Lymphoepithelioma-like Carcinoma of the Breast Synchronous with a High-Grade Invasive Ductal Carcinoma and Ductal Carcinoma in Situ in a Different Quadrant of the Same Breast: A Case Report
Abstract
:1. Introduction
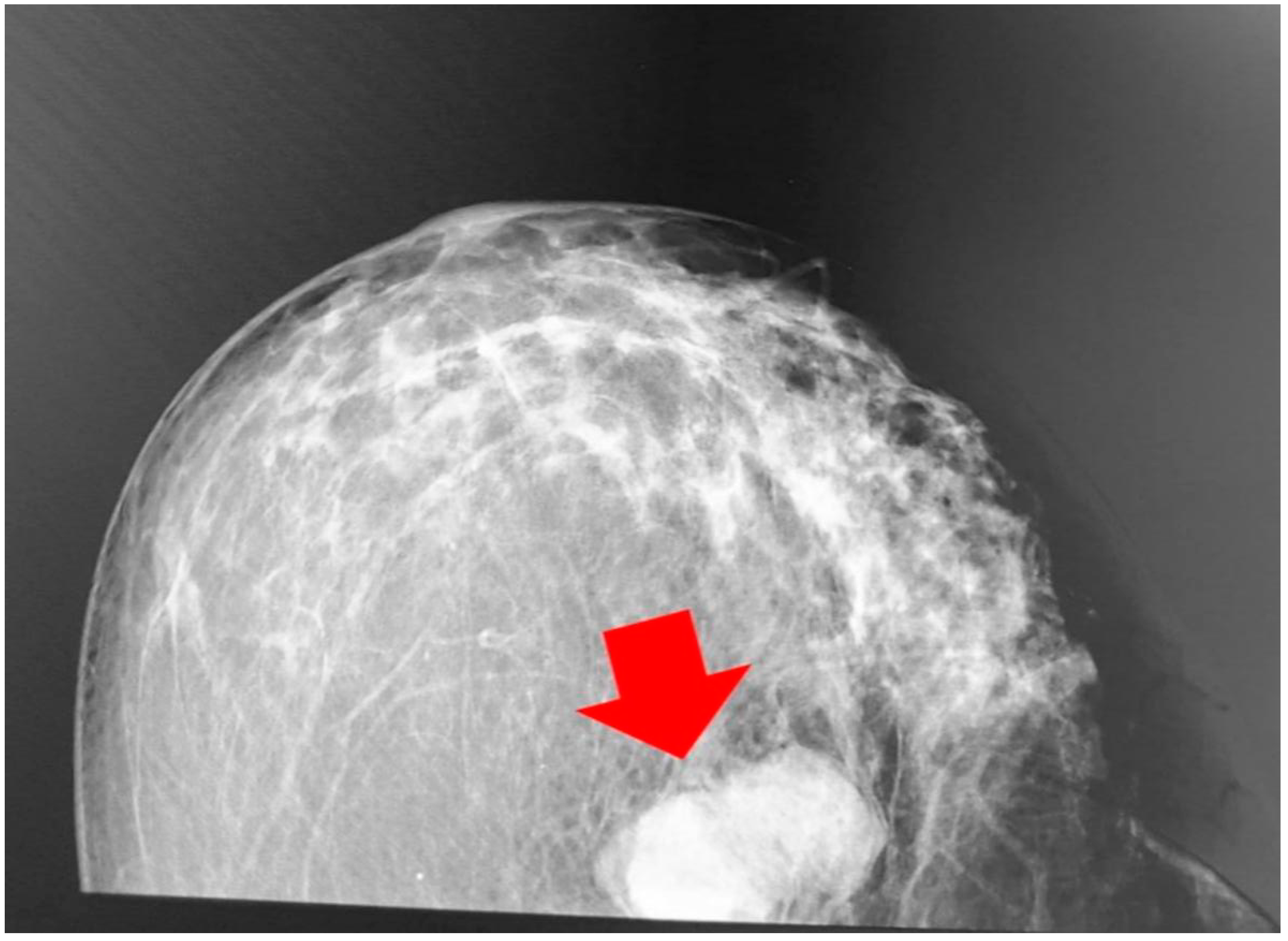
2. A Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Classification of Tumours Editorial Board Breast Tumours WHO Classification of Tumours, 5th ed.; IARC Press: Lyon, France, 2019; Volume 2. [Google Scholar]
- Tavassoli, F.A.; Devilee, P. Pathology and Genetics of Tumours of the Breast and Female Genital Organs; IARC Press: Lyon, France, 2003. [Google Scholar]
- Kumar, S.; Kumar, D. Lymphoepithelioma-like carcinoma of the breast. Mod. Pathol. 1994, 7, 129–131. [Google Scholar] [PubMed]
- Regaud, C.; Reverchon, L. A case of squamous epithelioma in the body of the superior maxillary. Rev. Laryngol. Otol. Rhinol. 1921, 42, 369–378. [Google Scholar]
- Schmincke, A. On the subject of lymphoepithelial tumours. Beitr. Pathol. Anat. 1921, 68, 161–170. [Google Scholar]
- Uesato, M.; Kono, T.; Shiratori, T.; Akutsu, Y.; Hoshino, I.; Murakami, K.; Horibe, D.; Maruyama, T.; Semba, Y.; Urahama, R.; et al. Lymphoepithelioma-like esophageal carcinoma with macroscopic reduction. World J. Gastrointest. Endosc. 2014, 6, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Sone, M.; Nakashima, T.; Nagasaka, T.; Itoh, A.; Yanagita, N. Lymphoepithelioma-like carcinoma of the larynx associated with an Epstein-Barr viral infection. Otolaryngol. Head Neck Surg. 1998, 119, 134–137. [Google Scholar] [CrossRef]
- Lin, L.; Lin, T.; Zeng, B. Primary lymphoepithelioma-like carcinoma of the lung: An unusual cancer and clinical outcomes of 14 patients. Oncol. Lett. 2017, 14, 3110–3116. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Hamada, T.; Sako, T.; Makihara, K.; Yamada, K.; Kashima, K.; Yokoyama, S.; Hirata, K.; Hachiya, Y.; Fukuyama, T.; et al. Lymphoepithelioma-like Carcinoma of the Stomach with Epithelioid Granulomas. Case Rep. Gastroenterol. 2010, 4, 361–368. [Google Scholar] [CrossRef]
- Sekihara, K.; Okuma, Y.; Kawamoto, H.; Hosomi, Y. Clinical outcome of thymic lymphoepithelioma-like carcinoma: Case report of a 14-year-old male. Oncol. Lett. 2014, 8, 2183–2186. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, S.; Pathak, S.D.; Saraswat, V.; Sarma, Y.S.; Bharadwaj, R.; Goorha, Y.K. Tracheal lymphoepithelioma-like carcinoma: A case report. Indian J. Cancer 2002, 39, 112–115. [Google Scholar]
- Lopez-Beltrán, A.; Luque, R.J.; Vicioso, L.; Anglada, F.; Requena, M.J.; Quintero, A.; Montironi, R. Lymphoepithelioma-like carcinoma of the urinary bladder: A clinicopathologic study of 13 cases. Virchows Arch. 2001, 438, 552–557. [Google Scholar] [CrossRef]
- Brun, J.; Randriambelomanana, J.; Cherier, L.; Lafon, M.; Trufflandier, N.; Le Bail, B. Lymphoepithelioma-like Carcinoma of the Ovary—A Case Report and Review of the Literature. Int. J. Gynecol. Pathol. 2010, 29, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Halpin, T.F.; Hunter, R.E.; Cohen, M.B. Lymphoepithelioma of the uterine cervix. Gynecol. Oncol. 1989, 34, 101–105. [Google Scholar] [CrossRef]
- Abedi, S.; Salama, S.; Alowami, S. Lymphoepithelioma-Like Carcinoma of the Skin: Case Report and Approach to Surgical Pathology Sign Out. Rare Tumors 2013, 5, e47. [Google Scholar]
- Shek, T.W.; Luk, I.S.; Ng, I.O.; Lo, C.Y. Lymphoepithelioma-like carcinoma of the thyroid gland: Lack of evidence of association with Epstein-Barr virus. Hum. Pathol. 1996, 27, 851–853. [Google Scholar] [CrossRef]
- Weiss, L.M.; Gaffey, M.J.; Shibata, D. Lymphoepithelioma-like carcinoma and its relationship to Epstein-Barr virus. Am. J. Clin. Pathol. 1991, 96, 156–158. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.J.; Pao, C.C.; Tseng, L.H.; Chang, C.T.; Lai, C.H.; Soong, Y.K.; Jyu-Jen, H. Lymphoepithelioma-like carcinoma of the uterine cervix: Association with Epstein-Barr virus and human papillomavirus. Cancer 1997, 80, 91–97. [Google Scholar] [CrossRef]
- Lakhani, S.; Ellis, I.; Schnitt, S.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Cree, I.A. WHO Classification of Tumours of the Breast, 4th ed.; IARC Press: Lyon, France, 2012. [Google Scholar]
- Cristina, S.; Boldorini, R.; Brustia, F.; Monga, G. Lymphoepithelioma-like carcinoma of the breast: An unusual pattern of infiltrating lobular carcinoma. Virchows Arch. 2000, 437, 198–202. [Google Scholar] [CrossRef]
- Pestereli, H.E.; Erdogan, O.; Kaya, R.; Karaveli, F.S. Lymphoepithelioma-like carcinoma of the breast. A newly recognized subtype of lobular carcinoma. Apmis 2002, 110, 447–450. [Google Scholar] [CrossRef]
- Sanati, S.; Ayala, A.; Middleton, L. Lymphoepithelioma-like carcinoma of the breast: Report of a case mimicking lymphoma. Ann. Diagn. Pathol. 2004, 8, 309–315. [Google Scholar] [CrossRef]
- O’Sullivan-Mejia, E.; Idowu, M.O.; Davis Masssey, H.; Cardenosa, G.; Grimes, M.M. Lymphoepithelioma-like carcinoma of the breast: Diagnosis by core needle biopsy. Breast J. 2009, 15, 658–660. [Google Scholar] [CrossRef]
- Dadmanesh, F.; Peterse, J.L.; Sapino, A.; Fonelli, A.; Eusebi, V. Lymphoepithelioma-like carcinoma of the breast: Lack of evidence of Epstein-Barr virus infection. Histopathology 2001, 38, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.; Chakkabat, P.; Goicochea, L.; Campassi, C.; Chumsri, S. Lymphoepithelioma-like carcinoma of the breast presenting as breast abscess. World J. Clin. Oncol. 2014, 5, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Abouelfad, D.; Yassen, N.; Amin, H.; Shabana, M. Lymphoepithelioma-Like Carcinoma of the Breast Mimicking Granulomatous Mastitis—Case Report and Review of the Literature. Asian Pac. J. Cancer Prev. 2017, 18, 1737–1741. [Google Scholar] [PubMed]
- Iezzoni, J.C.; Gaffey, M.J.; Weiss, L.M. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am. J. Clin. Pathol. 1995, 103, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Kulka, J.; Kovalszky, I.; Svastics, E.; Berta, M.; Füle, T. Lymphoepithelioma-like carcinoma of the breast: Not Epstein-Barr virus-, but human papilloma virus-positive. Hum. Pathol. 2008, 39, 298–301. [Google Scholar] [CrossRef]
- Nio, Y.; Tsuboi, K.; Tamaoki, M.; Maruyama, R. Lymphoepithelioma like carcinoma of the breast: A case report with a special analysis of an association with human papilloma virus. Anticancer Res. 2012, 32, 1435–1441. [Google Scholar]
- Koufopoulos, N.; Syrios, J.; Papanikolaou, A.; Misitzis, I.; Kapatou, K.; Dimas, D.; Khaldi, L. Lymphoepithelioma-like breast Carcinoma. Pol. J. Pathol. 2018, 69, 98–104. [Google Scholar] [CrossRef]
- Naidoo, P.; Chetty, R. Lymphoepithelioma-like carcinoma of the breast with associated sclerosing lymphocytic lobulitis. Arch. Pathol. Lab. Med. 2001, 125, 669–672. [Google Scholar] [CrossRef]
- Saleh, R.; DaCamara, P.; Radhi, J.; Boutross-Tadross, O. Lymphoepithelioma-like carcinoma of the breast mimicking nodular sclerosing Hodgkin’s lymphoma. Breast J. 2005, 11, 353–354. [Google Scholar] [CrossRef]
- Trihia, H.; Siatra, H.; Gklisty, H.; Diamantopoulos, P.; Arapantoni-Dadiotis, P.; Kalogerakos, K. Lymphoepithelioma-like carcinoma of the breast: Cytological and histological features and review of the literature. Acta Cytol. 2012, 56, 85–91. [Google Scholar] [CrossRef]
- Shet, T.; Pai, T.; Shetty, O.; Desai, S. Lymphoepithelioma-like carcinoma of breast- evaluation for Epstein-Barr virus-encoded RNA, human papillomavirus, and markers of basal cell differentiation. Ann. Diagn. Pathol. 2016, 25, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Fadila, K.; Faycal, A.; Lamiaa, J.; Mohammed, B.; Nabil, I. Lymphoepithelioma-like carcinoma of the breast: A case report and review of the literature. Pan Afr. Med. J. 2019, 32, 19. [Google Scholar] [CrossRef] [PubMed]
- Marinova, L.; Jordanova, B. Pathohistological and Immunohistochemical Analysis, Differential Diagnosis, Prognosis and Complex Treatment in a Rare Lymphoepithelioma-Like Breast Cancer. Adv. Bioeng. Biomed. Sci. Res. 2021, 4, 16–19. [Google Scholar]
- Ilvan, S.; Celik, V.; Akyildiz, E.U.; Bese, N.S. Lymphoepithelioma-like carcinoma of the breast: Is it a distinct entity? Clinico-pathological evaluation of two cases and review of the literature. Breast 2004, 13, 522–526. [Google Scholar] [CrossRef]
- Kurose, A.; Ichinohasama, R.; Kanno, H.; Kobayashi, T.; Ishida, M.; Nishinari, N.; Sawai, T. Lymphoepithelioma-like carcinoma of the breast. Report of a case with the first electron microscopic study and review of the literature. Virchows Arch. 2005, 447, 653–659. [Google Scholar] [CrossRef]
- Jeong, A.K.; Park, S.B.; Kim, Y.M.; Ko, B.K.; Yang, M.J.; Kwon, W.J.; Lee, J.H.; Weon, Y.C. Lymphoepithelioma-like carcinoma of the breast. J. Ultrasound Med. 2010, 29, 485–488. [Google Scholar] [CrossRef]
- Nankin, N.L.; Gondusky, C.J.; Abasolo, P.A.; Kalantari, B.N. Kalantari Lymphoepithelioma-like carcinoma of the breast. Radiol. Case Rep. 2015, 10, 963. [Google Scholar] [CrossRef] [Green Version]
- Dinniwell, R.; Hanna, W.M.; Mashhour, M.; Saad, R.S.; Czarnota, G.J. Lymhoepithelima-like carcinoma of the breast: A diagnostic and therapeutic challenge. Curr. Oncol. 2012, 19, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Coronel, M.T.; Perez-Sanchez, V.M.; Salazar-Campos, J.E.; Diaz-Molina, R.; Arce-Salinas, C.H. Lymphoepithelioma-like carcinoma of breast: A case report and review of the literature. Indian J. Pathol. Microbiol. 2019, 62, 125–128. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front Oncol. 2022, 12, 820968. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Sinelnikov, M.Y.; Zhang, X.; Cao, Y.; Lu, P. Robot-Assisted Minimally Invasive Breast Surgery: Recent Evidence with Comparative Clinical Outcomes. J. Clin. Med. 2022, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- ACR. BI-RADS® Atlas; D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanev, V.; Naneva, S.; Yordanov, A.; Strashilov, S.; Konsoulova, A.; Vasileva-Slaveva, M.; Betova, T.; Ivanov, I. Lymphoepithelioma-like Carcinoma of the Breast Synchronous with a High-Grade Invasive Ductal Carcinoma and Ductal Carcinoma in Situ in a Different Quadrant of the Same Breast: A Case Report. Medicina 2022, 58, 1146. https://doi.org/10.3390/medicina58091146
Nanev V, Naneva S, Yordanov A, Strashilov S, Konsoulova A, Vasileva-Slaveva M, Betova T, Ivanov I. Lymphoepithelioma-like Carcinoma of the Breast Synchronous with a High-Grade Invasive Ductal Carcinoma and Ductal Carcinoma in Situ in a Different Quadrant of the Same Breast: A Case Report. Medicina. 2022; 58(9):1146. https://doi.org/10.3390/medicina58091146
Chicago/Turabian StyleNanev, Vasil, Silvia Naneva, Angel Yordanov, Strahil Strashilov, Assia Konsoulova, Mariela Vasileva-Slaveva, Tatyana Betova, and Ivan Ivanov. 2022. "Lymphoepithelioma-like Carcinoma of the Breast Synchronous with a High-Grade Invasive Ductal Carcinoma and Ductal Carcinoma in Situ in a Different Quadrant of the Same Breast: A Case Report" Medicina 58, no. 9: 1146. https://doi.org/10.3390/medicina58091146
APA StyleNanev, V., Naneva, S., Yordanov, A., Strashilov, S., Konsoulova, A., Vasileva-Slaveva, M., Betova, T., & Ivanov, I. (2022). Lymphoepithelioma-like Carcinoma of the Breast Synchronous with a High-Grade Invasive Ductal Carcinoma and Ductal Carcinoma in Situ in a Different Quadrant of the Same Breast: A Case Report. Medicina, 58(9), 1146. https://doi.org/10.3390/medicina58091146






