The Possible Role of Electrical Stimulation in Osteoporosis: A Narrative Review
Abstract
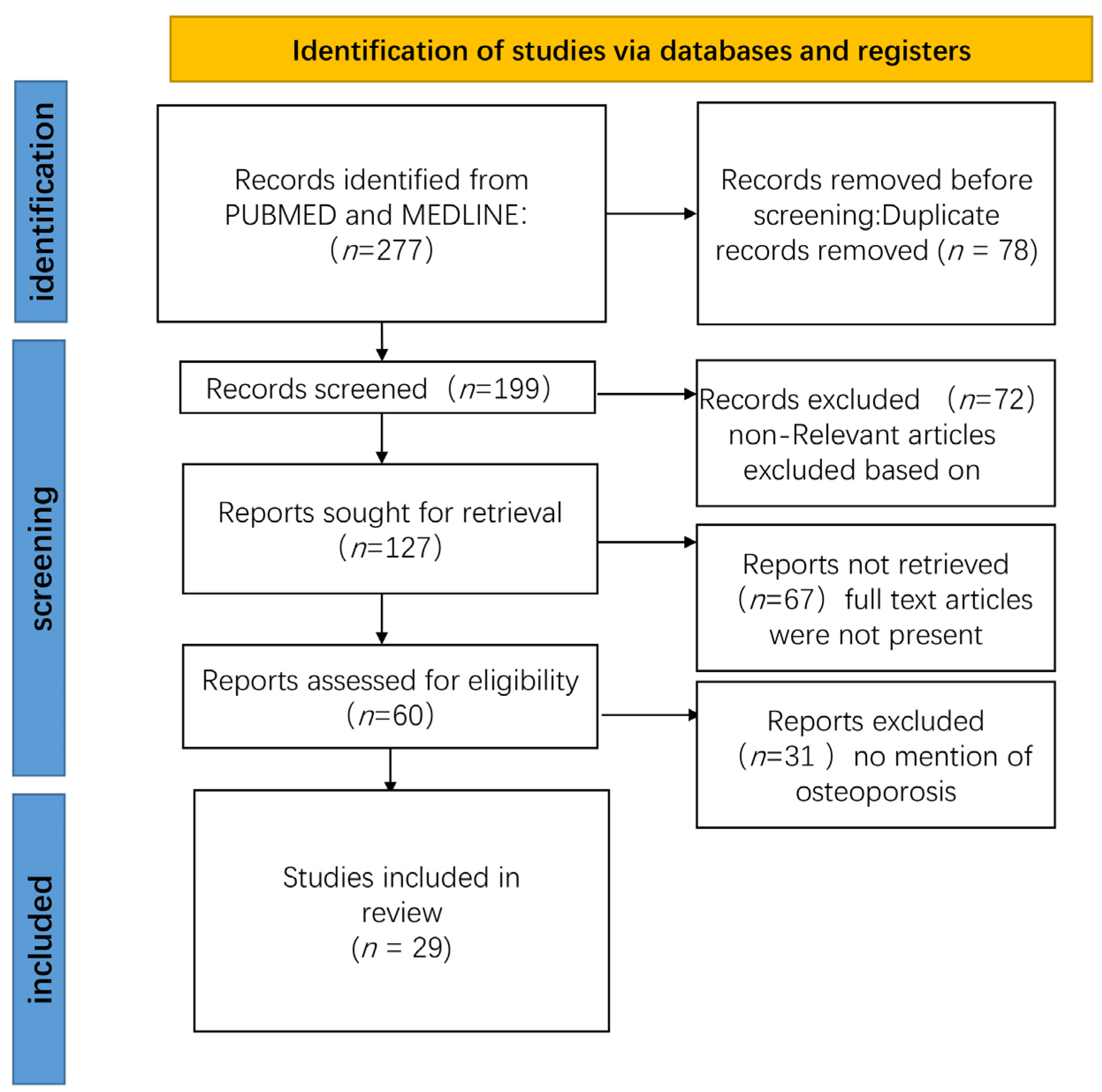
:1. Introduction
2. Methods
3. PEMFs for Osteoporosis
3.1. The Clinical Research into PEMFs in Osteoporosis
3.2. The Effects of a PEMF on Osteogenesis and Its Mechanism
| Researchers | Outcome | Mechanism | References |
|---|---|---|---|
| Wen-Fang He et al. | osteogenic differentiation | primary cilia | [37] |
| Yan-Fang Xie et al. | osteoblast mineralization | primary cilia-dependent | [38] |
| Juan-Li Yan et al. | osteoblast maturation | primary cilia | [39] |
| Qian Wang et al. | osteogenesis | H-type blood vessels | [44] |
| Tiantian Wang et al. | osteogenesis | H-type angiogenesis | [45] |
| Xi Shao et al. | osteogenesis | Wnt/β-catenin | [54] |
| Shaoyu Wu et al. | osteogenic differentiation | Wnt/Ca+ | [55] |
| Xi Shao et al. | bone formation | Wnt/β-catenin | [56] |
| Da Jing et al. | bone formation | Wnt/β-catenin | [57] |
| Jun Zhou et al. | osteogenesis | Wnt/β-catenin | [58] |
3.3. The Effects of a PEMF on Osteoclasts and Its Mechanism
4. DC for Osteoporosis
5. CC for Osteoporosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, C. Osteoporosis: Staying strong. Nature 2017, 550, S15–S17. [Google Scholar] [CrossRef]
- Leslie, W.D.; Crandall, C.J. Serial Bone Density Measurement for Osteoporosis Screening. JAMA 2021, 326, 1622–1623. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Al Jumaily, K.; Lin, M.; Siminoski, K.; Ye, C. Dual-energy X-ray absorptiometry scanner mismatch in follow-up bone mineral density testing. Osteoporos. Int. 2022, 33, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Quattrini, S.; Palpacelli, A.; Catassi, G.N.; Lionetti, M.E.; Catassi, C. Metabolic Bone Disease in Children with Intestinal Failure and Long-Term Parenteral Nutrition: A Systematic Review. Nutrients 2022, 14, 995. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Zhao, S.; Li, J.; Li, X.; Wang, Y.; Xie, D.; Zeng, C.; Lei, G.; Wei, J.; Li, H. Denosumab and Risk of Community-acquired Pneumonia: A Population-based Cohort Study. J. Clin. Endocrinol. Metab. 2022, 107, e3366–e3373. [Google Scholar] [CrossRef]
- Lippuner, K.; Rimmer, G.; Stuck, A.K.; Schwab, P.; Bock, O. Hospitalizations for major osteoporotic fractures in Switzerland: A long-term trend analysis between 1998 and 2018. Osteoporos. Int. 2022, 33, 2327–2335. [Google Scholar] [CrossRef]
- Kadri, A.; Binkley, N.; Daffner, S.D.; Anderson, P.A. Clinical risk factor status in patients with vertebral fracture but normal bone mineral density. Spine J. Off. J. N. Am. Spine Soc. 2022, 22, 1634–1641. [Google Scholar] [CrossRef]
- Leguy, D.; Magro, L.; Pierache, A.; Coiteux, V.; Agha, I.Y.; Cortet, B.; Legroux-Gerot, I. Changes in bone mineral density after allogenic stem cell transplantation. Jt. Bone Spine 2022, 89, 105373. [Google Scholar] [CrossRef]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef]
- Nakatoh, S.; Fujimori, K.; Ishii, S.; Tamaki, J.; Okimoto, N.; Ogawa, S.; Iki, M. Association of pharmacotherapy with the second hip fracture incidence in women: A retrospective analysis of the National Database of Health Insurance Claims and Specific Health Checkups of Japan. Geriatr. Gerontol. Int. 2022, 22, 930–937. [Google Scholar] [CrossRef]
- Dimai, H.P.; Fahrleitner-Pammer, A. Osteoporosis and Fragility Fractures: Currently available pharmacological options and future directions. Best Pract. Res. Clin. Rheumatol. 2022, 36, 101780. [Google Scholar] [CrossRef]
- Iida, H.; Sakai, Y.; Seki, T.; Watanabe, T.; Wakao, N.; Matsui, H.; Imagama, S. Bisphosphonate treatment is associated with decreased mortality rates in patients after osteoporotic vertebral fracture. Osteoporos. Int. 2022, 33, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S. Medical treatment of osteoporosis. Climacteric J. Int. Menopause Soc. 2022, 25, 43–49. [Google Scholar] [CrossRef]
- Akhter, S.; Qureshi, A.R.; Aleem, I.; El-Khechen, H.A.; Khan, S.; Sikder, O.; Khan, M.; Bhandari, M.; Aleem, I. Efficacy of Electrical Stimulation for Spinal Fusion: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 2020, 10, 4568. [Google Scholar] [CrossRef] [Green Version]
- Al-Jabri, T.; Tan, J.Y.Q.; Tong, G.Y.; Shenoy, R.; Kayani, B.; Parratt, T.; Khan, T. The role of electrical stimulation in the management of avascular necrosis of the femoral head in adults: A systematic review. BMC Musculoskelet. Disord. 2017, 18, 319. [Google Scholar] [CrossRef] [Green Version]
- Gan, J.C.; Glazer, P.A. Electrical stimulation therapies for spinal fusions: Current concepts. Eur. Spine J. 2006, 15, 1301–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.J.K.; Tai, Y.K.; Yap, J.L.Y.; Fong, C.H.H.; Loo, L.S.W.; Kukumberg, M.; Fröhlich, J.; Zhang, S.; Li, J.Z.; Wang, J.-W.; et al. Brief exposure to directionally-specific pulsed electromagnetic fields stimulates extracellular vesicle release and is antagonized by streptomycin: A potential regenerative medicine and food industry paradigm. Biomaterials 2022, 287, 121658. [Google Scholar] [CrossRef]
- Ganse, B.; Orth, M.; Roland, M.; Diebels, S.; Motzki, P.; Seelecke, S.; Kirsch, S.-M.; Welsch, F.; Andres, A.; Wickert, K.; et al. Concepts and clinical aspects of active implants for the treatment of bone fractures. Acta Biomater. 2022, 146, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, L.; Jiang, J.; Liu, Y.; Fan, Z.; Zhong, C.; He, C. Pulsed electromagnetic fields: Promising treatment for osteoporosis. Osteoporos. Int. 2019, 30, 267–276. [Google Scholar] [CrossRef]
- Abellaneda-Pérez, K.; Vaqué-Alcázar, L.; Perellón-Alfonso, R.; Solé-Padullés, C.; Bargalló, N.; Salvador, R.; Ruffini, G.; Nitsche, M.A.; Pascual-Leone, A.; Bartrés-Faz, D. Multifocal Transcranial Direct Current Stimulation Modulates Resting-State Functional Connectivity in Older Adults Depending on the Induced Current Density. Front. Aging Neurosci. 2021, 13, 725013. [Google Scholar] [CrossRef]
- Li, M.; Zhang, D.; Chen, Y.; Chai, X.; He, L.; Chen, Y.; Guo, J.; Sui, X. Discrimination and Recognition of Phantom Finger Sensation Through Transcutaneous Electrical Nerve Stimulation. Front. Neurosci. 2018, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Fornell, S.; Ribera, J.; Mella, M.; Carranza, A.; Serrano-Toledano, D.; Domecq, G. Effects of electrical stimulation in the treatment of osteonecrosis of the femoral head. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther. 2018, 28, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Goyal, K.; Borkholder, D.A.; Day, S.W. Dependence of Skin-Electrode Contact Impedance on Material and Skin Hydration. Sensors 2022, 22, 8510. [Google Scholar] [CrossRef]
- Ng, C.L.; Reaz, M.B.I.; Crespo, M.L.; Cicuttin, A.; Chowdhury, M.E.H. Characterization of capacitive electromyography biomedical sensor insulated with porous medical bandages. Sci. Rep. 2020, 10, 14891. [Google Scholar] [CrossRef]
- Zheng, Y.-N.; Yu, Z.; Mao, G.; Li, Y.; Pravarthana, D.; Asghar, W.; Liu, Y.; Qu, S.; Shang, J.; Li, R.-W. A Wearable Capacitive Sensor Based on Ring/Disk-Shaped Electrode and Porous Dielectric for Noncontact Healthcare Monitoring. Glob. Chall. 2020, 4, 1900079. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Menger, M.M.; Braun, B.J.; Schweizer, S.; Linnemann, C.; Falldorf, K.; Ronniger, M.; Wang, H.; Histing, T.; Nussler, A.K.; et al. Modulation of Macrophage Activity by Pulsed Electromagnetic Fields in the Context of Fracture Healing. Bioengineering 2021, 8, 167. [Google Scholar] [CrossRef]
- Androjna, C.; Fort, B.; Zborowski, M.; Midura, R.J. Pulsed electromagnetic field treatment enhances healing callus biomechanical properties in an animal model of osteoporotic fracture. Bioelectromagnetics 2014, 35, 396–405. [Google Scholar] [CrossRef]
- Tong, J.; Chen, Z.; Sun, G.; Zhou, J.; Zeng, Y.; Zhong, P.; Deng, C.; Chen, X.; Liu, L.; Wang, S.; et al. The Efficacy of Pulsed Electromagnetic Fields on Pain, Stiffness, and Physical Function in Osteoarthritis: A Systematic Review and Meta-Analysis. Pain Res. Manag. 2022, 2022, 9939891. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Q.; He, H.-C.; He, C.-Q.; Chen, J.; Yang, L. Clinical update of pulsed electromagnetic fields on osteoporosis. Chin. Med. J. 2008, 121, 2095–2099. [Google Scholar] [CrossRef]
- Catalano, A.; Loddo, S.; Bellone, F.; Pecora, C.; Lasco, A.; Morabito, N. Pulsed electromagnetic fields modulate bone metabolism via RANKL/OPG and Wnt/β-catenin pathways in women with postmenopausal osteoporosis: A pilot study. Bone 2018, 116, 42–46. [Google Scholar] [CrossRef]
- Liu, H.-F.; Yang, L.; He, H.-C.; Zhou, J.; Liu, Y.; Wang, C.-Y.; Wu, Y.-C.; He, C.-Q. Pulsed electromagnetic fields on postmenopausal osteoporosis in southwest China: A randomized, active-controlled clinical trial. Bioelectromagnetics 2013, 34, 323–332. [Google Scholar] [CrossRef]
- Tabrah, F.; Hoffmeier, M.; Gilbert, F., Jr.; Batkin, S.; Bassett, C.A. Bone density changes in osteoporosis-prone women exposed to pulsed electromagnetic fields (PEMFs). J. Bone Miner. Res. 1990, 5, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Amack, J.D. Structures and functions of cilia during vertebrate embryo development. Mol. Reprod. Dev. 2022, 89, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Klena, N.; Pigino, G. Structural Biology of Cilia and Intraflagellar Transport. Annu. Rev. Cell Dev. Biol. 2022, 38, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, S.; Ling, H.; Zhang, C.; Kong, Y. Primary Cilia: A Cellular Regulator of Articular Cartilage Degeneration. Stem Cells Int. 2022, 2022, 2560441. [Google Scholar] [CrossRef]
- He, W.; Qin, R.; Gao, Y.; Zhou, J.; Wei, J.; Liu, J.; Hou, X.; Ma, H.; Xian, C.J.; Li, X.; et al. The interdependent relationship between the nitric oxide signaling pathway and primary cilia in pulse electromagnetic field-stimulated osteoblastic differentiation. FASEB J. 2022, 36, e22376. [Google Scholar] [CrossRef]
- Xie, Y.-F.; Shi, W.-G.; Zhou, J.; Gao, Y.-H.; Li, S.-F.; Fang, Q.-Q.; Wang, M.-G.; Ma, H.-P.; Wang, J.-F.; Xian, C.J.; et al. Pulsed electromagnetic fields stimulate osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPRII localized at the base of primary cilium. Bone 2016, 93, 22–32. [Google Scholar] [CrossRef]
- Yan, J.-L.; Zhou, J.; Ma, H.-P.; Ma, X.-N.; Gao, Y.-H.; Shi, W.-G.; Fang, Q.-Q.; Ren, Q.; Xian, C.J.; Chen, K.-M. Pulsed electromagnetic fields promote osteoblast mineralization and maturation needing the existence of primary cilia. Mol. Cell. Endocrinol. 2015, 404, 132–140. [Google Scholar] [CrossRef]
- Liu, S.; Chen, T.; Wang, R.; Huang, H.; Fu, S.; Zhao, Y.; Wang, S.; Wan, L. Exploring the effect of the “quaternary regulation” theory of “peripheral nerve-angiogenesis-osteoclast-osteogenesis” on osteoporosis based on neuropeptides. Front. Endocrinol. 2022, 13, 908043. [Google Scholar] [CrossRef]
- Lin, X.; Xu, F.; Zhang, K.-W.; Qiu, W.-X.; Zhang, H.; Hao, Q.; Li, M.; Deng, X.-N.; Tian, Y.; Chen, Z.-H.; et al. Acacetin Prevents Bone Loss by Disrupting Osteoclast Formation and Promoting Type H Vessel Formation in Ovariectomy-Induced Osteoporosis. Front. Cell Dev. Biol. 2022, 10, 796227. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liao, L.; Hu, Y.; Xu, Y.; Zhang, Y.; Huo, F.; Tian, W.; Guo, W. Identification of Type H Vessels in Mice Mandibular Condyle. J. Dent. Res. 2021, 100, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Liao, L.; Su, X.; Shuai, Y.; Zhang, X.; Deng, Z.; Jin, Y. Declining histone acetyltransferase GCN5 represses BMSC-mediated angiogenesis during osteoporosis. FASEB J. 2017, 31, 4422–4433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhou, J.; Wang, X.; Xu, Y.; Liang, Z.; Gu, X.; He, C. Coupling induction of osteogenesis and type H vessels by pulsed electromagnetic fields in ovariectomy-induced osteoporosis in mice. Bone 2022, 154, 116211. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, L.; Liang, Z.; Bai, L.; Pei, H.; Zhang, T.; Wu, L.; Wang, L.; Wang, X.; You, X.; et al. Pulsed electromagnetic fields attenuate glucocorticoid-induced bone loss by targeting senescent LepR+ bone marrow mesenchymal stromal cells. Biomater. Adv. 2022, 133, 112635. [Google Scholar] [CrossRef]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef]
- Bats, M.-L.; Peghaire, C.; Delobel, V.; Dufourcq, P.; Couffinhal, T.; Duplàa, C. Wnt/frizzled Signaling in Endothelium: A Major Player in Blood-Retinal- and Blood-Brain-Barrier Integrity. Cold Spring Harb. Perspect. Med. 2022, 12, a041219. [Google Scholar] [CrossRef]
- Neuhaus, J.; Weimann, A.; Berndt-Paetz, M. Immunocytochemical Analysis of Endogenous Frizzled-(Co-)Receptor Interactions and Rapid Wnt Pathway Activation in Mammalian Cells. Int. J. Mol. Sci. 2021, 22, 12057. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef]
- Sardar, A.; Gautam, S.; Sinha, S.; Rai, D.; Tripathi, A.K.; Dhaniya, G.; Mishra, P.R.; Trivedi, R. Nanoparticles of naturally occurring PPAR-γ inhibitor betulinic acid ameliorates bone marrow adiposity and pathological bone loss in ovariectomized rats via Wnt/β-catenin pathway. Life Sci. 2022, 309, 121020. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, H.; China, S.P.; Cabriales, J.A.; Moininazeri, J.; Casteel, D.E.; Garcia, J.J.; Wong, V.W.; Chen, A.; Sah, R.L.; Boss, G.R.; et al. Protein Kinase G2 Is Essential for Skeletal Homeostasis and Adaptation to Mechanical Loading in Male but Not Female Mice. J. Bone Miner. Res. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Bo, Y.; Tian, Y.; Mao, L.; Xue, C.; Dong, P.; Wang, J. Docosahexaenoic Acid-Enriched Phosphatidylcholine Exerted Superior Effects to Triglyceride in Ameliorating Obesity-Induced Osteoporosis through Up-Regulating the Wnt/β-Catenin Pathway. J. Agric. Food Chem. 2022, 70, 13904–13912. [Google Scholar] [CrossRef]
- Shao, X.; Yang, Y.; Tan, Z.; Ding, Y.; Luo, E.; Jing, D.; Cai, J. Amelioration of bone fragility by pulsed electromagnetic fields in type 2 diabetic KK-Ay mice involving Wnt/β-catenin signaling. Am. J. Physiol. Metab. 2021, 320, E951–E966. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yu, Q.; Lai, A.; Tian, J. Pulsed electromagnetic field induces Ca(2+)-dependent osteoblastogenesis in C3H10T1/2 mesenchymal cells through the Wnt-Ca(2+)/Wnt-β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2018, 503, 715–721. [Google Scholar] [CrossRef]
- Shao, X.; Yan, Z.; Wang, D.; Yang, Y.; Ding, Y.; Luo, E.; Jing, D.; Cai, J. Pulsed Electromagnetic Fields Ameliorate Skeletal Deterioration in Bone Mass, Microarchitecture, and Strength by Enhancing Canonical Wnt Signaling-Mediated Bone Formation in Rats with Spinal Cord Injury. J. Neurotrauma 2021, 38, 765–776. [Google Scholar] [CrossRef]
- Jing, D.; Li, F.; Jiang, M.; Cai, J.; Wu, Y.; Xie, K.; Wu, X.; Tang, C.; Liu, J.; Guo, W.; et al. Pulsed electromagnetic fields improve bone microstructure and strength in ovariectomized rats through a Wnt/Lrp5/β-catenin signaling-associated mechanism. PLoS ONE 2013, 8, e79377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; He, H.; Yang, L.; Chen, S.; Guo, H.; Xia, L.; Liu, H.; Qin, Y.; Liu, C.; Wei, X.; et al. Effects of Pulsed Electromagnetic Fields on Bone Mass and Wnt/β-Catenin Signaling Pathway in Ovariectomized Rats. Arch. Med. Res. 2012, 43, 274–282. [Google Scholar] [CrossRef]
- Wang, P.; Tang, C.; Wu, J.; Yang, Y.; Yan, Z.; Liu, X.; Shao, X.; Zhai, M.; Gao, J.; Liang, S.; et al. Pulsed electromagnetic fields regulate osteocyte apoptosis, RANKL/OPG expression, and its control of osteoclastogenesis depending on the presence of primary cilia. J. Cell. Physiol. 2019, 234, 10588–10601. [Google Scholar] [CrossRef]
- Pi, Y.; Liang, H.; Yu, Q.; Yin, Y.; Xu, H.; Lei, Y.; Han, Z.; Tian, J. Low-frequency pulsed electromagnetic field inhibits RANKL-induced osteoclastic differentiation in RAW264.7 cells by scavenging reactive oxygen species. Mol. Med. Rep. 2019, 19, 4129–4136. [Google Scholar] [CrossRef]
- Lei, Y.; Su, J.; Xu, H.; Yu, Q.; Zhao, M.; Tian, J. Pulsed electromagnetic fields inhibit osteoclast differentiation in RAW264.7 macrophages via suppression of the protein kinase B/mammalian target of rapamycin signaling pathway. Mol. Med. Rep. 2018, 18, 447–454. [Google Scholar] [CrossRef]
- He, Z.; Selvamurugan, N.; Warshaw, J.; Partridge, N.C. Pulsed electromagnetic fields inhibit human osteoclast formation and gene expression via osteoblasts. Bone 2018, 106, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, J.; Yang, Y.; Zhai, M.; Shao, X.; Yan, Z.; Zhang, X.; Wu, Y.; Cao, L.; Sui, B.; et al. Differential intensity-dependent effects of pulsed electromagnetic fields on RANKL-induced osteoclast formation, apoptosis, and bone resorbing ability in RAW264.7 cells. Bioelectromagnetics 2017, 38, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, H.; Han, Z.; Chen, P.; Yu, Q.; Lei, Y.; Li, Z.; Zhao, M.; Tian, J. Pulsed electromagnetic field inhibits RANKL-dependent osteoclastic differentiation in RAW264.7 cells through the Ca 2+-calcineurin-NFATc1 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 482, 289–295. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Y.; Chen, J.; Zheng, S.; Huang, H.; Dong, X. Effects of pulsed electromagnetic fields on the expression of NFATc1 and CAII in mouse osteoclast-like cells. Aging Clin. Exp. Res. 2015, 27, 13–19. [Google Scholar] [CrossRef]
- Chang, K.; Chang, W.H.; Tsai, M.-T.; Shih, C. Pulsed Electromagnetic Fields Accelerate Apoptotic Rate in Osteoclasts. Connect. Tissue Res. 2006, 47, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Forsted, D.L.; Dalinka, M.K.; Mitchell, E.; Brighton, C.T.; Alavi, A. Radiologic Evaluation of the Treatment of Nonunion of Fractures by Electrical Stimulation. Radiology 1978, 128, 629–634. [Google Scholar] [CrossRef]
- Creasey, G.H.; Ho, C.H.; Triolo, R.J.; Gater, D.R.; DiMarco, A.F.; Bogie, K.M.; Keith, M.W. Clinical applications of electrical stimulation after spinal cord injury. J. Spinal Cord Med. 2004, 27, 365–375. [Google Scholar] [CrossRef]
- Iimura, K.; Watanabe, N.; Milliken, P.; Hsieh, Y.-H.; Lewis, S.J.; Sridhar, A.; Hotta, H. Chronic Electrical Stimulation of the Superior Laryngeal Nerve in the Rat: A Potential Therapeutic Approach for Postmenopausal Osteoporosis. Biomedicines 2020, 8, 369. [Google Scholar] [CrossRef]
- Lau, R.Y.-C.; Qian, X.; Po, K.-T.; Li, L.-M.; Guo, X. Response of Rat Tibia to Prolonged Unloading Under the Influence of Electrical Stimulation at the Dorsal Root Ganglion. Neuromodulation J. Int. Neuromodulation Soc. 2017, 20, 284–289. [Google Scholar] [CrossRef]
- Lau, Y.C.; Qian, X.; Po, K.T.; Li, L.M.; Guo, X. Electrical stimulation at the dorsal root ganglion preserves trabecular bone mass and microarchitecture of the tibia in hindlimb-unloaded rats. Osteoporos. Int. 2015, 26, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Piazzolla, A.; Solarino, G.; Bizzoca, D.; Garofalo, N.; Dicuonzo, F.; Setti, S.; Moretti, B. Capacitive coupling electric fields in the treatment of vertebral compression fractures. J. Biol. Regul. Homeost. Agents 2015, 29, 637–646. [Google Scholar]
- Rossini, M.; Viapiana, O.; Gatti, D.; de Terlizzi, F.; Adami, S. Capacitively Coupled Electric Field for Pain Relief in Patients with Vertebral Fractures and Chronic Pain. Clin. Orthop. Relat. Res. 2010, 468, 735–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manjhi, J.; Mathur, R.; Behari, J. Effect of low level capacitive-coupled pulsed electric field stimulation on mineral profile of weight-bearing bones in ovariectomized rats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 189–195. [Google Scholar] [CrossRef]
- Behari, J.; Behari, J. Changes in bone histology due to capacitive electric field stimulation of ovariectomized rat. Indian J. Med. Res. 2009, 130, 720–725. [Google Scholar]
- Brighton, C.T.; Luessenhop, C.P.; Pollack, S.R.; Steinberg, D.R.; Petrik, M.E.; Kaplan, F.S. Treatment of castration-induced osteoporosis by a capacitively coupled electrical signal in rat vertebrae. J. Bone Jt. Surg. Am. Vol. 1989, 71, 228–236. [Google Scholar] [CrossRef]
- Brighton, C.T.; Tadduni, G.T.; Goll, S.R.; Pollack, S.R. Treatment of denervation/disuse osteoporosis in the rat with a capacitively coupled electrical signal: Effects on bone formation and bone resorption. J. Orthop. Res. 1988, 6, 676–684. [Google Scholar] [CrossRef]
- Brighton, C.T.; Tadduni, G.T.; Pollack, S.R. Treatment of sciatic denervation disuse osteoporosis in the rat tibia with capacitively coupled electrical stimulation. Dose response and duty cycle. J. Bone Jt. Surg. Am. Vol. 1985, 67, 1022–1028. [Google Scholar] [CrossRef]
| Researchers | Outcome | Mechanism | References |
|---|---|---|---|
| Pan Wang et al. | osteoclast formation | RANKL/OPG | [59] |
| Ying Pi et al. | osteoclast differentiation | reactive oxygen | [60] |
| Yutian Lei et al. | osteoclast differentiation | Akt/mTOR | [61] |
| Zhiming He et al. | osteoclast formation | TGF-β | [62] |
| Pan Wang et al. | osteoclast formation | RANKL | [63] |
| Jie Zhang et al. | osteoclast differentiation | Ca2+ | [64] |
| Jianquan He et al. | osteoclast formation | NFATc1 | [65] |
| Kyle Chang et al. | osteoclast formation | apoptotic rate | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Luo, Y.; Xu, J.; Guo, C.; Shi, J.; Li, L.; Sun, X.; Kong, Q. The Possible Role of Electrical Stimulation in Osteoporosis: A Narrative Review. Medicina 2023, 59, 121. https://doi.org/10.3390/medicina59010121
Zhang W, Luo Y, Xu J, Guo C, Shi J, Li L, Sun X, Kong Q. The Possible Role of Electrical Stimulation in Osteoporosis: A Narrative Review. Medicina. 2023; 59(1):121. https://doi.org/10.3390/medicina59010121
Chicago/Turabian StyleZhang, Weifei, Yuanrui Luo, Jixuan Xu, Chuan Guo, Jing Shi, Lu Li, Xiao Sun, and Qingquan Kong. 2023. "The Possible Role of Electrical Stimulation in Osteoporosis: A Narrative Review" Medicina 59, no. 1: 121. https://doi.org/10.3390/medicina59010121
APA StyleZhang, W., Luo, Y., Xu, J., Guo, C., Shi, J., Li, L., Sun, X., & Kong, Q. (2023). The Possible Role of Electrical Stimulation in Osteoporosis: A Narrative Review. Medicina, 59(1), 121. https://doi.org/10.3390/medicina59010121





