrier, M.; Doormaal, F.; Robin, P.; Otten, H.; Salaun, P.; Büller, H.R.; Le Gal, G.; Es, N. Risk scores for occult cancer in patients with unprovoked venous thromboembolism: Results from an individual patient data meta-analysis. J. Thromb. Haemost. 2020, 18, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, S.; Cordeanu, E.-M.; Nouri, S.; Faller, A.-M.; Frantz, A.-S.; Mirea, C.; Bilbault, P.; Ohlmann, P.; Le Ray, I.; Stephan, D. Rivaroxaban versus standard anticoagulation for symptomatic venous thromboembolism (REMOTEV observational study): Analysis of 6-month outcomes. Int. J. Cardiol. 2017, 226, 103–109. [Google Scholar] [CrossRef]
- Gaertner, S.; Cordeanu, E.-M.; Mirea, C.; Frantz, A.-S.; Auger, C.; Bilbault, P.; Ohlmann, P.; Schini-Kerth, V.; Stephan, D. Increased risk and severity of unprovoked venous thromboembolism with clustering cardiovascular risk factors for atherosclerosis: Results of the REMOTEV registry. Int. J. Cardiol. 2018, 252, 169–174. [Google Scholar] [CrossRef]
- Cordeanu, M.; Gaertner, S.; Faller, A.; Mirea, C.; Le Ray, I.; Stephan, D. Prognostic value of the simplified PESI score in comparison with the 2014 ESC risk model in pulmonary embolism. Int. J. Cardiol. 2016, 220, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Cordeanu, E.-M.; Younes, W.; Canuet, M.; Mirea, C.; Faller, A.-M.; Frantz, A.-S.; Daglayan, A.; Gaertner, S.; Stephan, D. Real-life practices of chronic thromboembolic pulmonary hypertension screening: Results from the REMOTEV observational study. Clin. Respir. J. 2018, 12, 2303–2306. [Google Scholar] [CrossRef]
- Cordeanu, E.-M.; Lambach, H.; Heitz, M.; Di Cesare, J.; Mirea, C.; Faller, A.-M.; Cavaro, A.-C.; Frantz, A.-S.; Gaertner, S.; Schini-Kerth, V.; et al. Pulmonary Embolism and Coexisting Deep Vein Thrombosis: A Detrimental Association? J. Clin. Med. 2019, 8, 899. [Google Scholar] [CrossRef] [Green Version]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.R.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3080. [Google Scholar] [CrossRef] [Green Version]
- Van Doormaal, F.F.; Terpstra, W.; Van Der Griend, R.; Prins, M.H.; Nijziel, M.R.; Van De Ree, M.A.; Büller, H.R.; Dutilh, J.C.; Ten Cate-Hoek, A.; Van Den Heiligenberg, S.M.; et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted?: Screening for cancer in idiopathic VTE. J. Thromb. Haemost. 2011, 9, 79–84. [Google Scholar] [CrossRef]
- Jara-Palomares, L.; Otero, R.; Jimenez, D.; Carrier, M.; Tzoran, I.; Brenner, B.; Margeli, M.; Praena-Fernandez, J.M.; Grandone, E.; Monreal, M.; et al. Development of a Risk Prediction Score for Occult Cancer in Patients with VTE. Chest 2017, 151, 564–571. [Google Scholar] [CrossRef]
- van Es, N.; Le Gal, G.; Otten, H.-M.; Robin, P.; Piccioli, A.; Lecumberri, R.; Jara-Palomares, L.; Religa, P.; Rieu, V.; Rondina, M.; et al. Screening for Occult Cancer in Patients with Unprovoked Venous Thromboembolism: A Systematic Review and Meta-analysis of Individual Patient Data. Ann. Intern. Med. 2017, 167, 410–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robin, P.; Otten, H.M.; Delluc, A.; van Es, N.; Carrier, M.; Salaün, P.-Y.; Le Gal, G. Effect of occult cancer screening on mortality in patients with unprovoked venous thromboembolism. Thromb. Res. 2018, 171, 92–96. [Google Scholar] [CrossRef]
- Robin, P.; van Es, N.; Le Roux, P.-Y.; Rondina, M.; Lecumberri, R.; Beckers, M.; Le Gal, G.; Salaun, P.-Y. Performance of 18F-fluorodesoxyglucose positron-emission tomography/computed tomography for cancer screening in patients with unprovoked venous thromboembolism: Results from an individual patient data meta-analysis. Thromb. Res. 2020, 194, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Shestakovska, O.; Aboyans, V.; Alings, M.; Anand, S.S.; Avezum, A.; Berkowitz, S.D.; Bhatt, D.L.; et al. Bleeding and New Cancer Diagnosis in Patients with Atherosclerosis. Circulation 2019, 140, 1451–1459. [Google Scholar] [CrossRef]
- Coleman, C. Early Detection and Screening for Breast Cancer. Semin. Oncol. Nurs. 2017, 33, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Ademas: Association Pour le Dépistage des Maladies du Sein n.d. Available online: http://ademas-alsace.com/ademaspro/mises-a-jours-resultats.php (accessed on 21 September 2019).
- Oeffinger, K.C.; Fontham, E.T.H.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.-C.T.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update from the American Cancer Society. JAMA 2015, 314, 1599–1614. [Google Scholar] [CrossRef]
- Ihaddadene, R.; Corsi, D.J.; Lazo-Langner, A.; Shivakumar, S.; Zarychanski, R.; Tagalakis, V.; Solymoss, S.; Routhier, N.; Douketis, J.; Le Gal, G.; et al. Risk factors predictive of occult cancer detection in patients with unprovoked venous thromboembolism. Blood 2016, 127, 2035–2037. [Google Scholar] [CrossRef]
- Erdmann, A.; Ney, B.; Qanadli, S.D.; Calanca, L.; Mazzolai, L. Deep vein thrombosis in uncommon localisations. Rev. Med. Suisse 2017, 13, 2134–2137. [Google Scholar]
- Rosell, A.; Lundström, S.; Mackman, N.; Wallén, H.; Thålin, C. A clinical practice-based evaluation of the RIETE score in predicting occult cancer in patients with venous thromboembolism. J. Thromb. Thrombolysis 2019, 48, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Kraaijpoel, N.; Di Nisio, M.; Mulder, F.; van Es, N.; Beyer-Westendorf, J.; Carrier, M.; Garcia, D.; Grosso, M.; Kakkar, A.; Mercuri, M.; et al. Clinical Impact of Bleeding in Cancer-Associated Venous Thromboembolism: Results from the Hokusai VTE Cancer Study. Thromb. Haemost. 2018, 118, 1439–1449. [Google Scholar] [CrossRef]
- Bertoletti, L.; Robin, P.; Jara-Palomares, L.; Tromeur, C.; Pastre, J.; Prevot-Bitot, N.; Mouneh, T.; Le Gal, G.; Salaun, P.-Y.; MVTEP Investigators. Predicting the risk of cancer after unprovoked venous thromboembolism: External validation of the RIETE score. J. Thromb. Haemost. 2017, 15, 2184–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraaijpoel, N.; van Es, N.; Raskob, G.; Büller, H.; Carrier, M.; Zhang, G.; Lin, M.; Grosso, M.; Di Nisio, M. Risk Scores for Occult Cancer in Patients with Venous Thromboembolism: A Post Hoc Analysis of the Hokusai-VTE Study. Thromb. Haemost. 2018, 118, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Cronin-Fenton, D.P.; Søndergaard, F.; Pedersen, L.A.; Fryzek, J.P.; Cetin, K.; Acquavella, J.; Baron, J.A.; Sørensen, H.T. Hospitalisation for venous thromboembolism in cancer patients and the general population: A population-based cohort study in Denmark, 1997–2006. Br. J. Cancer 2010, 103, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Canonico, M.E.; Santoro, C.; Avvedimento, M.; Giugliano, G.; Mandoli, G.E.; Prastaro, M.; Franzone, A.; Piccolo, R.; Ilardi, F.; Cameli, M.; et al. Venous Thromboembolism and Cancer: A Comprehensive Review from Pathophysiology to Novel Treatment. Biomolecules 2022, 12, 259. [Google Scholar] [CrossRef] [PubMed]

| Total N = 993 M(IQR)/N(%) | Occult Cancer N = 53 M(IQR)/N(%) | Absence of Cancer N = 940 M(IQR)/N(%) | p | |
|---|---|---|---|---|
| Demographic data | ||||
| Age Age > 65 years | 67 (50–78) 519 (52.3) | 73 (66–82) 41 (77.4) | 66 (49–78) 478 (50.9) | 0.00119 0.0003 |
| Female | 528 (53.2) | 27 (50.9) | 501 (53.3) | 0.84 |
| BMI (N = 911) | 27 (24–31) | 27 (24.5–30) | 27 (23–31) | 0.66 |
| Cardiovascular risk factors | ||||
| Arterial hypertension | 486 (48.9) | 27 (50.9) | 459 (48.8) | 0.87 |
| History of smoking (active or former) (N = 965) Active | 403 (41.8) 158 (16.4) | 21 (40.4) 2 (3.8) | 382 (41.8) 156 (17.1) | 0.95 0.024 |
| Diabetes | 146 (14.7) | 6 (11.3) | 140 (14.9) | 0.60 |
| Dyslipidemia | 298 (30) | 16 (30.2) | 282 (30) | 1 |
| Obesity (N = 911) | 313 (33.3) | 15 (29.4) | 298 (33.5) | 0.65 |
| Héredity | 45 (4.5) | 1 (1.9) | 44 (4.7) | 0.50 |
| Comorbidities | ||||
| Ischemic heart disease | 44 (4.4) | 2 (3.8) | 42 (4.5) | 1 |
| Stroke/transient ischemic attack | 61 (6.1) | 4 (7.5) | 57 (6.1) | 0.56 |
| Lower-limb arteriopathy | 29 (2.9) | 2 (3.8) | 27 (2.9) | 0.66 |
| Chronic obstructive pulmonary disease | 52 (5.2) | 3 (5.7) | 49 (5.2) | 0.75 |
| Atrial fibrillation | 59 (5.9) | 3 (5.7) | 56 (5.9) | 1 |
| Chronic kidney disease | 513 (51.7) | 38 (71.7) | 475 (50.5) | 0.003 |
| 60 ≤ eGFR MDRD < 90 mL/min/1.73 m2 | 347 (34.9) | 27 (50.9) | 320 (34) | 0.018 |
| 30 ≤ eGFR MDRD < 60 mL/min/1.73 m2 | 138 (13.9) | 10 (18.9) | 128 (13.6) | 0.38 |
| 15 ≤ eGFR MDRD < 30 mL/min/1.73 m2 | 23 (2.3) | 1 (1.9) | 22 (2.3) | 1 |
| eGFR MDRD < 15 mL/min/1.73 m2 | 5 (0.5) | 0 | 5 (5.3) | 1 |
| Cognitive disorders | 105 (10.6) | 3 (5.7) | 102 (10.9) | 0.35 |
| Psychiatric disorders | 132 (13.3) | 5 (9.4) | 127 (13.5) | 0.53 |
| VTE risk factors | ||||
| Fracture/orthopedic surgery | 35 (3.5) | 1 (1.9) | 34 (3.6) | 1 |
| Surgery with anesthesia > 30 min | 78 (7.9) | 1 (1.9) | 78 (8.2) | 0.11 |
| VTE history | 319 (32.1) | 20 (37.7) | 299 (31.8) | 0.45 |
| Recent hospitalization for heart/respiratory failure < 3 months | ||||
| Myocardial infarction < 3 months | 12 (1.2) | 1 (1.9) | 11 (1.2) | 0.48 |
| Spinal cord injury | 7 (0.7) | 0 | 7 (7.4) | 1 |
| Thrombophilia | 1 (1) | 0 | 1 (1) | 1 |
| Paralysis | 49 (4.9) | 1 (1.9) | 48 (5.1) | 0.51 |
| Central catheter | 6 (0.6) | 0 | 6 (0.6) | 1 |
| Oral contraception/hormone replacement therapy/in vitro fertilization | 2 (0.2) | 0 | 2 (0.2) | 1 |
| Pregnancy | ||||
| Antithrombotic treatment at admission | ||||
| Antiplatelet | 179 (18) | 10 (18.9) | 169 (17.9) | 1 |
| Anticoagulant | 64 (6.4) | 7 (13.2) | 57 (6.1) | 0.07 |
| Total N = 993 M(IQR)/N(%) | Occult Cancer N = 53 M(IQR)/N(%) | Absence of Cancer N = 940 M(IQR)/N(%) | p | |
|---|---|---|---|---|
| Index event | ||||
| PE | 868 (87.4) | 46 (86.8) | 822 (87.4) | 1 |
| PE only | 276 (27.8) | 10 (18.9) | 266 (28.3) | 0.18 |
| PE + DVT | 592 (59.6) | 36 (67.9) | 556 (59.1) | 0.26 |
| DVT only | 125 (12.6) | 7 (13.2) | 118 (12.6) | 1 |
| D-dimer (N = 764) | 3280 (1800–7570) | 3200 (2085–7770) | 3280 (1800–7562) | 0.99 |
| Provoked | 340 (35.1) | 11 (33.3) | 329 (35.2) | 0.97 |
| Length of hospital stay | 6 (4–8) | 8 (6–13) | 6 (4–8) | <0.0001 |
| DVT extension | ||||
| DVT lower limbs | 683 (68.8) | 42 (79.2) | 641 (68.2) | 0.12 |
| Distal | 198 (19.9) | 15 (28.3) | 183 (19.5) | 0.16 |
| Proximal | 380 (38.3) | 19 (35.8) | 361 (38.4) | 0.82 |
| Bilateral | 105 (10.6) | 8 (15.1) | 97 (10.3) | 0.46 |
| USVT | 56 (5.6) | 4 (7.5) | 52 (5.5) | 0.53 |
| Concomitant DVT lower limbs and USVT | 22 (2.2) | 3 (5.7) | 19 (2) | 0.08 |
| Type of Exam | Total | Negative Exam | Positive Exam | Cancer Diagnosed | Cancer Diagnosed/Positive Exam | Cancer Diagnosed/Total |
|---|---|---|---|---|---|---|
| Limited screening examinations | ||||||
| CTPA | 627 | NA | NA | 16 | NA | 2.55% |
| Chest X-ray | 726 | 725 | 1 | 0 | 0 | 0 |
| FOBT | 492 | 354 | 138 | 2 | 1.4% | 0.41% |
| PSA | 372 | 322 | 50 | 5 | 10% | 1.34% |
| Mammograms | 190 | 188 | 2 | 2 | 100% | 1.05% |
| Extended screening examinations | ||||||
| Abdomino-pelvic US | 700 | 686 | 14 | 6 | 42.8% | 0.86% |
| TAP CT | 171 | 148 | 23 | 10 | 43.5% | 5.85% |
| SPEP | 591 | 564 | 27 | 4 | 14.8% | 0.67% |
| Variable | Univariate Analysis | Multivarate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | |
| Age > 65 years | 3.47 | 1.83–7.17 | 0.0003 | 2.58 | 1.26–5.72 | 0.0132 |
| Active smoking | 0.19 | 0.03–0.63 | 0.024 | 0.33 | 0.05–1.19 | 0.150 |
| Chronic kidney disease | 2.48 | 1.37–4.70 | 0.003 | 1.59 | 0.83–3.18 | 0.17 |
| Anticoagulation | 2.11 | 0.61–5.55 | 0.07 | 2.09 | 0.58–5.81 | 0.19 |
| Concomitant DVT of the lower limbs and USVT | 2.91 | 0.66–8.89 | 0.08 | 4.48 | 1.00–15.3 | 0.0280 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeanu, E.-M.; Jambert, L.; Tousch, J.; Mirea, C.; Delatte, A.; Younes, W.; Woehl, B.; Harter, C.; Frantz, A.-S.; Hamade, A.; et al. The Conundrum of Occult Cancer Screening in Venous Thromboembolism: Lessons from the REMOTEV Registry. Medicina 2022, 58, 913. https://doi.org/10.3390/medicina58070913
Cordeanu E-M, Jambert L, Tousch J, Mirea C, Delatte A, Younes W, Woehl B, Harter C, Frantz A-S, Hamade A, et al. The Conundrum of Occult Cancer Screening in Venous Thromboembolism: Lessons from the REMOTEV Registry. Medicina. 2022; 58(7):913. https://doi.org/10.3390/medicina58070913
Chicago/Turabian StyleCordeanu, Elena-Mihaela, Lucas Jambert, Jonathan Tousch, Corina Mirea, Alexandre Delatte, Waël Younes, Bastien Woehl, Claire Harter, Anne-Sophie Frantz, Amer Hamade, and et al. 2022. "The Conundrum of Occult Cancer Screening in Venous Thromboembolism: Lessons from the REMOTEV Registry" Medicina 58, no. 7: 913. https://doi.org/10.3390/medicina58070913






