Identification and In Silico Analysis of a Homozygous Nonsense Variant in TGM1 Gene Segregating with Congenital Ichthyosis in a Consanguineous Family
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Ethical Approval
2.2. Clinical and Family History Interviews, Sample Collection and Extraction of Genomic DNA
2.3. Amplification and Sequencing of TGM1 Gene
2.4. Computational Analysis
3. Results
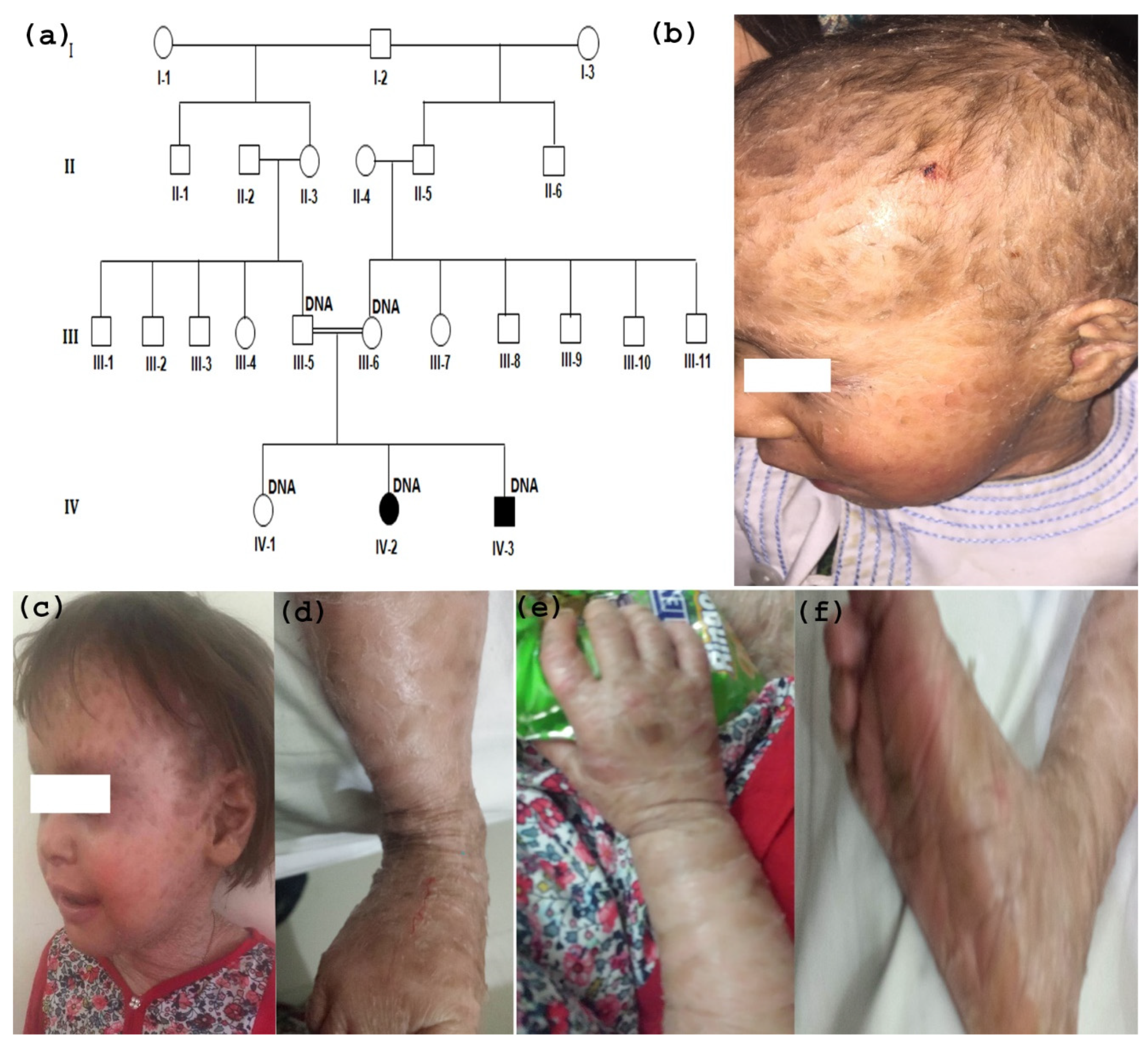
3.1. Clinical Description
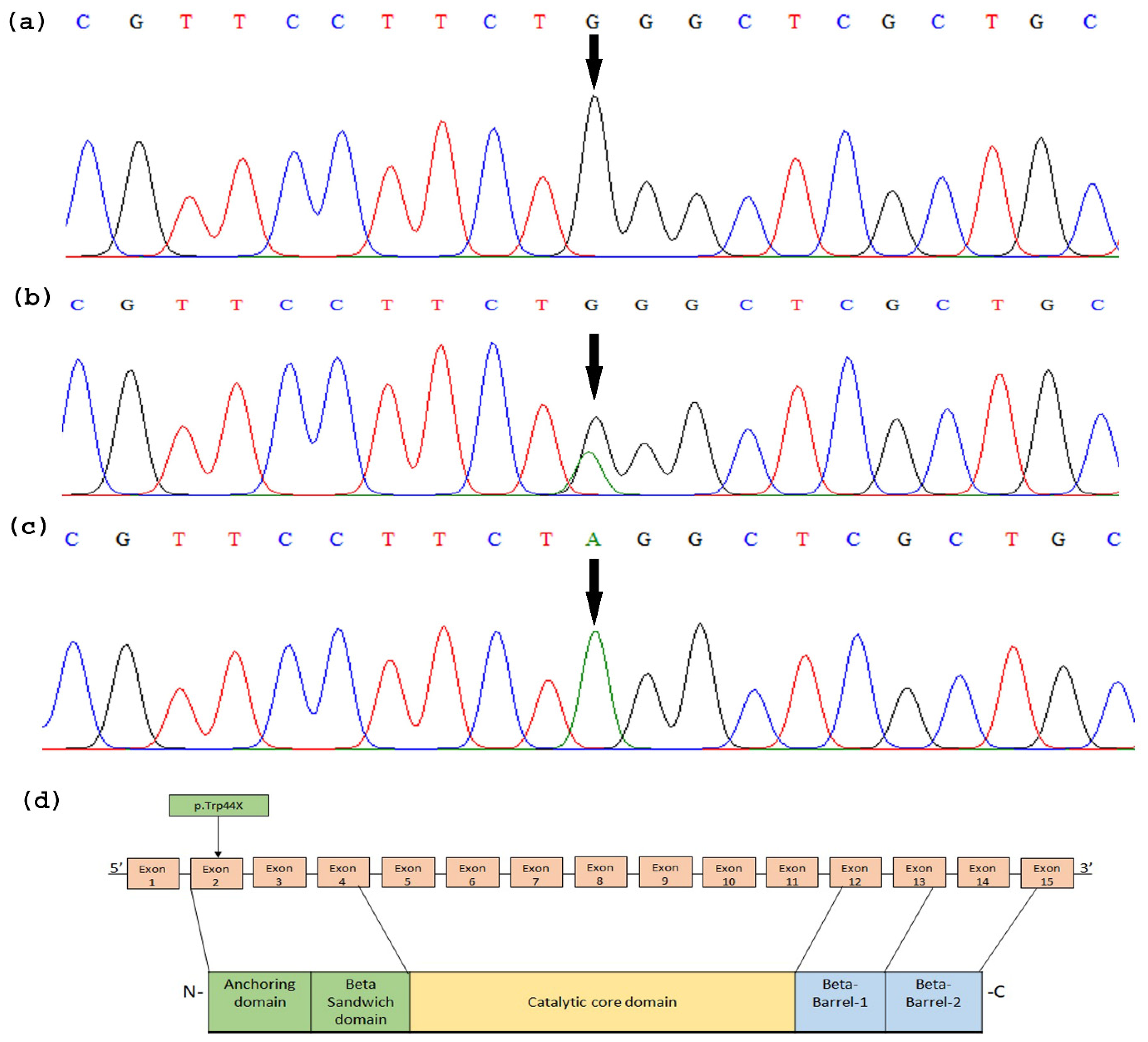
3.2. TGM1 Gene Sequencing
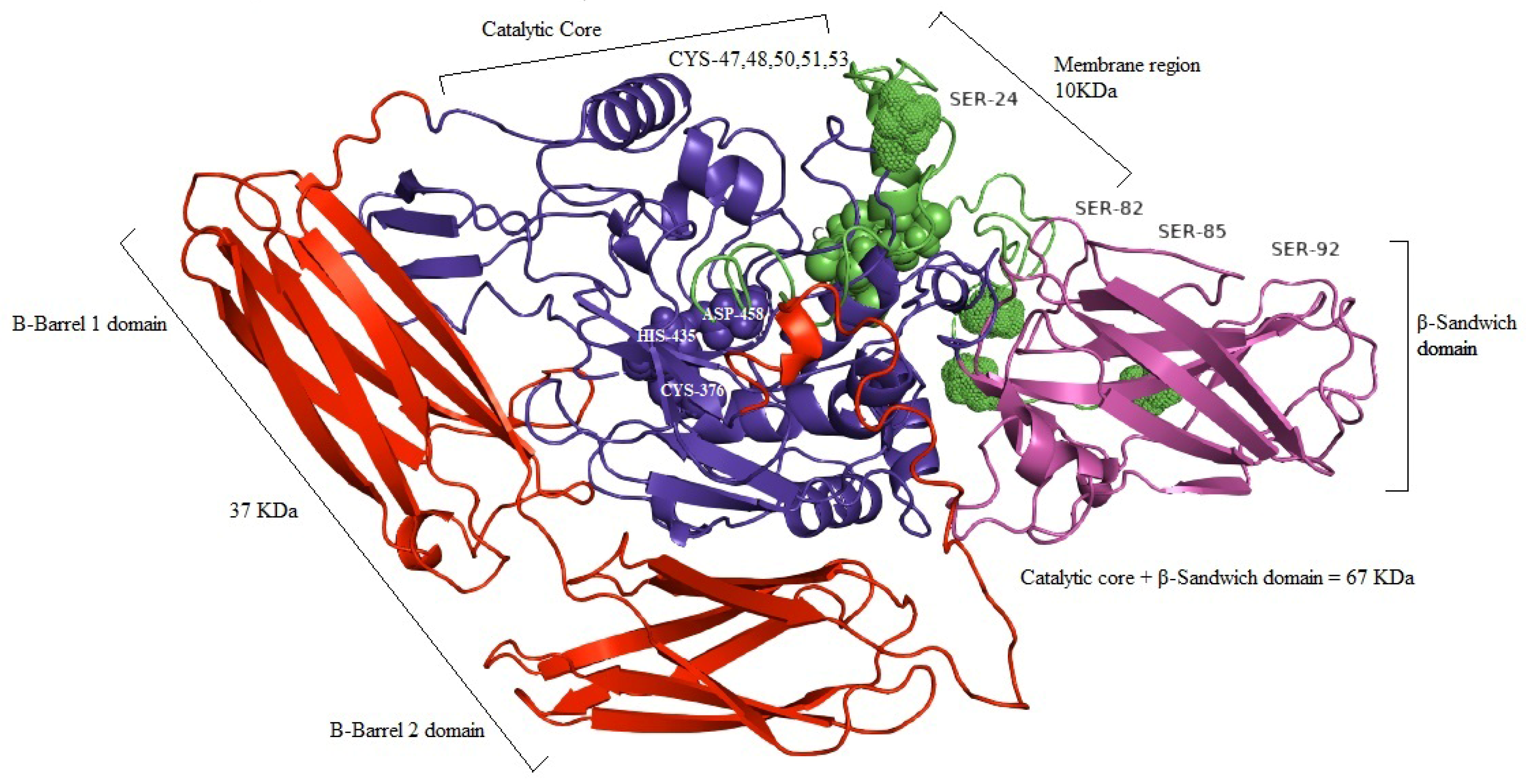
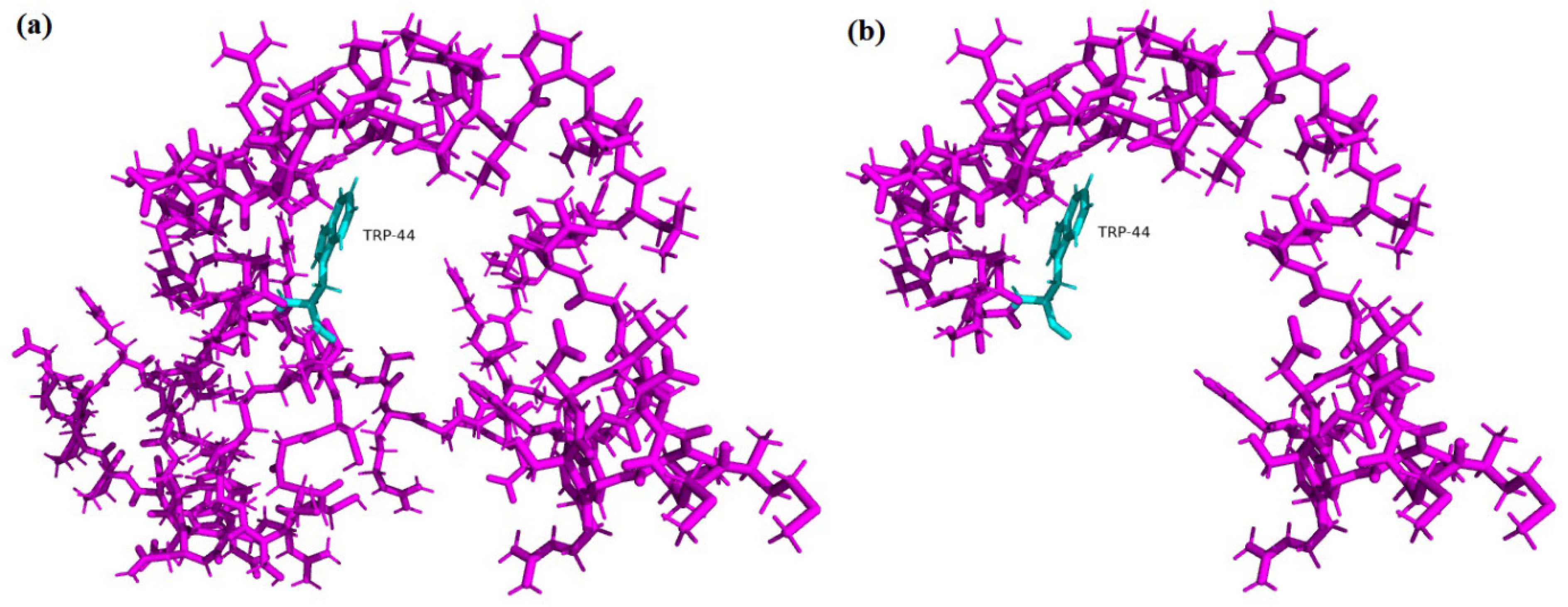
3.3. In Silico and computational Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Elias, P.M. Ichthyoses, Clinical, Biochemical, Pathogenic and Diagnostic Assessment; Karger Medical and Scientific Publishers: Basel, Switzerland, 2010; Volume 39. [Google Scholar]
- Oji, V.; Tadini, G.; Akiyama, M.; Bardon, C.B.; Bodemer, C.; Bourrat, E.; Coudiere, P.; DiGiovanna, J.J.; Elias, P.; Fischer, J.; et al. Revised nomenclature and classification of inherited ichthyoses: Results of the First Ichthyosis Consensus Conference in Sorèze 2009. J. Am. Acad. Dermatol. 2010, 63, 607–641. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.T.K.; Iizuka, H.; Shimizu, H.; Tomita, Y.M.Y.; Hashimoto, K. Comprehensive Handbook of Clinical Dermatology; Nakayamashoten: Tokyo, Japan, 2003; Volume 7, pp. 103–127. [Google Scholar]
- Oji, V.; Traupe, H. Ichthyosis: Clinical manifestations and practical treatment options. Am. J. Clin. Dermatol. 2009, 10, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Covarrubias, S.; González-Huerta, L. Analysis of the VCX3A, VCX2 and VCX3B genes shows that VCX3A gene deletion is not sufficient to result in mental retardation in X-linked ichthyosis. Br. J. Dermatol. 2008, 158, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Saral, S.; Vural, A.; Wollenberg, A.; Ruzicka, T. A practical approach to ichthyoses with systemic manifestations. Clin. Genet. 2016, 91, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Richard, G.; Adam, M.P.; Everman, D.B.; Mirzaa, G.M.; Pagon, R.A.; Wallace, S.E.; Bean, L.J.H.; Gripp, K.W.; Amemiya, A. Autosomal Recessive Congenital IchthyosisIn: GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Brown, S.; Relton, C.; Liao, H.; Zhao, Y.; Sandilands, A.; McLean, W.; Cordell, H.; Reynolds, N. Filaggrin haploinsufficiency is highly penetrant and is associated with increased severity of eczema: Further delineation of the skin phenotype in a prospective epidemiological study of 792 school children. Br. J. Dermatol. 2009, 161, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Bourrat, E. Genetics of Inherited Ichthyoses and Related Diseases. Acta Derm. Venereol. 2020, 100, 186–196. [Google Scholar] [CrossRef]
- Akiyama, M.; Sakai, K.; Yanagi, T.; Tabata, N.; Yamada, M.; Shimizu, H. Partially disturbed lamellar granule secretion in mild congenital ichthyosiform erythroderma with ALOX12B mutations. Br. J. Dermatol. 2010, 163, 201–204. [Google Scholar] [CrossRef]
- Sandilands, A.; Terron-Kwiatkowski, A.; Hull, P.; O’Regan, G.M.; Clayton, T.H.; Watson, R.M.; Carrick, T.; Evans, A.T.; Liao, H.; Zhao, Y.; et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat. Genet. 2007, 39, 650–654. [Google Scholar] [CrossRef]
- Samdani, A.J.; Naz, N.; Ahmed, N. Molecular studies of ichthyosis vulgaris in Pakistani families. J. Coll. Physicians Surg. Pak. 2010, 20, 644. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M. The Epidermal Permeability Barrier: From the Early Days at Harvard to Emerging Concepts. J. Investig. Dermatol. 2004, 122, xxxvi–xxxix. [Google Scholar] [CrossRef] [PubMed]
- Hand, J.L.; Runke, C.K.; Hodge, J.C. The phenotype spectrum of X-linked ichthyosis identified by chromosomal microarray. J. Am. Acad. Dermatol. 2015, 72, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, N.; Oiso, N.; Fukai, K.; Hanada, K.; Fujita, H.; Ishii, M. Deletion of distal promoter of VCXA in a patient with X-linked ichthyosis associated with borderline mental retardation. J. Dermatol. Sci. 2007, 45, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Selcer, K.W.; DiFrancesca, H.M.; Chandra, A.B.; Li, P.-K. Immunohistochemical analysis of steroid sulfatase in human tissues. J. Steroid Biochem. Mol. Biol. 2007, 105, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Sugiyama-Nakagiri, Y.; Sakai, K.; McMillan, J.R.; Goto, M.; Arita, K.; Tsuji-Abe, Y.; Tabata, N.; Matsuoka, K.; Sasaki, R.; et al. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J. Clin. Investig. 2005, 115, 1777–1784. [Google Scholar] [CrossRef]
- Karim, N.; Ullah, A.; Murtaza, G.; Naeem, M. Molecular Genetic Study of a Large Inbred Pakistani Family Affected with Autosomal Recessive Congenital Ichthyosis Through Whole Exome Sequencing. Genet. Test. Mol. Biomark. 2019, 23, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Steinert, P.M.; Marekov, L.N. The Proteins Elafin, Filaggrin, Keratin Intermediate Filaments, Loricrin, and Small Proline-rich Proteins 1 and 2 Are Isodipeptide Cross-linked Components of the Human Epidermal Cornified Cell Envelope. J. Biol. Chem. 1995, 270, 17702–17711. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M. Harlequin ichthyosis and other autosomal recessive congenital ichthyoses: The underlying genetic defects and pathomechanisms. J. Dermatol. Sci. 2006, 42, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pazos, L.; Ginarte, M.; Vega, A.; Toribio, J. Autosomal recessive congenital ichthyosis. Actas Dermosifiliogr. 2013, 104, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Eckl, K.-M.; de Juanes, S.; Kurtenbach, J.; Nätebus, M.; Lugassy, J.; Oji, V.; Traupe, H.; Preil, M.-L.; Martínez, F.; Smolle, J.; et al. Molecular Analysis of 250 Patients with Autosomal Recessive Congenital Ichthyosis: Evidence for Mutation Hotspots in ALOXE3 and Allelic Heterogeneity in ALOX12B. J. Investig. Dermatol. 2009, 129, 1421–1428. [Google Scholar] [CrossRef]
- Vahlquist, A.; Bygum, A.; Gånemo, A.; Virtanen, M.; Hellström-Pigg, M.; Strauss, G.; Brandrup, F.; Fischer, J. Genotypic and clinical spectrum of self-improving collodion ichthyosis: ALOX12B, ALOXE3, and TGM1 mutations in Scandinavian patients. J. Invest. Dermatol. 2010, 130, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Sawamura, D.; Shimizu, H. The clinical spectrum of nonbullous congenital ichthyosiform erythroderma and lamellar ichthyosis. Clin. Exp. Dermatol. 2003, 28, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Wajid, M.; Kurban, M.; Shimomura, Y.; Christiano, A.M. NIPAL4/ichthyin is expressed in the granular layer of human epidermis and mutated in two Pakistani families with autosomal recessive ichthyosis. J. Dermatol. 2010, 220, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Richard, G. Autosomal recessive congenital ichthyosis. In GeneReviews; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mefford, H.C., Stephens, K., Amemiya, A., Ledbetter, N., Eds.; University of Washington: Seattle, WA, USA, 2001; Last Update: Updated 2017 edn 23. [Google Scholar]
- Russell, L.J.; DiGiovanna, J.J.; Rogers, G.R.; Steinert, P.M.; Hashem, N.; Compton, J.G.; Bale, S.J. Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat. Genet. 1995, 9, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, T.; Akiyama, M. Inherited ichthyosis: Non-syndromic forms. J. Dermatol. 2016, 43, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J. Autosomal recessive congenital ichthyosis. J. Invest. Dermatol. 2009, 129, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; McDonnell, M.; Chen, X.-S.; Lakkis, M.M.; Li, H.; Isaacs, S.N.; Elsea, S.H.; Patel, P.I.; Funk, C.D. Human 12 (R)-lipoxygenase and the mouse ortholog molecular cloning, expression, and gene chromosomal assignment. J. Biol. Chem. 1998, 273, 33540–33547. [Google Scholar] [CrossRef]
- Heidt, M.; Fürstenberger, G.; Vogel, S.; Marks, F.; Krieg, P. Diversity of mouse lipoxygenases: Identification of a subfamily of epidermal isozymes exhibiting a differentiation-dependent mRNA expression pattern. Lipids 2000, 35, 701–707. [Google Scholar] [CrossRef]
- Krieg, P.; Fürstenberger, G. The role of lipoxygenases in epidermis. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1841, 390–400. [Google Scholar] [CrossRef]
- Annilo, T.; Shulenin, S.; Chen, Z.Q.; Arnould, I.; Prades, C.; Lemoine, C.; Maintoux- Larois, C.; Devaud, C.; Dean, M.; Denefle, P.; et al. Identification and characterization of a novel ABCA subfamily member, ABCA12, located in the lamellar ichthyosis region on 2q34. Cytogenet. Genome Res. 2002, 98, 169–176. [Google Scholar]
- Sakai, K.; Akiyama, M.; Sugiyama-Nakagiri, Y.; McMillan, J.R.; Sawamura, D.; Shimizu, H. Localization of ABCA12 from Golgi apparatus to lamellar granules in human upper epidermal keratinocytes. Exp. Dermatol. 2007, 16, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Lefévre, C.; Audebert, S.; Jobard, F.; Bouadjar, B.; Lakhdar, H.; Boughdene-Stambouli, O.; Blanchet-Bardon, C.; Heilig, R.; Foglio, M.; Weissenbach, J.M.; et al. Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum. Mol. Genet. 2003, 12, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Kelsell, P.D.; Norgett, E.E.; Unsworth, H.; Teh, M.-T.; Cullup, T.; Mein, C.A.; Dopping-Hepenstal, J.P.; Dale, A.B.; Tadini, G.; Fleckman, P.; et al. Mutations in ABCA12 Underlie the Severe Congenital Skin Disease Harlequin Ichthyosis. Am. J. Hum. Genet. 2005, 76, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.; Bouadjar, B.; Karaduman, A.; Jobard, F.; Saker, S.; Özguc, M.; Lathrop, M.; Prud’homme, J.F.; Fischer, J. Mutations in ichthyin a new gene on chromosome 5q33 in a new form of autosomal recessive congnital ichthyosis. Hum. Mol. Genet. 2004, 13, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Harting, M.; Brunetti-Pierri, N.; Chan, C.S.; Kirby, J.; Dishop, M.; Richard, G.; Scaglia, F.; Yan, A.C.; Levy, M.L. Self-healing collodion membrane and mild nonbullous congenital ichthyosiform erythroderma due to 2 novel mutations in the ALOX12B gene. Arch. Dermatol. 2008, 144, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Virolainen, E.; Wessman, M.; Hovatta, I.; Niemi, K.-M.; Ignatius, J.; Kere, J.; Peltonen, L.; Palotie, A. Assignment of a Novel Locus for Autosomal Recessive Congenital Ichthyosis to Chromosome 19p13.1-p13.2. Am. J. Hum. Genet. 2000, 66, 1132–1137. [Google Scholar] [CrossRef]
- Ohno, Y.; Nakamichi, S.; Ohkuni, A.; Kamiyama, N.; Naoe, A.; Tsujimura, H.; Yokose, U.; Sugiura, K.; Ishikawa, J.; Akiyama, M.; et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc. Natl. Acad. Sci. USA 2015, 112, 7707–7712. [Google Scholar] [CrossRef]
- Hotz, A.; Bourrat, E.; Küsel, J.; Oji, V.; Alter, S.; Hake, L.; Korbi, M.; Ott, H.; Hausser, I.; Zimmer, A.D.; et al. Mutation update for CYP4F22 variants associated with autosomal recessive congenital ichthyosis. Hum. Mutat. 2018, 39, 1305–1313. [Google Scholar] [CrossRef]
- Radner, F.P.; Marrakchi, S.; Kirchmeier, P.; Kim, G.J.; Ribierre, F.; Kamoun, B.; Abid, L.; Leipoldt, M.; Turki, H.; Schempp, W.; et al. Mutations in CERS3 cause autosomal recessive congenital ichthyosis in humans. PLoS Genet. 2013, 9, e1003536. [Google Scholar] [CrossRef]
- Eckl, K.-M.; Tidhar, R.; Thiele, H.; Oji, V.; Hausser, I.; Brodesser, S.; Preil, M.-L.; Önal-Akan, A.; Stock, F.; Müller, D.; et al. Impaired Epidermal Ceramide Synthesis Causes Autosomal Recessive Congenital Ichthyosis and Reveals the Importance of Ceramide Acyl Chain Length. J. Investig. Dermatol. 2013, 133, 2202–2211. [Google Scholar] [CrossRef]
- Grall, A.; Guaguere, E.; Planchais, S.; Grond, S.; Bourrat, E.; Hausser, I.; Hitte, C.; Le Gallo, M.; Derbois, C.; Kim, G.-J.; et al. PNPLA1 mutations cause autosomal recessive congenital ichthyosis in golden retriever dogs and humans. Nat. Genet. 2012, 44, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Grond, S.; Eichmann, T.O.; Dubrac, S.; Kolb, D.; Schmuth, M.; Fischer, J.; Crumrine, D.; Elias, P.M.; Haemmerle, G.; Zechner, R.; et al. PNPLA1 Deficiency in Mice and Humans Leads to a Defect in the Synthesis of Omega-O-Acylceramides. J. Investig. Dermatol. 2016, 137, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Anjo, T.; Kaneko, A.; Senoo, Y.; Shibata, A.; Takama, H.; Yokoyama, K.; Nishito, Y.; Ono, T.; Taya, C.; et al. PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat. Commun. 2017, 8, 14609. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Kamiyama, N.; Nakamichi, S.; Kihara, A. PNPLA1 is a transacylase essential for the generation of the skin barrier lipid ω-O-acylceramide. Nat. Commun. 2017, 8, 14610. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Ansar, M.; Mehmood, S.; Izoduwa, A.; Lee, K.; Nasir, A.; Abrar, M.; Ullah, A.; Aziz, A.; Smith, J.; et al. A novel missense variant in the PNPLA1 gene underlies congenital ichthyosis in three consanguineous families. J. Eur. Acad. Dermatol. Venereol. 2016, 30, e210–e213. [Google Scholar] [CrossRef] [PubMed]
- Fachal, L.; Rodríguez-Pazos, L.; Ginarte, M.; Carracedo, A.; Toribio, J.; Vega, A. Identification of a novel PNPLA1 mutation in a Spanish family with autosomal recessive congenital ichthyosis. Br. J. Dermatol. 2014, 170, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Pigg, M.; Bygum, A.; Gånemo, A.; Virtanen, M.; Brandrup, F.; Zimmer, A.; Hotz, A.; Vahlquist, A.; Fischer, J. Spectrum of Autosomal Recessive Congenital Ichthyosis in Scandinavia: Clinical Characteristics and Novel and Recurrent Mutations in 132 Patients. Acta Derm. Venereol. 2016, 96, 932–937. [Google Scholar] [CrossRef]
- Vahidnezhad, H.; Youssefian, L.; Saeidian, A.H.; Zeinali, S.; Mansouri, P.; Sotoudeh, S.; Barzegar, M.; Mohammadi-Asl, J.; Karamzadeh, R.; Abiri, M.; et al. Gene-Targeted Next Generation Sequencing Identifies PNPLA1 Mutations in Patients with a Phenotypic Spectrum of Autosomal Recessive Congenital Ichthyosis: The Impact of Consanguinity. J. Investig. Dermatol. 2017, 137, 678–685. [Google Scholar] [CrossRef]
- Hu, Q.; Yi, L.; Chen, K.; Zhou, J.; Chen, L.; Zeng, L.; Li, H. Analysis of clinical phenotype and TGM1 gene mutation in a child with neonatal congenital ichthyosis. Cmaj 2019, 36, 357–359. [Google Scholar]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Rigsby, R.E.; Parker, A.B. Using the PyMOL application to reinforce visual understanding of protein structure. Biochem. Mol. Biol. Educ. 2016, 44, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Farasat, S.; Wei, M.-H.; Herman, M.; Liewehr, D.J.; Steinberg, S.M.; Bale, S.J.; Fleckman, P.; Toro, J.R. Novel transglutaminase-1 mutations and genotype-phenotype investigations of 104 patients with autosomal recessive congenital ichthyosis in the USA. J. Med. Genet. 2009, 46, 103–111. [Google Scholar] [CrossRef]
- Rodríguez-Pazos, L.; Ginarte, M.; Vega-Gliemmo, A.; Toribio, J. Lamellar ichthyosis with a novel homozygous C-terminal mutation in the transglutaminase-1 gene. Int. J. Dermatol. 2009, 48, 1195–1197. [Google Scholar] [CrossRef] [PubMed]
- Hohl, D. Cornified Cell Envelope. Dermatology 1990, 180, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, C.S.; Birckbichler, P.J.; Rice, R.H. Transglutaminases: Multifunctional cross linking enzymes that stabilize tissues. FASEB J. 1991, 5, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Schmuth, M.; Uchida, Y.; Rice, R.H.; Behne, M.; Crumrine, D.; Feingold, K.R.; Holleran, W.M.; Pharm, D. Basis for the permeability barrier abnormality in lamellar ichthyosis. Exp. Dermatol. 2002, 11, 248–256. [Google Scholar] [CrossRef]
- Huber, M.; Rettler, I.; Bernasconi, K.; Frenk, E.; Lavrijsen, S.P.M.; Ponec, M.; Bon, A.; Lautenschlager, S.; Schorderet, D.F.; Hohl, D. Mutations of Keratinocyte Transglutaminase in Lamellar Ichthyosis. Science 1995, 267, 525–528. [Google Scholar] [CrossRef]
- Ishida-Yamamoto, A.; Iizuka, H. Structural organization of cornified cell envelopes and alterations in inherited skin disorders. Exp. Dermatol. 1998, 7, 1–10. [Google Scholar] [CrossRef]
- Hernández-Martín, A.; Garcia-Doval, I.; Aranegui, B.; de Unamuno, P.; Rodríguez-Pazos, L.; González-Enseñat, M.-A.; Vicente, A.; Martín-Santiago, A.; Garcia-Bravo, B.; Feito, M.; et al. Prevalence of autosomal recessive congenital ichthyosis: A population-based study using the capture-recapture method in Spain. J. Am. Acad. Dermatol. 2012, 67, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Terrinoni, A.; Serra, V.; Codispoti, A.; Talamonti, E.; Bui, L.; Palombo, R.; Sette, M.; Campione, E.; Didona, B.; Annicchiarico-Petruzzelli, M.; et al. Novel transglutaminase 1 mutations in patients affected by lamellar ichthyosis. Cell Death Dis. 2012, 3, e416. [Google Scholar] [CrossRef]
- Saat, H.; Sahin, I.; Duzkale, N.; Gonul, M.; Bahsi, T.; Can, B.; Coskun, F.O.; Ozkok, S.; Takir, M.; Oskayli, M.C.; et al. Genetic Etiology of Ichthyosis in Turkish Patients: Next-generation Sequencing Identified Seven Novel Mutations. Medeni. Med. J. 2022, 37, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Shan, B.; Guo, L.; Lv, S.; Li, F. Compound Heterozygous Mutations in TGM1 Causing a Severe Form of Lamellar Ichthyosis: A Case Report. Pharm. Pers. Med. 2022, 15, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Diep, Q.M.; Luong, L.H.; Tran, T.H.; Dinh, O.T.L.; Nguyen, H.Q.; Bui, T.H.; Ta, T.V.; Tran, V.K. A case of self-improving collodion ichthyosis in Vietnam. Pediatr. Dermatol. 2020, 37, 574–575. [Google Scholar] [CrossRef]
- Rajpopat, S.; Moss, C.; Mellerio, J.; Vahlquist, A.; Gånemo, A.; Hellstrom-Pigg, M.; Ilchyshyn, A.; Burrows, N.; Lestringant, G.; Taylor, A.; et al. Harlequin ichthyosis: A review of clinical and molecular findings in 45 cases. Arch. Dermatol. Res. 2011, 147, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [PubMed]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 2009, 77, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.U.; Lüthy, R.; Eisenberg, D. A Method to Identify Protein Sequences That Fold into a Known Three-Dimensional Structure. Science 1991, 253, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef]
- Hooft, R.W.W.; Vriend, G.; Sander, C.; Abola, E.E. Errors in protein structures. Nature 1996, 381, 272. [Google Scholar] [CrossRef]
- Ganemo, A.; Pigg, M.; Virtanen, M.; Kukk, T.; Raudsepp, H.; Rossman-Ringdahl, I.; Westermark, P.; Niemi, K.M.; Dahl, N.; Vahlquist, A. Autosomal recessive congenital ichthyosis in Sweden and Estonia: Clinical, genetic and ultrastructural findings in eighty-three patients. Acta. Derm.Venereol. 2003, 83, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Al-Naamani, A.; Al-Waily, A.; Al-Kindi, M.; Al-Awadi, M.; Al-Yahyaee, S.A. Transglutaminase-1 Mutations in Omani Families with Lamellar Ichthyosis. Med. Princ. Pr. 2013, 22, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Bourrat, E.; Blanchet-Bardon, C.; Derbois, C.; Cure, S.; Fischer, J. Specific TGM1 mutation profiles in bathing suit and self improving collodion ichthyoses: Phenotypic and genotypic data from 9 patients with dynamic phenotypes of autosomal recessive congenital ichthyosis. Arch. Dermatol. 2012, 148, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pazos, L.; Ginarte, M.; Fachal, L.; Toribio, J.; Carracedo, A.; Vega, A. Analysis of TGM1, ALOX12B, ALOXE3, NIPAL4 and CYP4F22 in autosomal recessive congenital ichthyosis from Galicia (NW Spain): Evidence of founder effects. Br. J. Dermatol. 2011, 165, 906–911. [Google Scholar] [CrossRef] [PubMed]
- İmren, I.G.; Tanacan, E.; Ceylaner, S.; Sümer, G.; Ekşioğlu, M. Mild lamellar ichthyosis with a truncated homozygousTGM1mutation in a pediatric patient from Turkey. Dermatol. Ther. 2020, 33, e14152. [Google Scholar] [CrossRef]
- Youssefian, L.; Vahidnezhad, H.; Saeidian, A.H.; Touati, A.; Sotoudeh, S.; Mahmoudi, H.; Mansouri, P.; Daneshpazhooh, M.; Aghazadeh, N.; Hesari, K.K.; et al. Autosomal recessive congenital ichthyosis: Genomic landscape and phenotypic spectrum in a cohort of 125 consanguineous families. Hum. Mutat. 2019, 40, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.L.; Farasat, S.; Steinbach, P.J.; Wei, M.-H.; Toure, O.; Fleckman, P.; Blake, P.; Bale, S.J.; Toro, J.R. Transglutaminase-1 gene mutations in autosomal recessive congenital ichthyosis: Summary of mutations (including 23 novel) and modeling of TGase-1. Hum. Mutat. 2009, 30, 537–547. [Google Scholar] [CrossRef]
- Fürstenberger, G.; Epp, N.; Eckl, K.-M.; Hennies, H.C.; Jørgensen, C.; Hallenborg, P.; Kristiansen, K.; Krieg, P. Role of epidermis-type lipoxygenases for skin barrier function and adipocyte differentiation. Prostaglandins Other Lipid Mediat. 2007, 82, 128–134. [Google Scholar] [CrossRef]
- Parmentier, L.; Blanchet-Bardon, C.; Nguyen, S.; Prud’Homme, J.-F.; Dubertret, L.; Weissenbach, J. Autosomal recessive lamellar ichthyosis: Identification of a new mutation in transglutaminase 1 and evidence for genetic heterogeneity. Hum. Mol. Genet. 1995, 4, 1391–1395. [Google Scholar] [CrossRef]
- Candi, E.; Melino, G.; Lahm, A.; Ceci, R.; Rossi, A.; Kim, I.G.; Ciani, B.; Steinert, P.M. Transglutaminase 1 mutations in lamellar ichthyosis Loss of activity due to failure of activation by proteolytic processing. J. Biol. Chem. 1998, 273, 13693–13702. [Google Scholar] [CrossRef]
- Ullah, R.; Ansar, M.; Durrani, Z.U.; Lee, K.; Santos-Cortez, R.L.P.; Muhammad, D.; Ali, M.; Zia, M.; Ayub, M.; Khan, S.; et al. Novel mutations in the genes TGM 1 and ALOXE 3 underlying autosomal recessive congenital ichthyosis. Int. J. Dermatol. 2016, 55, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Karim, N.; Shah, F.A.; Naeem, M. Novel TGM1 mutation in a Pakistani family affected with severe lamellar ichthyosis. Pediatr. Neonatol. 2018, 59, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Candi, E.; Melino, G.; Mei, G.; Tarcsa, E.; Chung, S.-I.; Marekov, L.N.; Steinert, P.M. Biochemical, Structural, and Transglutaminase Substrate Properties Of Human Loricrin, the Major Epidermal Cornified Cell Envelope Protein. J. Biol. Chem. 1995, 270, 26382–26390. [Google Scholar] [CrossRef] [PubMed]
- Candi, E.; Tarcsa, E.; Idler, W.W.; Kartasova, T.; Marekov, L.N.; Steinert, P.M. Transglutaminase Cross-linking Properties of the Small Proline-rich 1 Family of Cornified Cell Envelope Proteins. J. Biol. Chem. 1999, 270, 26382–26390. [Google Scholar] [CrossRef] [PubMed]
- Mazereeuw-Hautier, J.; Vahlquist, A.; Traupe, H.; Bygum, A.; Amaro, C.; Aldwin, M.; Audouze, A.; Bodemer, C.; Bourrat, E.; Diociaiuti, A.; et al. Management of congenital ichthyoses: European guidelines of care, part one. Br. J. Dermatol. 2019, 180, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Mazereeuw-Hautier, J.; Hernández-Martín, A.; O’Toole, E.A.; Bygum, A.; Amaro, C.; Aldwin, M.; Audouze, A.; Bodemer, C.; Bourrat, E.; Diociaiuti, A.; et al. Management of congenital ichthyoses: European guidelines of care, part two. Br. J. Dermatol. 2019, 180, 484–495. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almazroea, A.; Ijaz, A.; Aziz, A.; Mushtaq Yasinzai, M.; Rafiullah, R.; Rehman, F.U.; Daud, S.; Shaikh, R.; Ayub, M.; Wali, A. Identification and In Silico Analysis of a Homozygous Nonsense Variant in TGM1 Gene Segregating with Congenital Ichthyosis in a Consanguineous Family. Medicina 2023, 59, 103. https://doi.org/10.3390/medicina59010103
Almazroea A, Ijaz A, Aziz A, Mushtaq Yasinzai M, Rafiullah R, Rehman FU, Daud S, Shaikh R, Ayub M, Wali A. Identification and In Silico Analysis of a Homozygous Nonsense Variant in TGM1 Gene Segregating with Congenital Ichthyosis in a Consanguineous Family. Medicina. 2023; 59(1):103. https://doi.org/10.3390/medicina59010103
Chicago/Turabian StyleAlmazroea, Abdulhadi, Ambreen Ijaz, Abdul Aziz, Muhammad Mushtaq Yasinzai, Rafiullah Rafiullah, Fazal Ur Rehman, Shakeela Daud, Rozeena Shaikh, Muhammad Ayub, and Abdul Wali. 2023. "Identification and In Silico Analysis of a Homozygous Nonsense Variant in TGM1 Gene Segregating with Congenital Ichthyosis in a Consanguineous Family" Medicina 59, no. 1: 103. https://doi.org/10.3390/medicina59010103
APA StyleAlmazroea, A., Ijaz, A., Aziz, A., Mushtaq Yasinzai, M., Rafiullah, R., Rehman, F. U., Daud, S., Shaikh, R., Ayub, M., & Wali, A. (2023). Identification and In Silico Analysis of a Homozygous Nonsense Variant in TGM1 Gene Segregating with Congenital Ichthyosis in a Consanguineous Family. Medicina, 59(1), 103. https://doi.org/10.3390/medicina59010103




