The Necessity of a Locally Active Antidote in the Clinical Practice of Botulinum Neurotoxin Therapy: Short Communication
Abstract
:1. Introduction
2. Materials and Methods
| Indication | Number of Patients | Number of BoNT-ADEVs | Age MV/SD | Number of Females/Males | Dose per Session MV/SD | Percentage of BoNT-ADEVs |
|---|---|---|---|---|---|---|
| CD | 75 | 5 | 68/11 | 50/25 | 255/135 | 6.7 |
| HFS | 39 | 2 | 75/9 | 18/21 | 30/8.5 | 5.1 |
| BLE | 19 | 2 | 66/10 | 10/9 | 75/15 | 10.5 |
| SPAS | 18 | 1 | 65/16 | 11/7 | 320/180 | 5.6 |
| PAIN | 16 | 0 | 51/12 | 12/4 | 183/32 | 0.0 |
| OMD/OPD | 10 | 2 | 52/15 | 5/5 | 132/75 | 20.0 |
| LD/WC | 9 | 1 | 50/14 | 4/5 | 175/150 | 11.1 |
| MEIGE | 5 | 1 | 60/10 | 3/2 | 155/90 | 20.0 |
| HYPER | 4 | 1 | 70/20 | 0/4 | 200/50 | 25 |
| GEN DYS | 2 | 0 | 60/5 | 0/2 | 400/100 | 0.0 |
| Indication | n= | Number of Patients with BoNT-ADEVs | ADOT-BoNT-ADEV |
|---|---|---|---|
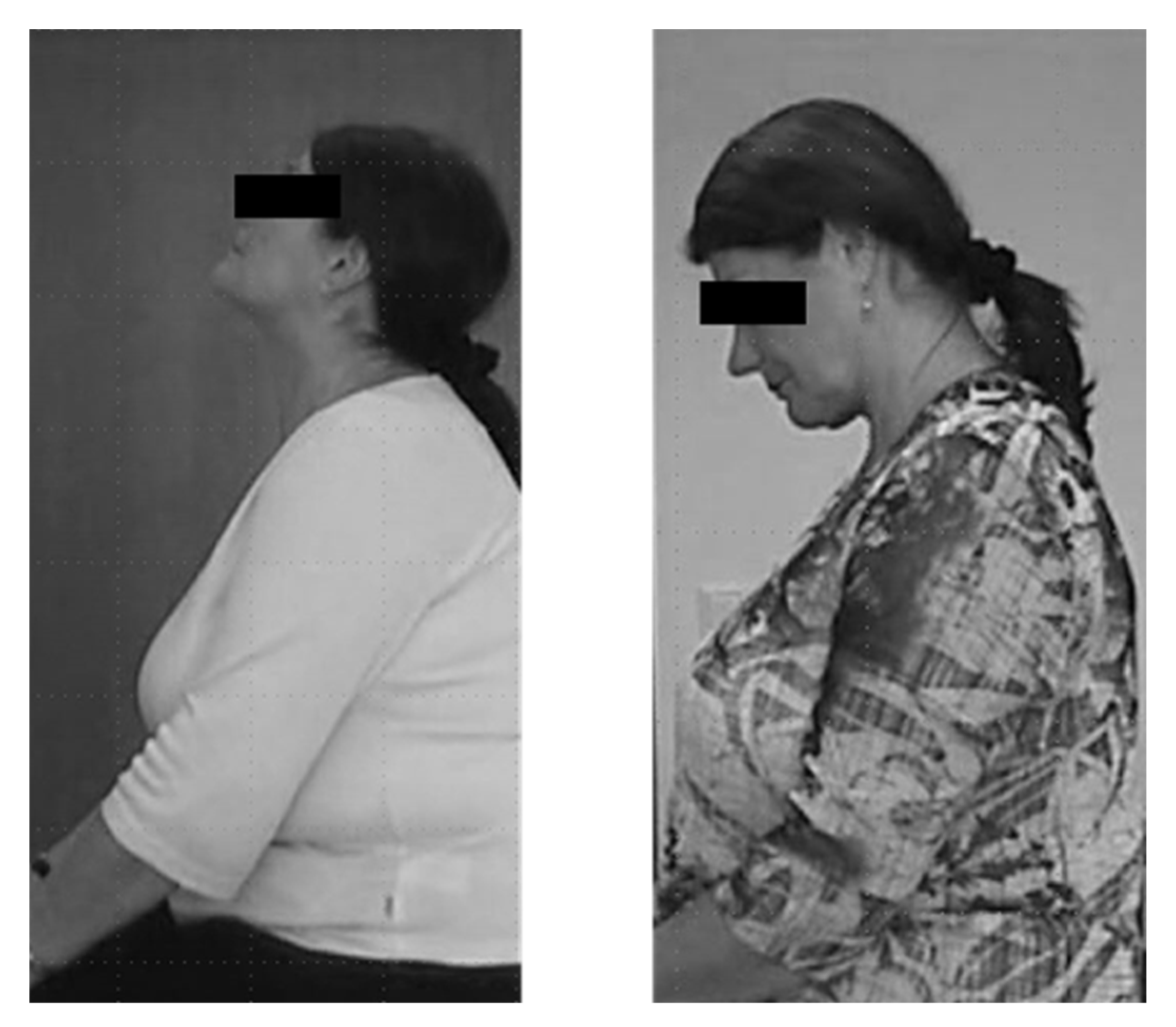
| CD | 5 | Patient 1, with retrocollis and retrocaput, experienced severe neck weakness after injection with only 100 U Dysport®. (Figure 1) | ADOT |
| Patient 2, with severe retrocaput, experienced difficulties in swallowing after injection of the deep neck muscles with 100 U Xeomin® per side | --- | ||
| Patient 3, with laterocollis and head tremor, experienced difficulties in swallowing after injection of the left lateral muscle group with 500 U Dysport®. | --- | ||
| Patient 4, with laterocollis und torticaput to the left side, experienced neck muscle weakness and muscle pain on the right side after injection of the left splenius and semispinalis capitis muscle with 200 U Xeomin®. | ADOT | ||
| Patient 5, with epsilon-glycan-positive dystonia, who was treated because of severe head jerks to the right side, experienced weakness of shoulder elevation after injection of the right splenius capitis and levator scapulae muscle with 200 U Xeomin®. | ADOT | ||
| HFS | 2 | Patient 1, with blepharospasm and additional hemifacial spasm on the left side. After injection of 60 U Dysport® into the right orbicularis oculi muscle, 10 U Dysport® per edge of the right eye lid, and 7.5 U Dysport® into the edges of the left eye lid, a moderate ptosis on the left side developed, despite dose reduction on the left side (Figure 2). | ADOT |
| Patient 2, with stable scheme and doses of 70 U Dysport on the left eye, reported significant ptosis of the left eye. | ADOT | ||
| BLE | 2 | Patient 1, with unusual combination of myasthenia gravis and blepharospasm, was injected with 30 U Botox® per orbicularis oculi muscle. Approx. 3 weeks after injection, she reported double vision for 2 to 3 weeks. | --- |
| Patient 2, with tonic and phasic muscle contraction of the orbicularis oculi muscle, was injected with 17.5 U Botox per oo and 5 U per edge of the eye lid, per side. This scheme was used without any complications for years. She reported severe ptosis on both eyes after intensive sun exposure for several hours, approx. 60 min after injection. | ADOT | ||
| SPAS | 1 | Patient with stiff knee gait after stroke, was injected with 500 U per quadriceps muscle. He claimed to have difficulty standing up and climbing stairs. | ADOT |
| OMD/OPG | 2 | Patient 1, with jaw opening dystonia. After injection of the pterygoid muscles with 30 U Xeomin®, from the outside, a paresis of jaw opening occurred. | ADOT |
| Patient 2, with complex tongue movement, had received 3 × 10 U Botox® per side of the tongue. Approx. 3 days later, the patient reported having difficulties in swallowing for 4–5 weeks. The localization of the side effect was difficult to determine. | --- | ||
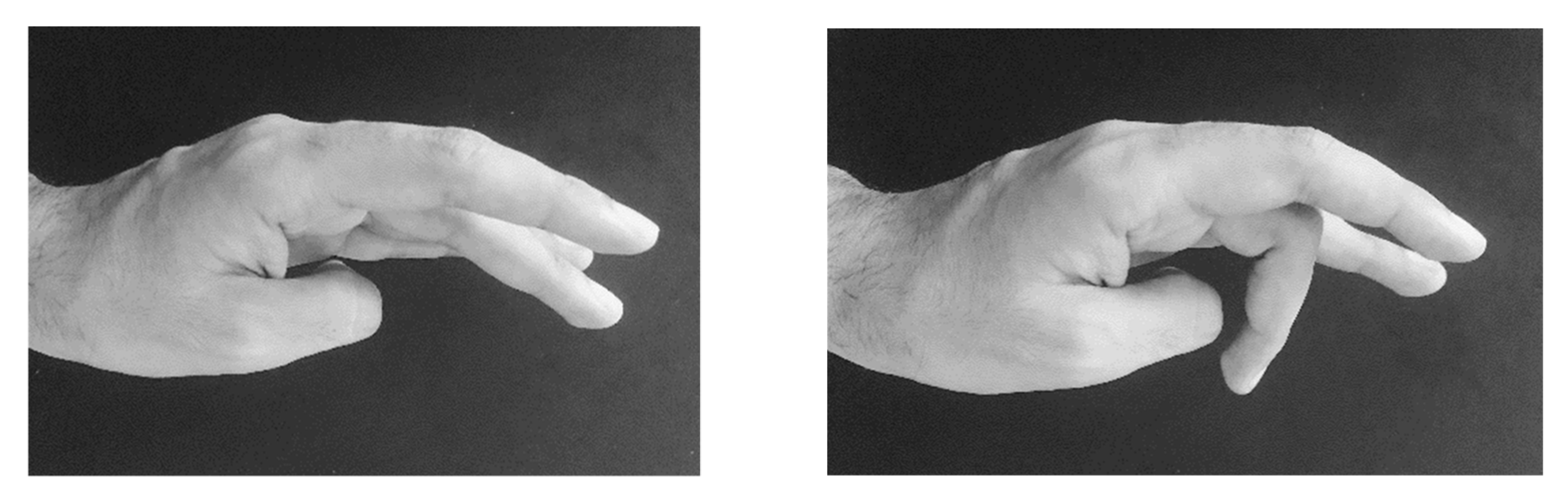
| LD/WC | 1 | Patient with writer´s cramp of extensor type. After 10 U Dysport® into the extensor indicis and 10 U Dysport® into the extensor digitorum communis, muscle severe paresis of the middle finger extensor occurred (Figure 3). | ADOT |
| MEIGE | 1 | Patient with involvement of the masseter muscle on both sides, experienced a moderate reduction in bite strength after injection of 30 U Botox® per masseter muscle. | ADOT |
| HYPER | 1 | Patient with carcinoma and removal of the submandibular glands, suffered from severe hypersalivation. After treatment with 100 U Xeomin® per parotid gland, he reported having a very dry mouth. | ADOT |

3. Results
3.1. Demographical and Treatment-Related Data
3.2. ADEVs and BoNT-ADEVs
4. Discussion
4.1. Safety and Efficacy of BoNT/A Injections
4.2. Two Types of BoNT-ADEVs
4.3. Combined Use of BoNT Injections and Antidote Application
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrero, B.A.; Ecklung, A.E.; Streett, C.S.; Ford, D.F.; King, J.K. Experimental botulism in monkeys—A clinical pathological study. Exp. Mol. Pathol. 1967, 6, 84–95. [Google Scholar] [CrossRef]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum toxin as a biological weapon: Medical and public health management. JAMA 2001, 285, 1059–1070, Erratum in JAMA 2001, 285, 2081. [Google Scholar] [CrossRef] [PubMed]
- McCarty, C.L.; Angelo, K.; Beer, K.D.; Cibulskas-White, K.; Quinn, K.; de Fijter, S.; Bokanyi, R.; St Germain, E.; Baransi, K.; Barlow, K.; et al. Large Outbreak of Botulism Associated with a Church Potluck Meal—Ohio, 2015. Morb. Mortal. Wkly. Rep. 2015, 64, 802–803. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.L.; Hatheway, C.; Becher, J.; Swerdlow, D.L. Botulism Surveillance and Emergency Response: A Public Health Strategy for a Global Challenge. JAMA 1997, 278, 433–435. [Google Scholar] [CrossRef]
- Hooper, R.R. The covert use of chemical and biological warfare against United States Strategic Forces. Mil. Med. 1983, 148, 901–902. [Google Scholar] [CrossRef]
- Scott, A.B. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology 1980, 87, 1044–1049. [Google Scholar] [CrossRef]
- Yiannakopoulou, E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology 2015, 95, 65–69. [Google Scholar] [CrossRef]
- Pirazzini, M.; Tehran, D.A.; Zanetti, G.; Lista, F.; Binz, T.; Shone, C.C.; Rossetto, O.; Montecucco, C. The thioredoxin reductase—redox system cleaves the interchain disulphide bond of botulinum neurotoxins on the cytosolic surface of synaptic vesicles. Toxicon 2015, 107, 32–36. [Google Scholar] [CrossRef]
- Pirazzini, M.; Bordin, F.; Rossetto, O.; Shone, C.C.; Binz, T.; Montecucco, C. The thioredoxin reductase-thioredoxin system is involved in the entry of tetanus and botulinum neurotoxins in the cytosol of nerve terminals. FEBS Lett. 2013, 587, 150–155. [Google Scholar] [CrossRef]
- Schiavo, G.; Rossetto, O.; Catsicas, S.; Delaureto, P.P.; Dasgupta, B.R.; Benfenati, F.; Montecucco, C.J. Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. Biol. Chem. 1993, 268, 23784–23787. [Google Scholar] [CrossRef]
- McNutt, P.M.; Vazquez-Cintron, E.J.; Tenezaca, L.; Ondeck, C.A.; Kelly, K.E.; Mangkhalakhili, M.; Machamer, J.B.; Angeles, C.A.; Glotfelty, E.J.; Cika, J.; et al. Neuronal delivery of antibodies has therapeutic effects in animal models of botulism. Sci. Transl. Med. 2021, 13, eabd7789. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, R.; Muroi, Y.; Unno, T.; Ishii, T. Ouabain exacerbates botulinum neurotoxin-induced muscle paralysis via progression of muscle atrophy in mice. J. Toxicol. Sci. 2010, 35, 795–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, G.E.; Wood, R.M. Effects of 3,4-Diaminopyridine in Cynomolgus Monkeys Poisoned with Type A Botulinum Toxin. Aminopyridines and Similarly Acting Drugs; Elsevier: Amsterdam, The Netherlands, 1982; p. 313. ISBN 9780080280004. [Google Scholar] [CrossRef]
- Adler, M.; Scovill, J.; Parker, G.; Lebeda, F.J.; Piotrowski, J.; Deshpande, S.S. Antagonism of botulinum toxin-induced muscle weakness by 3,4-diaminopyridine in rat phrenic nerve-hemidiaphragm preparation. Toxicon 1995, 33, 527–537, ISSN 0041-0101. [Google Scholar] [CrossRef]
- Molgo, J.; Meunier, F.A.; Poulain, B. Effects of 3,4-diaminopyridine on quantal acetylcholine release from neuromuscular junctions paralysed in vivo with botulinum type-F-toxin. Toxicon 1996, 34, 1092. [Google Scholar] [CrossRef]
- Adler, M.; Capacio, B.; Deshpande, S.S. Antagonism of botulinum toxin A-mediated muscle paralysis by 3,4-diaminopyridine delivered via osmotic minipumps. Toxicon 2000, 38, 1381–1388. [Google Scholar] [CrossRef]
- Bradford, A.B.; Machamer, J.B.; Russo, T.M.; McNutt, P.M. 3,4-Diaminopyridine Reverses Paralysis in Botulinum Neurotoxin-intoxicated Diaphragms through Two Functionally Distinct Mechanisms. Available online: http://www.elsevier.com/open-access/userlicense/1.0/ (accessed on 23 May 2022).
- Machamer, J.B.; Vazquez-Cintron, E.J.; O’Brien, S.W.; Kelly, K.E.; Altvater, A.C.; Pagarigan, K.T.; Dubee, P.B.; Ondeck, C.A.; McNutt, P.M. Antidotal treatment of botulism in rats by continuous infusion with 3,4-diaminopyridine. Mol. Med. 2022, 28, 61. [Google Scholar] [CrossRef]
- Friggeri, A.; Marçon, F.; Marciniak, S.; Lemaire-Hurtel, A.S.; Seydi, A.; Ammenouche, N.; Levrard, M.; Mahjoub, Y.; Airapetian, N.; Tinturier, F.; et al. 3,4-Diaminopyridine may improve neuromuscular block during botulism. Crit. Care 2013, 17, 449. [Google Scholar] [CrossRef] [Green Version]
- Bremer, P.T.; Pellett, S.; Carolan, J.P.; Tepp, W.H.; Eubanks, L.M.; Allen, K.N.; Johnson, E.A.; Janda, K.D. Metal Ions Effectively Ablate the Action of Botulinum Neurotoxin A. J. Am. Chem. Soc. 2017, 139, 7264–7272. [Google Scholar] [CrossRef]
- Hefter, H.; Samadzadeh, S. Effective Treatment of Neurological Symptoms with Normal Doses of Botulinum Neurotoxin in Wilson’s Disease: Six Cases and Literature Review. Toxins 2021, 13, 241. [Google Scholar] [CrossRef]
- Contarino, M.F.; Van Den Dool, J.; Balash, Y.; Bhatia, K.; Giladi, N.; Koelman, J.H.; Lokkegaard, A.; Marti, M.J.; Postma, M.; Relja, M.; et al. Clinical Practice: Evidence-Based Recommendations for the Treatment of Cervical Dystonia with Botulinum Toxin. Front. Neurol. 2017, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Hefter, H.; Hartmann, C.J.; Kahlen, U.; Samadzadeh, S.; Rosenthal, D.; Moll, M. Clinical Improvement After Treatment With IncobotulinumtoxinA (XEOMIN®) in Patients With Cervical Dystonia Resistant to Botulinum Toxin Preparations Containing Complexing Proteins. Front. Neurol. 2021, 12, 636590. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royal College of Physicians BSoRM. Spasticity in Adults: Management Using Botulinum Toxin; National Guidelines; Royal College of Physicians: London, UK, 2018; pp. 1–72. [Google Scholar]
- Benecke, R.; Jost, W.H.; Kanovsky, P.; Ruzicka, E.; Comes, G.; Grafe, S. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology 2005, 64, 1949–1951. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, T. Adverse events associated with botulinum toxin A injection in the treatment of the upper and lower limbs muscles spasticity and dystonia: A systematic review with meta-analysis. Open Sci. Framew. 2020. [Google Scholar] [CrossRef]
- Benecke, R. Xeomin® in the treatment of cervical dystonia. Europ. J. Neurol. 2009, 16 (Suppl. 2), 6–10. [Google Scholar] [CrossRef]
- Hefter, H.; Benecke, R.; Erbguth, F.; Jost, W.; Reichel, G.; Wissel, J. An open-label cohort study of the improvement of quality of life and pain in de novo cervical dystonia patients after injections with 500 U botulinum toxin A (Dysport). BMJ Open 2013, 3, e001853. [Google Scholar] [CrossRef] [Green Version]
- Duarte, G.S.; Rodrigues, F.B.; Ferreira, J.J.; Costa, J. Adverse events with botulinum toxin treatment in cervical dystonia: How much should we blame placebo? Park. Rel. Disord. 2018, 56, 16–19. [Google Scholar] [CrossRef]
- Scheps, D.; López de la Paz, M.; Jurk, M.; Hofmann, F.; Frevert, J. Design of modified botulinum neurotoxin A1 variants with a shorter persistence of paralysis and duration of action. Toxicon 2017, 139, 101–108. [Google Scholar] [CrossRef]
- Eleopra, R.; Tugnoli, V.; Quatrale, R.; Rossetto, O.; Montecucco, C.; Dressler, D. Clinical use of Non-A botulinum toxins: Botulinum toxin type C and botulinum toxin type F. Neurotocicity Res. 2006, 9, 127–131. [Google Scholar] [CrossRef]
- Adler, M.; Deshpande, S.S.; Apland, J.P. Simultaneous or sequential administration of botulinum neurotoxin E does not reduce the paralysis caused by botulinum neurotoxin A in rat EDL muscle. Botulinum. J. 2011, 1, 442–456. [Google Scholar] [CrossRef]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Hefter, H.; Tezayak, O.; Rosenthal, D. Long-term outcome of neurological Wilson´s disease. Park. Rel. Disord. 2019, 49, 48–53. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefter, H.; Samadzadeh, S. The Necessity of a Locally Active Antidote in the Clinical Practice of Botulinum Neurotoxin Therapy: Short Communication. Medicina 2022, 58, 935. https://doi.org/10.3390/medicina58070935
Hefter H, Samadzadeh S. The Necessity of a Locally Active Antidote in the Clinical Practice of Botulinum Neurotoxin Therapy: Short Communication. Medicina. 2022; 58(7):935. https://doi.org/10.3390/medicina58070935
Chicago/Turabian StyleHefter, Harald, and Sara Samadzadeh. 2022. "The Necessity of a Locally Active Antidote in the Clinical Practice of Botulinum Neurotoxin Therapy: Short Communication" Medicina 58, no. 7: 935. https://doi.org/10.3390/medicina58070935
APA StyleHefter, H., & Samadzadeh, S. (2022). The Necessity of a Locally Active Antidote in the Clinical Practice of Botulinum Neurotoxin Therapy: Short Communication. Medicina, 58(7), 935. https://doi.org/10.3390/medicina58070935






