Correlation between Endometriosis and Selected Allergic and Autoimmune Diseases and Eating Habits
Abstract
:1. Introduction
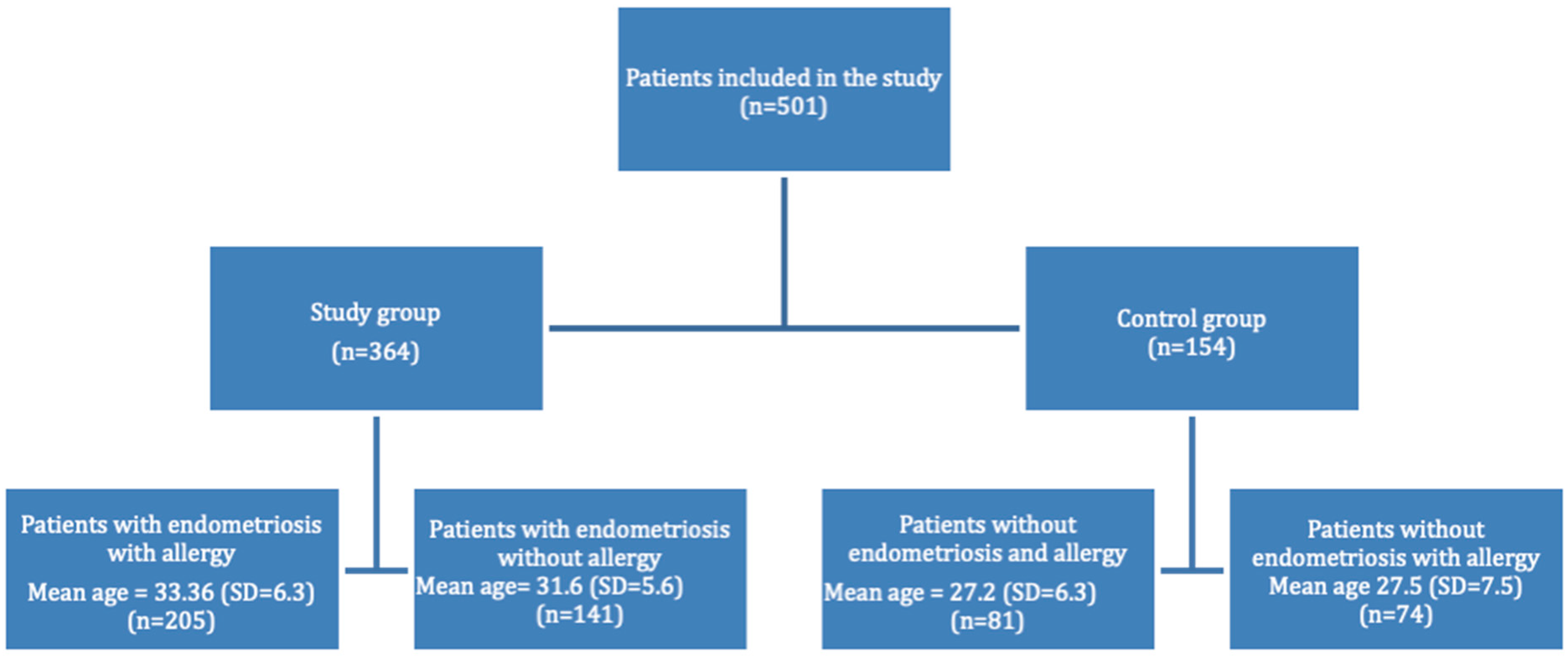
2. Materials and Methods
3. Results
- Smoking
- Obstetrical complications
- Allergies
- Food allergies
- Gastrointestinal tract symptoms
- Asthma
- Medical history
4. Discussion
- Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czyzyk, A.; Podfigurna, A.; Szeliga, A.; Meczekalski, B. Update on endometriosis pathogenesis. Minerva Ginecol. 2017, 69, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filip, L.; Duică, F.; Prădatu, A.; Crețoiu, D.; Suciu, N.; Crețoiu, S.M.; Predescu, D.-V.; Varlas, V.N.; Voinea, S.-C. Endometriosis Associated Infertility: A Critical Review and Analysis on Etiopathogenesis and Therapeutic Approaches. Medicina 2020, 56, 460. [Google Scholar] [CrossRef] [PubMed]
- De Ziegler, D.; Borghese, B.; Chapron, C. Endometriosis and infertility: Pathophysiology and management. Lancet 2010, 376, 730–738. [Google Scholar] [CrossRef]
- Zubrzycka, A.; Zubrzycki, M.; Perdas, E.; Zubrzycka, M. Genetic, Epigenetic, and Steroidogenic Modulation Mechanisms in Endometriosis. J. Clin. Med. 2020, 9, 1309. [Google Scholar] [CrossRef]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef]
- Bungum, H.F.; Vestergaard, C.; Knudsen, U.B. Endometriosis and type 1 allergies/immediate type hypersensitivity: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 209–215. [Google Scholar] [CrossRef]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Millar, S.R.; Navarro, P.; Harrington, J.M.; Perry, I.J.; Phillips, C.M. Dietary Quality Determined by the Healthy Eating Index-2015 and Biomarkers of Chronic Low-Grade Inflammation: A Cross-Sectional Analysis in Middle-to-Older Aged Adults. Nutrients 2021, 13, 222. [Google Scholar] [CrossRef]
- Missmer, S.A.; Chavarro, J.E.; Malspeis, S.; Bertone-Johnson, E.R.; Hornstein, M.D. A prospective study of dietary fat consumption and endometriosis risk. Hum. Reprod. 2010, 25, 1528–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helbig, M.; Vesper, A.S.; Beyer, I.; Fehm, T. Does Nutrition Affect Endometriosis? Geburtshilfe Frauenheilkd 2021, 81, 191–199. [Google Scholar] [CrossRef]
- Jurkiewicz-Przondziono, J.; Lemm, M.; Kwiatkowska-Pamuła, A.; Ziółko, E.; Wójtowicz, M.K. Influence of diet on the risk of developing endometriosis. Ginekol. Pol. 2017, 88, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; De Carolis, C.; Man, G.C.W.; Wang, C.C. The link between immunity, autoimmunity and endometriosis: A literature update. Autoimmun. Rev. 2018, 17, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Iwabe, T.; Terakawa, N. Role of cytokines in endometriosis. Fertil. Steril. 2001, 76, 1–10. [Google Scholar] [CrossRef]
- Pencovich, N.; Luk, J.; Hantisteanu, S.; Hornstein, M.D.; Fainaru, O. The development of endometriosis in a murine model is dependent on the presence of dendritic cells. Reprod. Biomed. Online 2014, 28, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Yuk, J.S.; Shin, J.S.; Shin, J.Y.; Oh, E.; Kim, H.; Park, W.I. Nickel Allergy Is a Risk Factor for Endometriosis: An 11-Year Population-Based Nested Case-Control Study. PLoS ONE 2015, 10, e0139388. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Johansen, J.D.; Carlsen, B.C.; Menné, T. Prevalence of nickel and cobalt allergy among female patients with dermatitis before and after Danish government regulation: A 23-year retrospective study. J. Am. Acad. Dermatol. 2009, 61, 799–805. [Google Scholar] [CrossRef]
- Schink, M.; Konturek, P.C.; Herbert, S.L.; Renner, S.P.; Burghaus, S.; Blum, S.; Fasching, P.A.; Neurath, M.F.; Zopf, Y. Different nutrient intake and prevalence of gastrointestinal comorbidities in women with endometriosis. J. Physiol. Pharmacol. 2019, 70, 255–268. [Google Scholar]
- Nodler, J.L.; Harris, H.R.; Chavarro, J.E.; Frazier, A.L.; Missmer, S.A. Dairy consumption during adolescence and endometriosis risk. Am. J. Obstet. Gynecol. 2020, 222, 257.e1–257.e16. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Peters, U.; De Roos, A.J.; Scholes, D.; Holt, V.L. Diet and risk of endometriosis in a population-based case-control study. Br. J. Nutr. 2011, 105, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Correale, J.; Ysrraelit, M.C.; Gaitán, M.I. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain 2009, 132, 1146–1160. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, E.S.; Hawrylowicz, C.M. The impact of vitamin D on regulatory T cells. Curr. Allergy Asthma. Rep. 2011, 11, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Harris, H.R.; Vitonis, A.F.; Chavarro, J.E.; Missmer, S.A. A prospective cohort study of meat and fish consumption and endometriosis risk. Am. J. Obstet. Gynecol. 2018, 219, e1–e178. [Google Scholar] [CrossRef]
- Parazzini, F.; Chiaffarino, F.; Surace, M.; Chatenoud, L.; Cipriani, S.; Chiantera, V.; Benzi, G.; Fedele, L. Selected food intake and risk of endometriosis. Hum. Reprod. 2004, 19, 1755–1759. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi, M.; Jahangiri, N.; Sadatmahalleh, S.J.; Aliani, F.; Akhoond, M. Diet and The Risk of Endometriosis in Iranian Women: A Case-Control Study. Int. J. Fertil. Steril. 2020, 14, 193–200. [Google Scholar]
- Maroun, P.; Cooper, M.J.; Reid, G.D.; Keirse, M.J. Relevance of gastrointestinal symptoms in endometriosis. Aust. N. Z. J. Obstet. Gynaecol. 2009, 49, 411–414. [Google Scholar] [CrossRef]
- Ballweg, M.L. Impact of endometriosis on women’s health: Comparative historical data show that the earlier the onset, the more severe the disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 201–218. [Google Scholar] [CrossRef]
- Kynyk, J.A.; Mastronarde, J.G.; McCallister, J.W. Asthma, the sex difference. Curr. Opin. Pulm. Med. 2011, 17, 6–11. [Google Scholar] [CrossRef]
- McCallister, J.W.; Mastronarde, J.G. Sex differences in asthma. J. Asthma 2008, 45, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Petrera, P.; Colombo, B.M.; Navaratnarajah, R.; Parisi, M.; Anserini, P.; Remorgida, V.; Ragni, N. Asthma in women with endometriosis. Hum. Reporod. 2005, 20, 3514–3517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinaii, N.; Cleary, S.D.; Ballweg, M.L.; Nieman, L.K.; Stratton, P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum. Reprod. 2002, 17, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Smorgick, N.; Marsh, C.A.; As-Sanie, S.; Smith, Y.R.; Quint, E.H. Prevalence of pain syndromes, mood conditions, and asthma in adolescents and young women with endometriosis. J. Pediatr. Adolesc. Gynecol. 2013, 26, 171–175. [Google Scholar] [CrossRef]
- Podgaec, S.; Abrao, M.S.; Dias, J.A., Jr.; Rizzo, L.V.; de Oliveira, R.M.; Baracat, E.C. Endometriosis: An inflammatory disease with a Th2 immune response component. Hum. Reprod. 2007, 22, 1373–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Su, S.; Liao, W.; Huang, C.; Hsu, C.Y.; Chen, H.; Wu, T.; Ho, W.; Wu, C. Asthma is associated with endometriosis: A retrospective population-based cohort study. Respir. Med. 2017, 132, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Zervou, M.I.; Vlachakis, D.; Papageorgiou, L.; Eliopoulos, E.; Goulielmos, G.N. Increased risk of rheumatoid arthritis in patients with endometriosis: Genetic aspects. Rheumatology 2022, keac143. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Yang, Y.C.; Hsu, C.Y.; Shen, Y.C. Risk of Rheumatoid Arthritis in Patients with Endometriosis: A Nationwide Population-Based Cohort Study. J. Women’s Health 2021, 30, 160–1164. [Google Scholar] [CrossRef]
- Shi, J.L.; Zheng, Z.M.; Chen, M.; Shen, H.H.; Li, M.Q.; Shao, J. IL-17: An important pathogenic factor in endometriosis. Int. J. Med. Sci. 2022, 19, 769–778. [Google Scholar] [CrossRef]
- Hsu, A.L.; Khachikyan, I.; Stratton, P. Invasive and noninvasive methods for the diagnosis of endometriosis. Clin. Obstet. Gynecol. 2010, 53, 413–419. [Google Scholar] [CrossRef] [PubMed]
| Study Group | Control Group | Study Group vs. Control Group (p =) | |||
|---|---|---|---|---|---|
| Group | Endometriosis and Allergy | Endometriosis without Allergy | Healthy with Allergy | Healthy without Allergy | |
| Mean no. of pregnancies | 0.80 (SD = 1.0) | 0.80 (SD = 1.0) | 0.48 (SD = 0.95) | 0.55 (SD = 0.91) | 0.0067 |
| Mean no. of miscarriages | 0.23 (SD = 0.59) | 0.206 (SD = 0.56) | 0.0864 (SD = 0.39) | 0.0812 (SD = 0.32) | 0.0253 |
| Mean no. of CC | 0.34 (SD = 0.63) | 0.3243 (SD = 0.65) | 0.2037 (SD = 0.49) | 0.2642 (SD = 0.56) | 0.1252 |
| Mean no. of premature births | 0.13 (SD = 0.42) | 0.1081 (SD = 0.34) | 0.0926 (SD = 0.29) | 0.0741 (SD = 0.27) | 0.6968 |
| Mean no. of late births | 0.13 (SD = 0.37) | 0.1091 (SD = 0.31) | 0.1481 (SD = 0.36) | 0.1132 (SD = 0.48) | 0.6328 |
| Type of Problem | Patients with Endometriosis [%] | Healthy Patients [%] | p Value |
|---|---|---|---|
| Abdominal pain (<6 months ago) | 79.47% (n = 151) | 41.33% (n = 31) | p = 0.000 |
| Abdominal pain (lasting > 1 day/week) | 60.31% (n = 117) | 26.39% (n = 19) | p = 0.0000 |
| Abdominal pain resolving after defecation | 42.54% (n = 77) | 25.76% (n = 17) | p = 0.0162 |
| Abdominal pain with a change in bowel movements | 49.14% (n = 86) | 36.49% (n = 27) | p = 0.06676 |
| Perianal lesions | 21.95% (n = 45) | 11.11% (n = 9) | p = 0.03481 |
| Bowel movement changes | 75.12% (n = 154) | 50.62% (n= 41) | p = 0.00006 |
| Mouth lesions | 27.32% (n = 56) | 18.52% (n = 15) | p = 0.1207 |
| Body mass loss | 11.71% (n = 24) | 9.88% (n = 8) | p = 0.658 |
| Study Group | Control Group | |||
|---|---|---|---|---|
| Endometriosis and Allergy Diagnosed Patients | Only Endometriosis without Allergy Patients | Allergic Patients without Endometriosis | Non-Allergic Patients without Endometriosis | |
| Healthy mother | 82.09% (n = 110) | 94.90% (n = 93) | 92.59% (n = 50) | 100% (n = 60) |
| Mother diagnosed with endometriosis | 17.91% (n = 24) | 5.10% (n = 5) | 7.41% (n = 4) | 0% (n = 0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowakowska, A.; Kwas, K.; Fornalczyk, A.; Wilczyński, J.; Szubert, M. Correlation between Endometriosis and Selected Allergic and Autoimmune Diseases and Eating Habits. Medicina 2022, 58, 1038. https://doi.org/10.3390/medicina58081038
Nowakowska A, Kwas K, Fornalczyk A, Wilczyński J, Szubert M. Correlation between Endometriosis and Selected Allergic and Autoimmune Diseases and Eating Habits. Medicina. 2022; 58(8):1038. https://doi.org/10.3390/medicina58081038
Chicago/Turabian StyleNowakowska, Aleksandra, Katarzyna Kwas, Angelika Fornalczyk, Jacek Wilczyński, and Maria Szubert. 2022. "Correlation between Endometriosis and Selected Allergic and Autoimmune Diseases and Eating Habits" Medicina 58, no. 8: 1038. https://doi.org/10.3390/medicina58081038
APA StyleNowakowska, A., Kwas, K., Fornalczyk, A., Wilczyński, J., & Szubert, M. (2022). Correlation between Endometriosis and Selected Allergic and Autoimmune Diseases and Eating Habits. Medicina, 58(8), 1038. https://doi.org/10.3390/medicina58081038






