The Prevalence and Factors Associated with the Prescription of Opioids for Head/Neck Pain after Elective Craniotomy for Tumor Resection/Vascular Repair: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Institutional Practice
2.4. Data Collection
2.5. Outcome Measures
2.6. Data Analysis and Statistics
3. Results
3.1. Study Sample Characteristics
3.2. Discharge and Follow-Up Opioid Prescriptions
3.3. Factors Associated with Opioid Prescription at Hospital Discharge
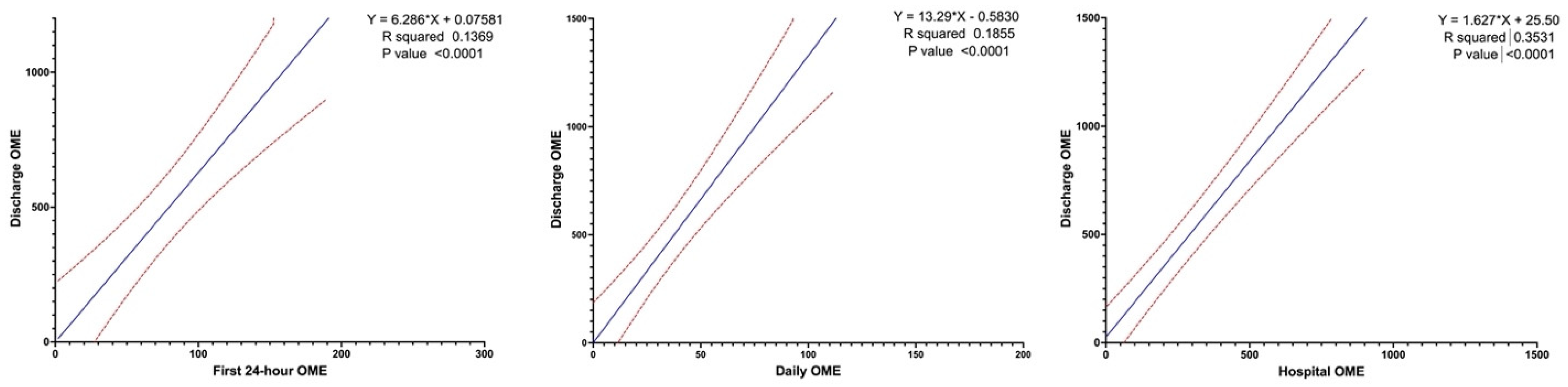
3.4. Factors Associated with Discharge OME
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Florence, C.S.; Zhou, C.; Luo, F.; Xu, L. The Economic Burden of Prescription Opioid Overdose, Abuse, and Dependence in the United States, 2013. Med. Care 2016, 54, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Mattson, C.L.; Tanz, L.J.; Quinn, K.; Kariisa, M.; Patel, P.; Davis, N.L. Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths—United States, 2013–2019. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.; Kariisa, M.; Seth, P.; Smith, H.; Davis, N.L. Drug and Opioid-Involved Overdose Deaths—United States, 2017–2018. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Vadivelu, N.; Kai, A.M.; Kodumudi, V.; Sramcik, J.; Kaye, A.D. The Opioid Crisis: A Comprehensive Overview. Curr. Pain Headache Rep. 2018, 22, 16. [Google Scholar] [CrossRef]
- Flexman, A.M.; Ng, J.L.; Gelb, A.W. Acute and chronic pain following craniotomy. Curr. Opin. Anaesthesiol. 2010, 23, 551–557. [Google Scholar] [CrossRef]
- Kessler, E.R.; Shah, M.; Gruschkus, S.K.; Raju, A. Cost and Quality Implications of Opioid-Based Postsurgical Pain Control Using Administrative Claims Data from a Large Health System: Opioid-Related Adverse Events and Their Impact on Clinical and Economic Outcomes. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 383–391. [Google Scholar] [CrossRef]
- Dunbar, P.J.; Visco, E.; Lam, A.M. Craniotomy Procedures Are Associated with Less Analgesic Requirements than Other Surgical Procedures. Anesthesia Analg. 1999, 88, 335–340. [Google Scholar] [CrossRef]
- Rocha-Filho, P.; Gherpelli, J.; De Siqueira, J.; Rabello, G. Post-craniotomy headache: A proposed revision of IHS diagnostic criteria. Cephalalgia 2010. [Google Scholar] [CrossRef]
- Lutman, B.; Bloom, J.; Nussenblatt, B.; Romo, V. A Contemporary Perspective on the Management of Post-Craniotomy Headache and Pain. Curr. Pain Headache Rep. 2018, 22, 69. [Google Scholar] [CrossRef]
- Ribeiro, M.D.C.D.O.; Pereira, C.U.; Mc Sallum, A.; Martins-Filho, P.R.S.; DeSantana, J.M.; Nunes, M.D.S.; Hora, E.C. Immediate post-craniotomy headache. Cephalalgia 2013, 33, 897–905. [Google Scholar] [CrossRef]
- Shibata, Y.; Hatayama, T.; Matsuda, M.; Yamazaki, T.; Komatsu, Y.; Endo, K.; Akutsu, H. Epidemiology of post-suboccipital craniotomy headache: A multicentre retrospective study. J. Perioper. Pr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Garg, R.; Sheshadri, V.; Venkatraghavan, L.; Bergese, S.D.; Cappellani, R.B.; Schaller, B. Perioperative Factors Contributing the Post-Craniotomy Pain: A Synthesis of Concepts. Front. Med. 2017, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, A.; Berkow, L.C.; Stevens, R.D.; Mirski, M.; Thompson, R.E.; White, E.D.; Weingart, J.D.; Long, D.M.; Yaster, M. Prospective evaluation of pain and analgesic use following major elective intracranial surgery. J. Neurosurg. 2007, 106, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Nemergut, E.C.; Durieux, M.E.; Missaghi, N.B.; Himmelseher, S. Pain management after craniotomy. Best Pr. Res. Clin. Anaesthesiol. 2007, 21, 557–573. [Google Scholar] [CrossRef]
- Tanskanen, P.; Kyttä, J.; Randell, T. Patient-controlled analgesia with oxycodone in the treatment of postcraniotomy pain. Acta Anaesthesiol. Scand. 1999, 43, 42–45. [Google Scholar] [CrossRef]
- Verchére, E.; Grenier, B.; Mesli, A.; Siao, D.; Sesay, M.; Maurette, P. Postoperative Pain Management After Supratentorial Craniotomy. J. Neurosurg. Anesthesiol. 2002, 14, 96–101. [Google Scholar] [CrossRef]
- Galvin, I.M.; Levy, R.; Day, A.G.; Gilron, I. Pharmacological interventions for the prevention of acute postoperative pain in adults following brain surgery. Cochrane Database Syst. Rev. 2019, 2019, cd011931. [Google Scholar] [CrossRef]
- College Station, TX: StataCorp LLC. STATA Version 15, Stata Statistical Software. Available online: //www.stata.com/support/faqs/resources/citing-software-documentation-faqs/ (accessed on 3 November 2022).
- Rstudio. Available online: https://www.rstudio.com/ (accessed on 1 January 2020).
- Hah, J.M.; Bateman, B.T.; Ratliff, J.; Curtin, C.; Sun, E. Chronic Opioid Use After Surgery. Obstet. Anesthesia Dig. 2017, 125, 1733–1740. [Google Scholar] [CrossRef]
- Brandal, D.; Keller, M.S.; Lee, C.; Grogan, T.; Fujimoto, Y.; Gricourt, Y.; Yamada, T.; Rahman, S.; Hofer, I.; Kazanjian, K.; et al. Impact of Enhanced Recovery After Surgery and Opioid-Free Anesthesia on Opioid Prescriptions at Discharge From the Hospital. Obstet. Anesthesia Dig. 2017, 125, 1784–1792. [Google Scholar] [CrossRef]
- Maciel, C.B.; Barlow, B.; Lucke-Wold, B.; Gobinathan, A.; Abu-Mowis, Z.; Peethala, M.M.; Merck, L.H.; Aspide, R.; Dickinson, K.; Miao, G.; et al. Acute Headache Management for Patients with Subarachnoid Hemorrhage: An International Survey of Health Care Providers. Neurocritical Care 2022. [Google Scholar] [CrossRef]
- Wilson, B.R.; Grogan, T.R.; Schulman, N.J.; Kim, W.; Gabel, E.; Wang, A.C. Early Postoperative Opioid Requirement is Associated With Later Pain Control Needs After Supratentorial Craniotomies. J. Neurosurg. Anesthesiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- McCullough, I.L.; Shteamer, J.W.; Erwood, A.M.; Spektor, B.; Boorman, D.W.M.; Sharifpour, M.; Olson, J.J.; Papangelou, A. Opioid-Free Anesthesia for Craniotomy. J. Neurosurg. Anesthesiol. 2021, 35, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Sussman, J.; Calderon, E.; Ubl, D.S.; Croome, K.P.; Taner, C.B.; Heimbach, J.K.; Moss, A.; Habermann, E.B.; Mathur, A.K.; Bs, J.S.; et al. Variation in opioid prescribing patterns after abdominal transplant surgery. Clin. Transplant. 2021, 35, 14439. [Google Scholar] [CrossRef]
- Mohanty, S.B.; Shin, M.B.; Casper, D.; Saifi, C. Postoperative Prescription of Low-dose Narcotics Yields Equivalent Pain Outcomes Compared to High-dose Narcotics in Opioid-naïve Patients Undergoing Spine Surgery. Spine 2021, 46, 1748–1757. [Google Scholar] [CrossRef]
- Gardner, W.T.; MacDonald, D.R.W.; Kennedy, M.J.; Faulkner, A.C.; McIntyre, J.R.; Forget, P.; Aitken, S.A.; Stevenson, I.M.; on behalf of the SCORE Collaborative. Opioid Prescription Following Wrist and Ankle Fracture Fixation in Scotland—Tradition Prevails. J. Clin. Med. 2022, 11, 468. [Google Scholar] [CrossRef]
- Valencia, L.; Becerra, Á.; Ojeda, N.; Domínguez, A.; Prados, M.; González-Martín, J.M.; Rodríguez-Pérez, A. Effect of Preoperative Anxiety on Postoperative Pain after Craniotomy. J. Clin. Med. 2022, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Ayyawar, H.; Bharti, N.; Panda, N.; Chhabra, R. A Comparative Study of Opioid-free Anesthesia With Opioid-base Anesthesia for Recovery Characteristics and Postoperative Cognitive Dysfunction After Trans-sphenoidal Resection of Pituitary Tumors. J. Neurosurg. Anesthesiol. 2022, 35, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khanna, R.; Onyewuenyi, A.C.; Panos, N.; Breslin, R.; Sani, S. Efficacy of an opioid-sparing analgesic protocol in pain control after less invasive cranial neurosurgery. PAIN Rep. 2021, 6, e948. [Google Scholar] [CrossRef]
- Neville, I.S.; Ureña, F.M.; Quadros, D.G.; Solla, D.; Lima, M.F.; Simões, C.; Vicentin, E.; Ribeiro, U.; Amorim, R.L.O.; Paiva, W.; et al. Safety and costs analysis of early hospital discharge after brain tumour surgery: A pilot study. BMC Surg. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Venkatraghavan, L.; Bharadwaj, S.; Au, K.; Bernstein, M.; Manninen, P. Same-day discharge after craniotomy for supratentorial tumour surgery: A retrospective observational single-centre study Congé le jour même après craniotomie pour chirurgie sur tumeur sus-tentorielle: Étude observationnelle rétrospective d’un centre hospitalier. Can. J. Anaesth. 2016, 63, 1245–1257. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Iacopino, D.G.; Azzarello, G.; Gaggiotti, C.; Graziano, F.; Gulì, C.; Pino, M.A.; Maugeri, R. End-of-Life Care in High-Grade Glioma Patients. The Palliative and Supportive Perspective. Brain Sci. 2018, 8, 125. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 273) | |
|---|---|
| Age in years, Median [IQR] | 54.0 [41; 63] |
| Female Sex | 173 (63.4%) |
| ASA Class, Median [IQR] | 3 [2; 3] |
| Commercial Insurance | 144 (52.7%) |
| Type of Case | |
| Tumor resection | 174 (63.7%) |
| Posterior-fossa tumor | 77(44.3%) |
| Anterior/middle fossa skull base tumor | 75(43.1%) |
| Convexity/parasagittal tumor | 22(12.6%) |
| Vascular repair | 99 (36.3%) |
| Cerebral aneurysms | 47(47.5%) |
| Arterio-venous malformations/cavernous malformations | 30(30.3%) |
| Cerebral bypass procedures | 22(22.2%) |
| Location | |
| Infratentorial | 62 (22.7%) |
| Supratentorial | 211 (77.3%) |
| Race Category | |
| Non-White | 59 (21.6%) |
| White | 214 (78.4%) |
| Ethnicity Category | |
| Hispanic or Latino | 26 (9.5%) |
| Not Hispanic or Latino | 246 (90.1%) |
| Declined/Unknown | 1 (0.4%) |
| Language Category | |
| English | 249 (91.2%) |
| Non-English | 24 (8.8%) |
| History of Chronic Pain | 161 (59.0%) |
| History of a Depression/Anxiety Disorder | 123 (45.1%) |
| History of Substance Abuse | 60 (22.0%) |
| Preoperative Opioid Use | 23 (8.4%) |
| Preoperative OME (n = 23) | 55 [27; 64] |
| Length Of Surgical Procedure, Median [IQR] | 331.6 [257; 399] |
| Hospital LOS, Median [IQR] | 6.00 [4; 12] |
| No Opiate Prescribed | Opiates Prescribed | Univariable Analysis p-Value | Multivariable Analysis OR [95% CI] | Multivariable Analysis p-Value | |
|---|---|---|---|---|---|
| n = 77 | n = 196 | ||||
| Age in years | 54.3 (16.0) | 51.3 (15.4) | 0.158 | ||
| Sex | 0.079 | ||||
| Female | 42 (54.5%) | 131 (66.8%) | |||
| Male | 35 (45.5%) | 65 (33.2%) | |||
| ASA physical class | 0.671 | ||||
| 1 | 2 (2.60%) | 5 (2.55%) | |||
| 2 | 19 (24.7%) | 57 (29.1%) | |||
| 3 | 44 (57.1%) | 113 (57.7%) | |||
| 4 | 12 (15.6%) | 21 (10.7%) | |||
| Insurance carrier | 0.569 | ||||
| Commercial | 38 (49.4%) | 106 (54.1%) | |||
| Non-commercial | 39 (50.6%) | 90 (45.9%) | |||
| Type of surgical procedure | 0.471 | ||||
| Tumor | 46 (59.7%) | 128 (65.3%) | |||
| Vascular | 31 (40.3%) | 68 (34.7%) | |||
| Race | 1 | ||||
| Non-White | 16 (20.8%) | 42 (21.4%) | |||
| White | 61 (79.2%) | 154 (78.6%) | |||
| Ethnicity | 0.871 | ||||
| Declined/Unknown | 0 (0.00%) | 1 (0.51%) | |||
| Hispanic or Latino | 8 (10.4%) | 18 (9.18%) | |||
| Not Hispanic or Latino | 69 (89.6%) | 177 (90.3%) | |||
| Language | 0.771 | ||||
| English | 66 (85.7%) | 163 (83.2%) | |||
| Non-English | 6 (7.79%) | 15 (7.65%) | |||
| History of chronic pain: | 0.007 | ||||
| 0 | 42 (54.5%) | 70 (35.7%) | Ref | ||
| 1 | 35 (45.5%) | 126 (64.3%) | 1.87 [1.06,3.29] | 0.03 | |
| Psychiatry history: | 0.625 | ||||
| 0 | 40 (51.9%) | 110 (56.1%) | |||
| 1 | 37 (48.1%) | 86 (43.9%) | |||
| History of substance abuse: | 1 | ||||
| 0 | 60 (77.9%) | 153 (78.1%) | |||
| 1 | 17 (22.1%) | 43 (21.9%) | |||
| Preoperative opioid use | 0.12 (0.32) | 0.07 (0.26) | 0.33 | ||
| 0 | 68 (88.3%) | 182 (92.9%) | |||
| 1 | 9 (11.7%) | 14 (7.14%) | |||
| Preoperative OME | 55.0 (27.8) | 75.2 (124) | 0.637 | ||
| Length of Procedure | 341 (115) | 328 (102) | 0.38 | ||
| Intraoperative ketamine | 0.085 | ||||
| infusion | |||||
| 0 | 46 (59.7%) | 140 (71.4%) | |||
| 1 | 31 (40.3%) | 56 (28.6%) | |||
| Maximum hospital pain score | 7.25 (2.50) | 7.61 (1.92) | 0.197 | ||
| Total Hospital OME | 495 (1287) | 274 (348) | 0.103 | ||
| Hospital LOS | 15.6 (14.7) | 8.07 (7.76) | <0.001 | 0.97 [0.93,1] | 0.075 |
| Hospital LOS category | <0.001 | ||||
| 1–4 days | 8 (10.4%) | 64 (32.7%) | Ref | ||
| 5–12 days | 38 (49.4%) | 105 (53.6%) | 0.35 [0.15,0.8) | 0.013 | |
| >12 days | 31 (40.3%) | 27 (13.8%) | 0.12 [0.05,0.29] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-Y.; Shenoy, V.S.; Fong, C.T.; Walters, A.M.; Sekhar, L.; Curatolo, M.; Vavilala, M.S.; Lele, A.V. The Prevalence and Factors Associated with the Prescription of Opioids for Head/Neck Pain after Elective Craniotomy for Tumor Resection/Vascular Repair: A Retrospective Cohort Study. Medicina 2023, 59, 28. https://doi.org/10.3390/medicina59010028
Wang W-Y, Shenoy VS, Fong CT, Walters AM, Sekhar L, Curatolo M, Vavilala MS, Lele AV. The Prevalence and Factors Associated with the Prescription of Opioids for Head/Neck Pain after Elective Craniotomy for Tumor Resection/Vascular Repair: A Retrospective Cohort Study. Medicina. 2023; 59(1):28. https://doi.org/10.3390/medicina59010028
Chicago/Turabian StyleWang, Wei-Yun, Varadaraya Satyanarayan Shenoy, Christine T. Fong, Andrew M. Walters, Laligam Sekhar, Michele Curatolo, Monica S. Vavilala, and Abhijit V. Lele. 2023. "The Prevalence and Factors Associated with the Prescription of Opioids for Head/Neck Pain after Elective Craniotomy for Tumor Resection/Vascular Repair: A Retrospective Cohort Study" Medicina 59, no. 1: 28. https://doi.org/10.3390/medicina59010028
APA StyleWang, W.-Y., Shenoy, V. S., Fong, C. T., Walters, A. M., Sekhar, L., Curatolo, M., Vavilala, M. S., & Lele, A. V. (2023). The Prevalence and Factors Associated with the Prescription of Opioids for Head/Neck Pain after Elective Craniotomy for Tumor Resection/Vascular Repair: A Retrospective Cohort Study. Medicina, 59(1), 28. https://doi.org/10.3390/medicina59010028






