Gastrectomy with or without Complete Omentectomy for Advanced Gastric Cancer: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
- -
- PubMed/MEDLINE
- -
- Scopus
- -
- Cochrane Library
- -
- Web of Science
2.2. Inclusion Criteria
2.3. Outcomes
2.4. Data Extraction
- -
- Demographic data [Author’s surname and year of publication, study type, study country, population size, gender and age, body mass index (BMI)];
- -
- Surgical data [surgical approach, surgical procedure];
- -
- Histopathological data [primary GC T stage, lymphadenectomy extension];
- -
- Outcomes data [3-year and 5-year OSs, 3-year and 5-year DFSs, overall and specific recurrence rates, harvested lymph nodes number, overall and major complications].
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
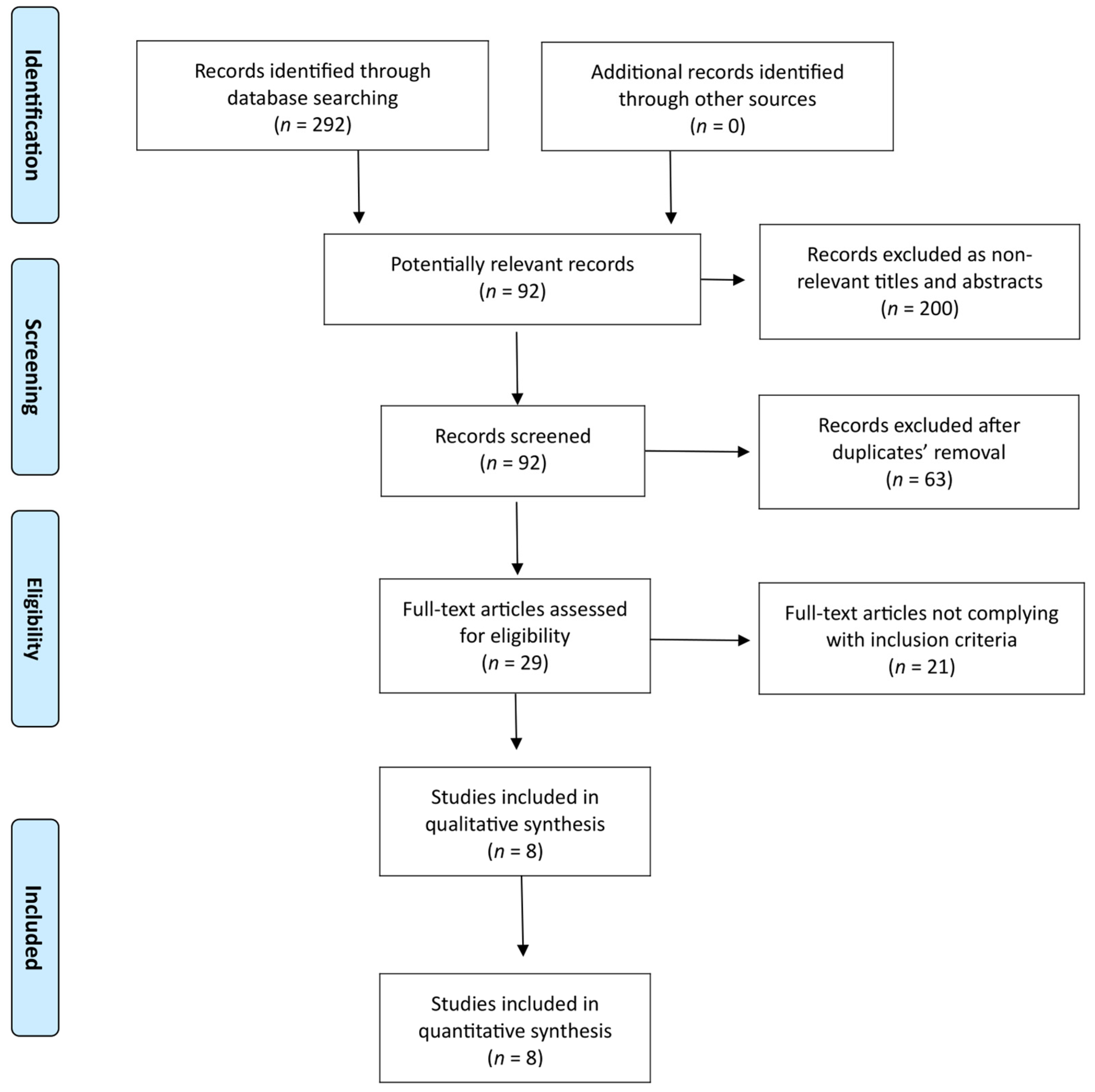
3.1. Search Results and Study Characteristics
3.2. Study and General Population Characteristics
3.3. Meta-Analyses Results
3.3.1. Overall Survival
3.3.2. Disease-Free Survival
3.3.3. Recurrences
3.3.4. Operative Time
3.3.5. Estimated Blood Loss
3.3.6. Number of Lymph Nodes Harvested
3.3.7. Overall Complications
3.3.8. Major Complications
3.3.9. Subgroup Analysis
3.3.10. Publication Bias
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Hu, Y.; Yoon, S.S. Extent of gastrectomy and lymphadenectomy for gastric adenocarcinoma. Surg. Oncol. 2022, 40, 101689. [Google Scholar] [CrossRef]
- Zizzo, M.; Zanelli, M.; Sanguedolce, F.; Torricelli, F.; Morini, A.; Tumiati, D.; Mereu, F.; Zuliani, A.L.; Palicelli, A.; Ascani, S.; et al. Robotic versus Laparoscopic Gastrectomy for Gastric Cancer: An Updated Systematic Review. Medicina 2022, 58, 834. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Hispán, E.; Pedregal, M.; Cristobal, I.; García-Foncillas, J.; Caramés, C. Immunotherapy for Peritoneal Metastases from Gastric Cancer: Rationale, Current Practice and Ongoing Trials. J. Clin. Med. 2021, 10, 4649. [Google Scholar] [CrossRef]
- Zhu, A.; Yin, G.; Liu, X.; Kong, W.; Zhang, Y.; Shan, Y.; Ying, R.; Zhang, J.; Zhou, C. Efficiency of complete omentectomy in patients with resectable gastric cancer: A meta-analysis and systematic review. BMC Gastroenterol. 2021, 21, 346. [Google Scholar] [CrossRef]
- Lin, H.W.; Loh, E.W.; Shen, S.C.; Tam, K.W. Gastrectomy with or without omentectomy for gastric cancer: A systematic review and meta-analysis. Surgery 2022, 171, 1281–1289. [Google Scholar] [CrossRef]
- Marano, L.; Polom, K.; Bartoli, A.; Spaziani, A.; De Luca, R.; Lorenzon, L.; Di Martino, N.; Marrelli, D.; Roviello, F.; Castagnoli, G. Oncologic Effectiveness and Safety of Bursectomy in Patients with Advanced Gastric Cancer: A Systematic Review and Updated Meta-Analysis. J. Investig. Surg. 2018, 31, 529–538. [Google Scholar] [CrossRef]
- Nie, R.C.; Yuan, S.Q.; Chen, S.; Yan, S.M.; Chen, Y.M.; Chen, X.J.; Chen, G.M.; Zhou, Z.W.; Chen, Y.B.; Li, Y.F. Bursectomy for advanced gastric cancer: An update meta-analysis. World J. Surg. Oncol. 2018, 16, 66. [Google Scholar] [CrossRef]
- Xiong, B.; Ma, L.; Huang, W.; Cheng, Y.; Luo, H.; Wang, K. Efficiency of bursectomy in patients with resectable gastric cancer: An updated meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 1483–1492. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Wang, K. Bursectomy in Gastric Cancer Surgery: A Meta-Analysis. Rev. Investig. Clin. 2019, 71, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Perivoliotis, K.; Baloyiannis, I.; Symeonidis, D.; Tepetes, K. The role of bursectomy in the surgical management of gastric cancer: A meta-analysis and systematic review. Updates Surg. 2020, 72, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Doki, Y.; Mizusawa, J.; Terashima, M.; Katai, H.; Yoshikawa, T.; Kimura, Y.; Takiguchi, S.; Nishida, Y.; Fukushima, N.; et al. Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): A phase 3, open-label, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2018, 3, 460–468. [Google Scholar] [CrossRef]
- Chai, S.W.; Wang, S.H.; Wang, C.Y.; Chen, Y.C.; Soong, R.S.; Huang, T.S. Partial Versus Total Omentectomy in Patients with Gastric Cancer: A Systemic Review and Meta-Analysis. Cancers 2021, 13, 4971. [Google Scholar] [CrossRef]
- Platell, C.; Cooper, D.; Papadimitriou, J.M.; Hall, J.C. The omentum. World J. Gastroenterol. 2000, 6, 169–176. [Google Scholar]
- Hasegawa, S.; Kunisaki, C.; Ono, H.; Oshima, T.; Fujii, S.; Taguri, M.; Morita, S.; Sato, T.; Yamada, R.; Yukawa, N.; et al. Omentum-preserving gastrectomy for advanced gastric cancer: A propensity-matched retrospective cohort study. Gastric Cancer 2013, 16, 383–388. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, J.H.; Kim, W. A comparison of total versus partial omentectomy for advanced gastric cancer in laparoscopic gastrectomy. World J. Surg. Oncol. 2014, 12, 64. [Google Scholar] [CrossRef]
- Young, S.; DiFronzo, L.A.; Ahuja, A.; Keim, L.; Papenfuss, C.; O’Connor, V.; Dehal, A. Performing Omentectomy during Gastrectomy Does Not Improve Survival: A Multi-Center Analysis of 471 Patients with Gastric Adenocarcinoma. J. Gastrointest. Surg. 2020, 24, 2856–2858. [Google Scholar] [CrossRef]
- Ri, M.; Nunobe, S.; Honda, M.; Akimoto, E.; Kinoshita, T.; Hori, S.; Aizawa, M.; Yabusaki, H.; Isobe, Y.; Kawakubo, H.; et al. Gastrectomy with or without omentectomy for cT3-4 gastric cancer: A multicentre cohort study. Br. J. Surg. 2020, 107, 1640–1647. [Google Scholar] [CrossRef]
- Sakimura, Y.; Inaki, N.; Tsuji, T.; Kadoya, S.; Bando, H. Long-term outcomes of omentum-preserving versus resecting gastrectomy for locally advanced gastric cancer with propensity score analysis. Sci. Rep. 2020, 10, 16305. [Google Scholar] [CrossRef]
- Seo, W.J.; Choi, S.; Roh, C.K.; Cho, M.; Kim, Y.M.; Kim, H.I.; Hyung, W.J. Omentum preservation as an oncologically comparable and surgically superior alternative to total omentectomy during radical gastrectomy for T3-T4 gastric cancer. Surgery 2021, 170, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; He, F.Q.; Liao, M.S.; Yang, C.; Chen, X.-D. Gastrectomy with omentum preservation versus gastrectomy with omentectomy for locally advanced gastric cancer: A systematic review and meta-analysis. Int. J. Surg. 2021, 96, 106176. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, M.; Shibuya, N.; Takagi, K.; Hachiya, H.; Tago, K.; Matsumoto, T.; Shimizu, T.; Aoki, T.; Kubota, K. Omentectomy Does Not Affect the Postoperative Outcome of Patients with Locally Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. J. Surg. Res. 2021, 264, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, M.; Zhou, Y.; Jiang, H.; Xu, L.; Hu, Z.; Liu, Y.; Jiang, Y.; Li, X. Efficacy of Omentum-Preserving Gastrectomy for Patients with Gastric Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 710814. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Liu, H.D.; Chen, Z.H.; Jin, T.; Hu, J.K.; Yang, K. Comparison of Survival and Safety Between Total Omentectomy and Partial Omentectomy for Gastric Cancer: A Meta-Analysis. Front. Surg. 2021, 8, 708545. [Google Scholar] [CrossRef]
- Kong, M.; Chen, H.; Zhang, R.; Sheng, H.; Li, L. Overall Survival Advantage of Omentum Preservation over Omentectomy for Advanced Gastric Cancer: A Systematic Review and Meta-Analysis. World J. Surg. 2022, 46, 1952–1961. [Google Scholar] [CrossRef]
- Murakami, H.; Yamada, T.; Taguri, M.; Hasegawa, S.; Yamanaka, T.; Rino, Y.; Mushiake, H.; Oshima, T.; Matsukawa, H.; Tani, K.; et al. Short-Term Outcomes from a Randomized Screening Phase II Non-inferiority Trial Comparing Omentectomy and Omentum Preservation for Locally Advanced Gastric Cancer: The TOP-G Trial. World J. Surg. 2021, 45, 1803–1811. [Google Scholar] [CrossRef]
- Lu, S.; Yang, Z.Y.; Yan, C.; Liu, W.T.; Ni, Z.T.; Yao, X.X.; Hua, Z.C.; Feng, R.H.; Zheng, Y.N.; Wang, Z.Q.; et al. A randomized controlled trial to evaluate omentum-preserving gastrectomy for patients with T1-T3 gastric cancer. Future Oncol. 2021, 17, 3301–3307. [Google Scholar] [CrossRef]
- Sato, Y.; Yamada, T.; Yoshikawa, T.; Machida, R.; Mizusawa, J.; Katayama, H.; Tokunaga, M.; Boku, N.; Terashima, M.; Stomach Cancer Study Group/Japan Clinical Oncology Group. Randomized controlled Phase III trial to evaluate omentum preserving gastrectomy for patients with advanced gastric cancer (JCOG1711, ROAD-GC). Jpn. J. Clin. Oncol. 2020, 50, 1321–1324. [Google Scholar] [CrossRef]
- Lee, S.; Suh, Y.S.; Kang, S.H.; Won, Y.; Park, Y.S.; Ahn, S.H.; Kim, H.H. Should total omentectomy be performed for advanced gastric cancer?: The role of omentectomy during laparoscopic gastrectomy for advanced gastric cancer. Surg. Endosc. 2022, 36, 6937–6948. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Review Manager (RevMan). Available online: https://training.cochrane.org/online-learning/core-software/revman (accessed on 3 June 2022).
- Cochrane Handbook for Systematic Reviews of Interventions [Version 5.1.0]. Available online: https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm (accessed on 3 June 2022).
- Zizzo, M.; Ugoletti, L.; Manzini, L.; Castro Ruiz, C.; Nita, G.E.; Zanelli, M.; De Marco, L.; Besutti, G.; Scalzone, R.; Sassatelli, R.; et al. Management of duodenal stump fistula after gastrectomy for malignant disease: A systematic review of the literature. BMC Surg. 2019, 19, 55. [Google Scholar]
- Agnes, A.; Biondi, A.; Persiani, R.; Laurino, A.; Reddavid, R.; De Giuli, M.; Sicoli, F.; Cananzi, F.; De Pascale, S.; Fumagalli, U.; et al. Development of the PERI-Gastric (PEritoneal Recurrence Index) and PERI-Gram (Peritoneal Recurrence Index NomoGRAM) for predicting the risk of metachronous peritoneal carcinomatosis after gastrectomy with curative intent for gastric cancer. Gastric Cancer 2022, 25, 629–639. [Google Scholar] [CrossRef]
- Guner, A.; Yildirim, R. Surgical management of metastatic gastric cancer: Moving beyond the guidelines. Transl. Gastroenterol. Hepatol. 2019, 4, 58. [Google Scholar] [CrossRef]
- Krist, L.F.; Koenen, H.; Calame, W.; van der Harten, J.J.; van der Linder, J.C.; Eestermans, I.L.; Meyer, S.; Beelen, R.H. Ontogeny of milky spots in the human greater omentum: An immunochemical study. Anat. Rec. 1997, 249, 399–404. [Google Scholar] [CrossRef]
- Ackermann, P.C.; De Wet, P.D.; Loots, G.P. Microcirculation of the rat omentum studied by means of corrosion casts. Acta Anat. 1991, 140, 146–149. [Google Scholar] [CrossRef]
- Vanvugt, E.; Vanrijthoven, E.A.M.; Kamperdijk, E.W.A.; Beelen, R.H.J. Omental milky spots in the local immune response in the peritoneal cavity of rats. Anat. Rec. 1996, 244, 235–245. [Google Scholar] [CrossRef]
- Wijffels, J.F.; Hendrickx, R.J.; Steenbergen, J.J.; Eestermans, I.L.; Beelen, R.H. Milky spots in the mouse omentum may play an important role in the origin of peritoneal macrophages. Res. Immunol. 1992, 143, 401–409. [Google Scholar] [CrossRef]
- Shimotsuma, M.; Shields, J.W.; Simpson Morgan, M.W.; Sakuyama, A.; Shirasu, M.; Hagiwara, A.; Takahashi, T. Morpho physiological function and role of omental milky spots as omentum associated lymphoid tissue (OALT) in the peritoneal cavity. Lymphology 1993, 26, 90–101. [Google Scholar] [PubMed]
- Shimotsuma, M.; Kawata, M.; Hagiwara, A.; Takahashi, T. Milky spots in the human greater omentum. Macroscopic and histological identification. Acta Anat. 1989, 136, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Naito, M.; Umezu, H.; Moriyama, H.; Takatsuka, H.; Takahashi, K.; Shultz, L.D. Macrophage differentiation and expression of macrophage colony stimulatingfactor in murine milky spots and omentum after macrophage elimination. J. Leukoc. Biol. 1997, 61, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Dux, K. Proliferative activity of macrophages in the greater omentum of the mouse in relation to the early postnatal development of the vascular structures. J. Leukoc. Biol. 1986, 40, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Hagiwara, A.; Shimotsuma, M.; Sakakura, C.; Osaki, K.; Sasaki, S.; Ohyama, T.; Ohgaki, M.; Imanishi, T.; Yamazaki, J.; et al. Role of milky spots as selective implantation sites for malignant cells in peritoneal dissemination in mice. J. Cancer Res. Clin. Oncol. 1996, 122, 590–595. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Takhashi, T.; Hagiwara, A.; Shimotsuma, M.; Sakakura, C.; Osaki, K.; Sasaki, S.; Shirasu, M.; Sakakibara, T.; Ohyama, T. Site-specific implantation in the milky spots of malignant cells in peritoneal dissemination: Immunohistochemical observation in mice inoculated intraperitoneally with bromodeoxyuridine labelled cells. Br. J. Cancer 1995, 71, 468–472. [Google Scholar] [CrossRef]
- Kersy, O.; Loewenstein, S.; Lubezky, N.; Sher, O.; Simon, N.B.; Klausner, J.M.; Lahat, G. Omental Tissue-Mediated Tumorigenesis of Gastric Cancer Peritoneal Metastases. Front. Oncol. 2019, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, R.J.; Loizidou, M.; Cooper, A.J.; Alexander, P.; Taylor, I. Importance of the omentum in the development of intra- abdominal metastases. Br. J. Surg. 1991, 78, 117–119. [Google Scholar] [CrossRef]
- Weese, J.L.; Ottery, F.D.; Emoto, S.E. Does omentectomy prevent malignant small bowel obstruction. Clin. Exp. Metastasis 1988, 6, 319–324. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- De Manzoni, G.; Marrelli, D.; Baiocchi, G.L.; Morgagni, P.; Saragoni, L.; Degiuli, M.; Donini, A.; Fumagalli, U.; Mazzei, M.A.; Pacelli, F.; et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer 2017, 20, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.H.; Zhang, X.T.; Li, Y.F.; Tang, L.; Qu, X.J.; Ying, J.E.; Zhang, J.; Sun, L.Y.; Lin, R.B.; Qiu, H.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. 2021, 41, 747–795. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Jongerius, E.J.; Boerma, D.; Seldenrijk, K.A.; Meijer, S.L.; Scheepers, J.J.; Smedts, F.; Lagarde, S.M.; Balague Ponz, O.; van Berge Henegouwen, M.I.; van Sandick, J.W.; et al. Role of omentectomy as part of radical surgery for gastric cancer. Br. J. Surg. 2016, 103, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.H.; Yeh, Y.S.; Tsai, H.L.; Huang, C.W.; Chang, T.K.; Su, W.C.; Wang, J.Y. Neoadjuvant Chemoradiotherapy for Locally Advanced Gastric Cancer: Where Are We at? Cancers 2022, 14, 3026. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.; Johnston, E.; Starling, N. Neoadjuvant and Adjuvant Therapy Approaches to Gastric Cancer. Curr. Treat. Options Oncol. 2022, 23, 1247–1268. [Google Scholar] [CrossRef]
- Lumish, M.A.; Ku, G.Y. Approach to Resectable Gastric Cancer: Evolving Paradigm of Neoadjuvant and Adjuvant Treatment. Curr. Treat. Options Oncol. 2022, 23, 1044–1058. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- Schuhmacher, C.; Gretschel, S.; Lordick, F.; Reichardt, P.; Hohenberger, W.; Eisenberger, C.F.; Haag, C.; Mauer, M.E.; Hasan, B.; Welch, J.; et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J. Clin. Oncol. 2010, 28, 5210–5218. [Google Scholar] [CrossRef]
- Kang, Y.K.; Yook, J.H.; Park, Y.K.; Lee, J.S.; Kim, Y.W.; Kim, J.Y.; Ryu, M.H.; Rha, S.Y.; Chung, I.J.; Kim, I.H.; et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J. Clin. Oncol. 2021, 39, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, H.; Li, Z.; Xue, Y.; Wang, Y.; Zhou, Z.; Yu, J.; Bu, Z.; Chen, L.; Du, Y.; et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): An open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021, 22, 1081–1092, Erratum in Lancet Oncol. 2021, 22, e347. [Google Scholar] [CrossRef] [PubMed]




| Authors/Year | Study Type | Study Country | Study Period | Group | Patient Population, n | Gender, n | Age (Years), Mean or Median | BMI (kg/m2), Mean or Median | |
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | ||||||||
| Lee et al./2022 [30] | Retrospective PSM | Korea | 2014–2018 | CO | 174 | 122 | 52 | 59.9 ± 12.7 | 23.1 ± 3.7 |
| NCO | 248 | 177 | 71 | 61.6 ± 13.3 | 23.8 ± 3.1 | ||||
| Seo et al./2021 [21] | Retrospective PSM | Korea | 2003–2015 | CO | 225 | 131 | 94 | 59 (49–70) | 23.5 (21.1–25.6) |
| NCO | 225 | 137 | 88 | 56 (49–67) | 22.9 (21.0–24.9) | ||||
| Murakami et al./2021 [27] | RCT | Japan | 2011–2018 | CO | 122 | 89 | 33 | 71 (30–90) | 22.4 (14.8–31.8) |
| NCO | 125 | 89 | 36 | 74 (45–89) | 22.2 (14.5–32.1) | ||||
| Sakimura et al./2020 [20] | Retrospective PSM | Japan | 2008–2017 | CO | 70 | 46 | 24 | 65.0 (37–90) | 22.2 (15.8–30.3) |
| NCO | 70 | 48 | 22 | 66.5 (42–94) | 22.4 (16.4–32.6) | ||||
| Ri et al./2020 [19] | Retrospective PSM | Japan | 2006–2012 | CO | 263 | 176 | 87 | 66.7 ± 11 | 22.4 ± 3.6 |
| NCO | 263 | 181 | 82 | 65.7 ± 12.9 | 22.5 ± 3.4 | ||||
| Young et al./2020 [18] | Retrospective | USA | 2008–2016 | CO | 90 | 62 | 28 | 69.5 (62–77) | 27.4 ± 6.1 |
| NCO | 381 | 217 | 164 | 68 (58–76) | 26.2 ± 5.3 | ||||
| Kim et al./2014 [17] | Retrospective PSM | Korea | 2004–2011 | CO | 80 | 56 | 24 | 60.9 ± 11.2 | n/a |
| NCO | 66 | 50 | 16 | 62.2 ± 11 | n/a | ||||
| Hasegawa et al./2013 [16] | Retrospective PSM | Japan | 2000–2009 | CO | 98 | 72 | 26 | 69.0 (40–91) | n/a |
| NCO | 98 | 72 | 26 | 68.7 (45–91) | n/a | ||||
| Authors/Year | Group | Patient Population, n | Surgical Approach, n | Surgical Procedure, n | Lymphadenectomy, n | pT Stage, n | pN Stage, n | pTNM Stage, n | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Open | MIS | DG | TG | D1 | D1+ | D2 | D2+ | T0 | T1 | T2 | T3 | T4 | N0 | N1 | N2 | N3 | I | II | III | IV | |||
| Lee et al./2022 [30] | CO | 174 | 0 | 174 | 101 | 73 | n/a | n/a | n/a | n/a | 0 | 0 | 22 | 78 | 74 | 42 | 19 | 39 | 74 | 10 | 43 | 121 | 0 |
| NCO | 248 | 0 | 248 | 157 | 91 | n/a | n/a | n/a | n/a | 0 | 0 | 45 | 119 | 84 | 65 | 35 | 62 | 86 | 21 | 77 | 150 | 0 | |
| Seo et al./2021 [21] | CO | 225 | 60 | 165 | 167 | 58 | 0 | 25 | 200 | 0 | 0 | 0 | 0 | 100 | 125 | 75 | 42 | 42 | 66 | 0 | 95 | 130 | 0 |
| NCO | 225 | 69 | 156 | 169 | 56 | 0 | 22 | 203 | 0 | 0 | 0 | 0 | 111 | 114 | 73 | 47 | 42 | 63 | 0 | 99 | 126 | 0 | |
| Murakami et al./2021 [27] | CO | 122 | 122 | 0 | 73 | 49 | 0 | 0 | 122 | 0 | 0 | 20 | 21 | 42 | 39 | 44 | 29 | 25 | 24 | 26 | 48 | 41 | 7 |
| NCO | 125 | 125 | 0 | 81 | 44 | 0 | 0 | 125 | 0 | 0 | 31 | 21 | 31 | 42 | 54 | 25 | 17 | 29 | 38 | 40 | 40 | 7 | |
| Sakimura et al./2020 [20] | CO | 70 | 41 | 29 | 45 | 25 | 0 | 9 | 61 | 1 | 5 | 12 | 32 | 20 | 22 | 14 | 14 | 20 | n/a | n/a | n/a | n/a | |
| NCO | 70 | 25 | 45 | 44 | 26 | 0 | 14 | 56 | 0 | 6 | 16 | 27 | 21 | 29 | 14 | 9 | 18 | n/a | n/a | n/a | n/a | ||
| Ri et al./2020 [19] | CO | 263 | 263 | 0 | 156 | 107 | 11 | 146 | 106 | 0 | 47 | 216 | 148 | 0 | 115 | 0 | 28 | 101 | 129 | 5 | |||
| NCO | 263 | 263 | 0 | 151 | 112 | 8 | 146 | 109 | 0 | 48 | 215 | 145 | 0 | 118 | 0 | 29 | 96 | 131 | 7 | ||||
| Young et al./2020 [18] | CO | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 41 | 47 | 53 | n/a | n/a | n/a | n/a | |||
| NCO | 381 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 184 | 176 | 205 | n/a | n/a | n/a | n/a | ||||
| Kim et al./2014 [17] | CO | 80 | 0 | 80 | 61 | 19 | 0 | 2 | 78 | 0 | 0 | 0 | 28 | 52 | 0 | 40 | 14 | 13 | 13 | 17 | 39 | 24 | 0 |
| NCO | 66 | 0 | 66 | 54 | 12 | 0 | 5 | 61 | 0 | 0 | 0 | 37 | 29 | 0 | 34 | 8 | 16 | 8 | 23 | 26 | 17 | 0 | |
| Hasegawa et al./2013 [16] | CO | 98 | 98 | 0 | 52 | 46 | 0 | 12 | 86 | 0 | 0 | 0 | 30 | 34 | 34 | 39 | 25 | 18 | 16 | 16 | 40 | 42 | 0 |
| NCO | 98 | 84 | 14 | 61 | 37 | 0 | 13 | 85 | 0 | 0 | 0 | 34 | 30 | 34 | 41 | 23 | 17 | 17 | 21 | 36 | 41 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zizzo, M.; Zanelli, M.; Sanguedolce, F.; Palicelli, A.; Ascani, S.; Morini, A.; Tumiati, D.; Mereu, F.; Zuliani, A.L.; Nardecchia, M.; et al. Gastrectomy with or without Complete Omentectomy for Advanced Gastric Cancer: A Meta-Analysis. Medicina 2022, 58, 1241. https://doi.org/10.3390/medicina58091241
Zizzo M, Zanelli M, Sanguedolce F, Palicelli A, Ascani S, Morini A, Tumiati D, Mereu F, Zuliani AL, Nardecchia M, et al. Gastrectomy with or without Complete Omentectomy for Advanced Gastric Cancer: A Meta-Analysis. Medicina. 2022; 58(9):1241. https://doi.org/10.3390/medicina58091241
Chicago/Turabian StyleZizzo, Maurizio, Magda Zanelli, Francesca Sanguedolce, Andrea Palicelli, Stefano Ascani, Andrea Morini, David Tumiati, Federica Mereu, Antonia Lavinia Zuliani, Melissa Nardecchia, and et al. 2022. "Gastrectomy with or without Complete Omentectomy for Advanced Gastric Cancer: A Meta-Analysis" Medicina 58, no. 9: 1241. https://doi.org/10.3390/medicina58091241








