Absolute and Functional Iron Deficiency in Colon Cancer: A Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Levin, T.R.; Corley, D.A.; Jensen, C.D.; Schottinger, J.E.; Quinn, V.P.; Zauber, A.G.; Lee, J.K.; Zhao, W.K.; Udaltsova, N.; Ghai, N.R.; et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large Community-Based Population. Gastroenterology 2018, 155, 1383–1391.e5. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Phipps, O.; Brookes, M.J.; Al-Hassi, H.O. Iron deficiency, immunology, and colorectal cancer. Nutr. Rev. 2021, 79, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Aksan, A.; Farrag, K.; Aksan, S.; Schroeder, O.; Stein, J. Flipside of the Coin: Iron Deficiency and Colorectal Cancer. Front. Immunol. 2021, 12, 635899. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.L. Dietary iron and colorectal cancer risk. Free Radic. Biol. Med. 1992, 12, 161–168. [Google Scholar] [CrossRef]
- Ashmore, J.H.; Rogers, C.J.; Kelleher, S.L.; Lesko, S.M.; Hartman, T.J. Dietary Iron and Colorectal Cancer Risk: A Review of Human Population Studies. Crit. Rev. Food Sci. Nutr. 2016, 56, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.M.; Richardson, K.L.; Kabir, T.D.; Trinder, D.; Ganss, R.; Leedman, P.J. Altered Iron Metabolism and Impact in Cancer Biology, Metastasis, and Immunology. Front. Oncol. 2020, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Sasazuki, S.; Inoue, M.; Iwasaki, M.; Shimazu, T.; Sawada, N.; Yamaji, T.; Takachi, R.; Tsugane, S.; Japan Public Health Center–Based Prospective Study Group. Zinc and heme iron intakes and risk of colorectal cancer: A population-based prospective cohort study in Japan. Am. J. Clin. Nutr. 2012, 96, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron-induced carcinogenesis: The role of redox regulation. Free Radic. Biol. Med. 1996, 20, 553–566. [Google Scholar] [CrossRef]
- Stone, H.; Almilaji, O.; John, C.; Smith, C.; Surgenor, S.L.; Ayres, L.; Williams, E.J.; Snook, J. The dedicated iron deficiency anaemia clinic: A 15-year experience. Front. Gastroenterol. 2022, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Almilaji, O.; Parry, S.D.; Docherty, S.; Snook, J. Evidence for improved prognosis of colorectal cancer diagnosed following the detection of iron deficiency anaemia. Sci. Rep. 2021, 11, 13055. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, G. Role of hepcidin in the pathophysiology and diagnosis of anemia. Blood Res. 2013, 48, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Liakopoulos, V.; Antoniadi, G.; Stefanidis, I. Which is the best way for estimating transferrin saturation? Ren. Fail. 2010, 32, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Motta, I. Anemia in Clinical Practice-Definition and Classification: Does Hemoglobin Change With Aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ploug, M.; Kroijer, R.; Qvist, N.; Lindahl, C.H.; Knudsen, T. Iron deficiency in colorectal cancer patients: A cohort study on prevalence and associations. Color. Dis. 2020, 23, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Gafter-Gvili, A.; Schechter, A.; Rozen-Zvi, B. Iron Deficiency Anemia in Chronic Kidney Disease. Acta Haematol. 2019, 142, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Grotto, H.Z. Anaemia of cancer: An overview of mechanisms involved in its pathogenesis. Med. Oncol. 2008, 25, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Busti, F.; Marchi, G.; Ugolini, S.; Castagna, A.; Girelli, D. Anemia and iron deficiency in cancer patients: Role of iron replacement therapy. Pharmaceuticals 2018, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Evstatiev, R.; Kornek, G.; Aapro, M.; Bauernhofer, T.; Buxhofer-Ausch, V.; Fridrik, M.; Geissler, D.; Geissler, K.; Gisslinger, H.; et al. Iron metabolism and iron supplementation in cancer patients. Wien. Klin. Wochenschr. 2015, 127, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J.; Dekker, J.W.T.; Harlaar, J.J.; Jeekel, J.; Schipperus, M.; Zwaginga, J.J. The role of preoperative iron deficiency in colorectal cancer patients: Prevalence and treatment. Int. J. Color. Dis. 2017, 32, 1617–1624. [Google Scholar] [CrossRef]
- Beale, A.L.; Penney, M.D.; Allison, M.C. The prevalence of iron deficiency among patients presenting with colorectal cancer. Colorectal Dis. 2005, 7, 398–402. [Google Scholar] [CrossRef]
- Sadahiro, S.; Suzuki, T.; Tokunaga, N.; Mukai, M.; Tajima, T.; Makuuchi, H.; Saito, T. Anemia in patients with colorectal cancer. Int. J. Color. Dis. 1998, 33, 488–494. [Google Scholar] [CrossRef]
- Väyrynen, J.P.; Tuomisto, A.; Väyrynen, S.A.; Klintrup, K.; Karhu, T.; Mäkelä, J.; Herzig, K.-H.; Karttunen, T.J.; Mäkinen, M.J. Preoperative anemia in colorectal cancer: Relationships with tumor characteristics, systemic inflammation, and survival. Sci. Rep. 2018, 8, 1126. [Google Scholar] [CrossRef] [PubMed]
- Almilaji, O.; Parry, S.; Docherty, S.; Snook, J. Colorectal Cancer and the Blood Loss Paradox. Front. Gastroenterol. 2022, 13, 381–385. [Google Scholar] [CrossRef]
- Ludwig, H.; Müldür, E.; Endler, G.; Hübl, W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann. Oncol. 2013, 24, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.; Mashhadi, M.A.; Mohammadi, M.; Ebrahimi, M.; Allahyari, A. Absolute and Functional Iron Deficiency Anemia among Different Tumors in Cancer Patients in South Part of Iran, 2014. Int. J. Hematol. Stem Cell Res. 2017, 11, 192–198. [Google Scholar]
- Gvirtzman, R.; Livovsky, D.M.; Tahover, E.; Goldin, E.; Koslowsky, B. Anemia can predict the prognosis of colorectal cancer in the pre-operative stage: A retrospective analysis. World J. Surg. Oncol. 2021, 19, 341. [Google Scholar] [CrossRef] [PubMed]
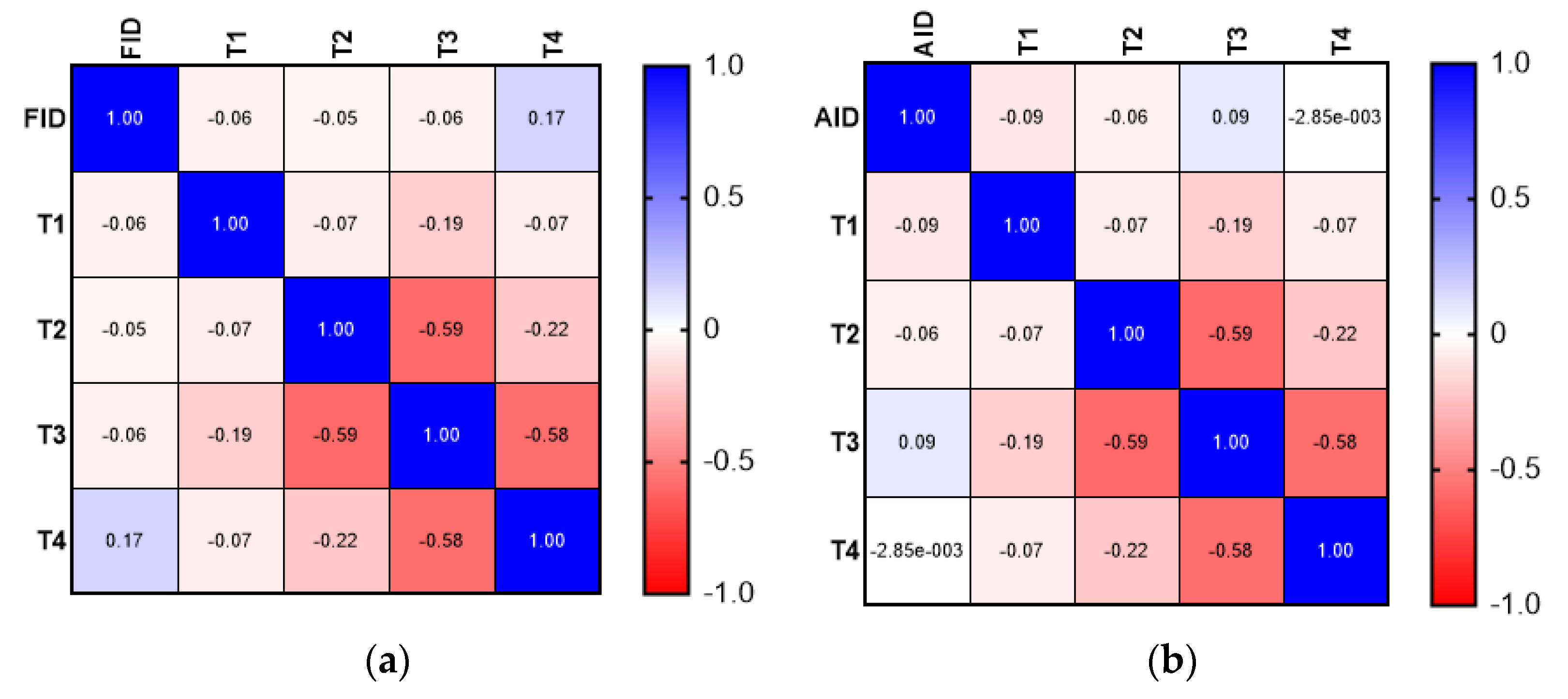
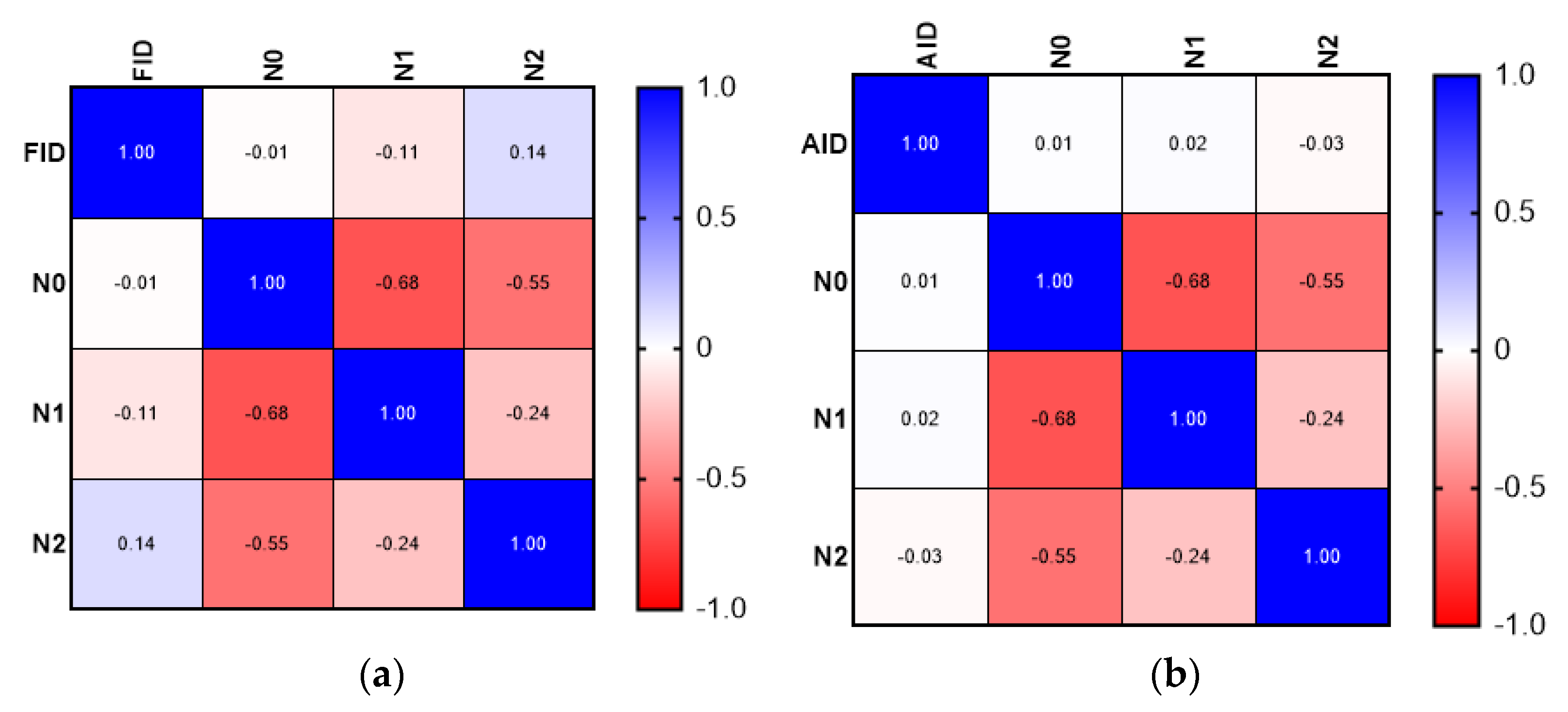
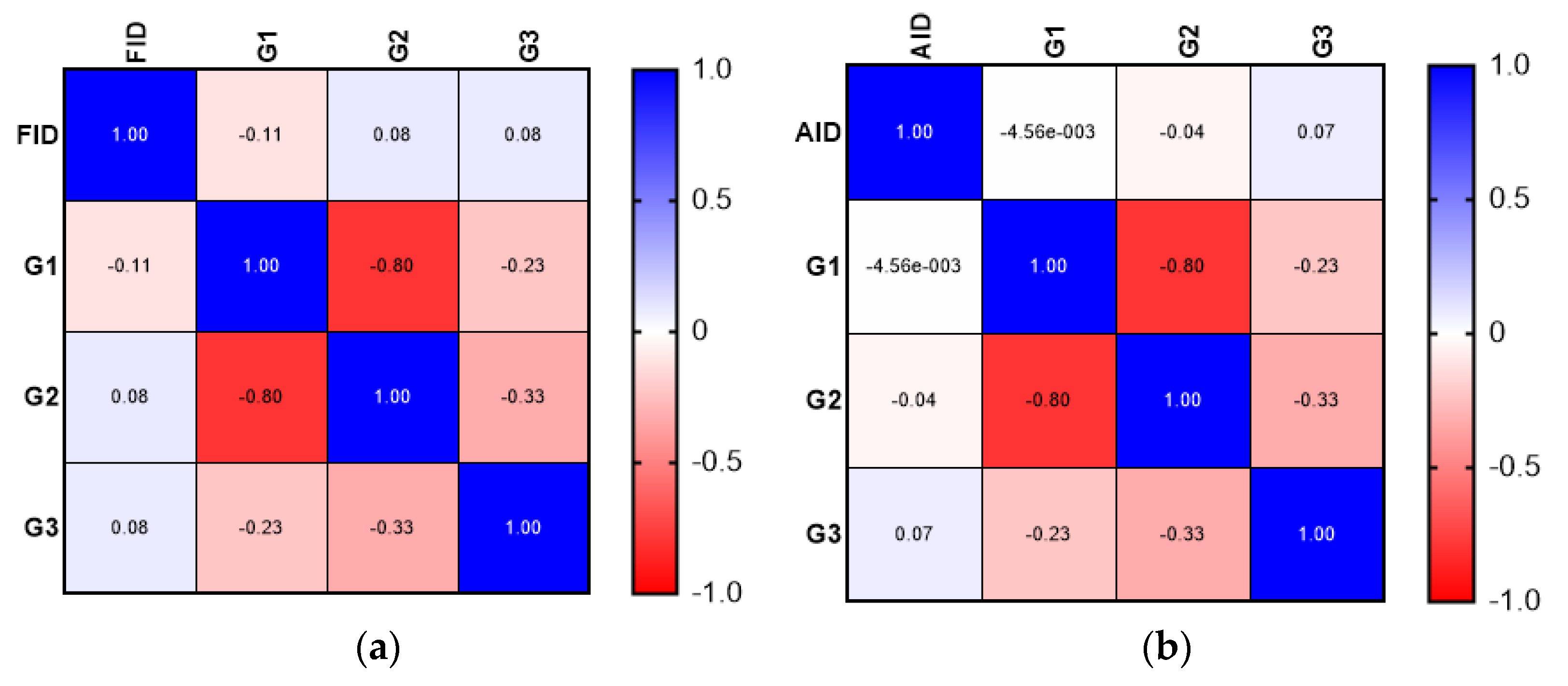

| Type of Deficiency | Mean ± SD (Years) |
|---|---|
| FID | 68.20 ± 9.09 |
| AID | 70.15 ± 11.72 |
| Anemia | 69.00 ± 10.19 |
| No anemia | 70.22 ± 9.34 |
| Overall | 69.51 ± 9.82 |
| Variable | Number of Cases (Percentage) |
|---|---|
| Location of tumor | |
| Right colon | 45 (34.88%) |
| Cecum | 11 (8.52%) |
| Ascending | 34 (26.35%) |
| Transverse colon | 23 (17.82%) |
| Left colon | 61 (47.28%) |
| Descending | 23 (17.82%) |
| Sigmoid | 38 (29.45%) |
| Differentiation | |
| G1 | 46 (35.6%) |
| G2 | 69 (53.5%) |
| G3 | 14 (10.9%) |
| T stage | |
| T1 | 3 (2.32%) |
| T2 | 24 (18.61%) |
| T3 | 79 (61.25%) |
| T4 | 23 (17.82%) |
| N stage | |
| N0 | 79 (61.24%) |
| N1 | 29 (22.48%) |
| N2 | 21 (16.28%) |
| M stage | |
| M0 | 118 (91.48%) |
| M1 | 11 (8.52%) |
| Lymphatic invasion | |
| L0 | 90 (69.77%) |
| L1 | 39 (30.23%) |
| Venous invasion | |
| V0 | 103 (79.85%) |
| V1 | 26 (20.15%) |
| Perineural invasion | |
| Pn0 | 102 (79.07%) |
| Pn1 | 27 (20.93%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Făgărășan, V.; Andraș, D.; Amarinei, G.; Seicean, R.I.; Bințințan, V.V.; Dindelegan, G.C.; Căinap, C.I. Absolute and Functional Iron Deficiency in Colon Cancer: A Cohort Study. Medicina 2022, 58, 1202. https://doi.org/10.3390/medicina58091202
Făgărășan V, Andraș D, Amarinei G, Seicean RI, Bințințan VV, Dindelegan GC, Căinap CI. Absolute and Functional Iron Deficiency in Colon Cancer: A Cohort Study. Medicina. 2022; 58(9):1202. https://doi.org/10.3390/medicina58091202
Chicago/Turabian StyleFăgărășan, Vlad, David Andraș, Giorgiana Amarinei, Radu Ioan Seicean, Vasile Virgil Bințințan, George Calin Dindelegan, and Calin Ioan Căinap. 2022. "Absolute and Functional Iron Deficiency in Colon Cancer: A Cohort Study" Medicina 58, no. 9: 1202. https://doi.org/10.3390/medicina58091202
APA StyleFăgărășan, V., Andraș, D., Amarinei, G., Seicean, R. I., Bințințan, V. V., Dindelegan, G. C., & Căinap, C. I. (2022). Absolute and Functional Iron Deficiency in Colon Cancer: A Cohort Study. Medicina, 58(9), 1202. https://doi.org/10.3390/medicina58091202







