Knee Diameter and Cross-Sectional Area as Biomarkers for Cartilage Knee Degeneration on Magnetic Resonance Images
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design
2.3. Inclusion and Exclusion Criteria
2.4. Image Analysis
- (1)
- On the sagittal PD-weighted images, the patellar ligament and the max. pole to pole distance in the patella were measured to calculate the Insall-Salvati index (Figure 1).
- (2)
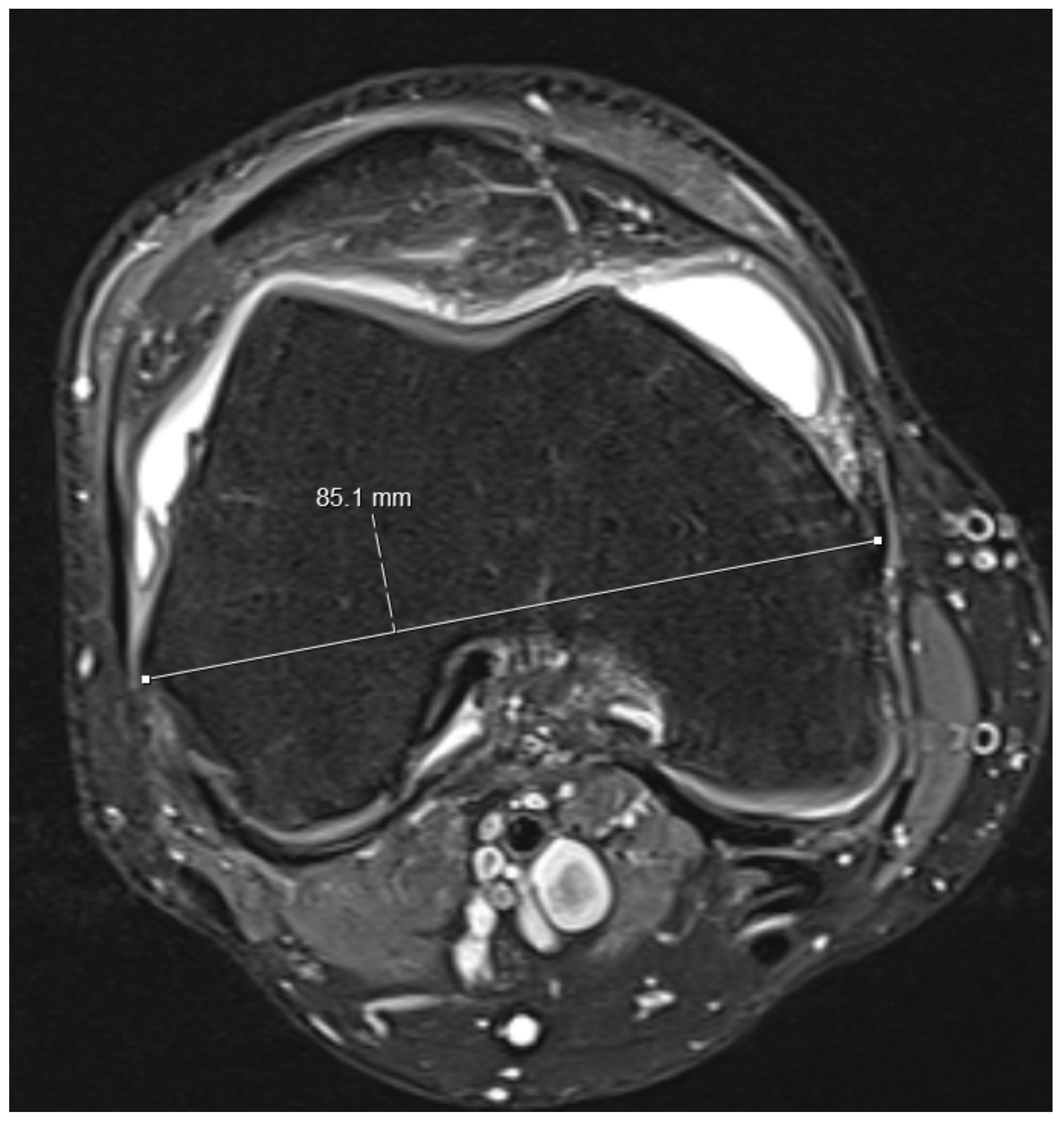
- Knee diameters were measured on axial PD-weighted images at the exact level of the patella upper pole (strictly antero-posterior = vertical, and medio-lateral = horizontal, independent of the leg position; Figure 2). The larger of these 2 diameters was taken as “maximal diameter”.
- (3)
- The whole knee cross-sectional area at the same level was automatically calculated by the Osirix software (Figure 2),
- (4)
- The largest axial diameter of the distal femur at the level of maximal condyle diameter was measured and used for normalization (Figure 3).
2.5. Statistical Analysis
3. Results
3.1. Demographics and Average Measurements
3.2. Logistic Regression for Binary Variables
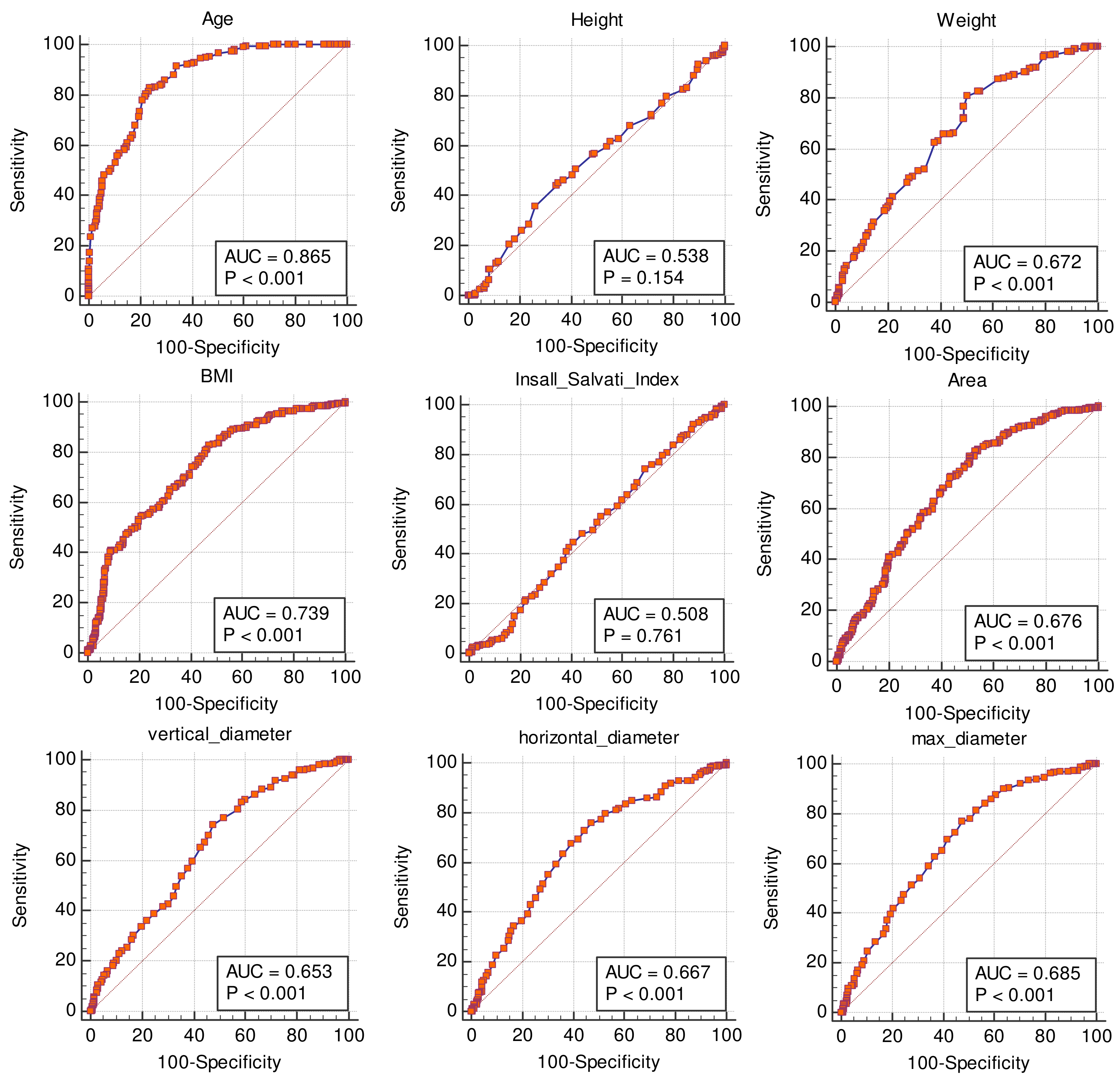
3.3. Receiver Operating Characteristic Curves (ROC) for Non-Binary Variables
3.4. Comparisons of ROC Curves
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.L.; Bae, W.C.; Chun, J.; Gratz, K.R.; Lotz, M.; Sah, R.L. Biomechanics of cartilage articulation: Effects of lubrication and degeneration on shear deformation. Arthritis Rheum. 2008, 58, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Axford, J.; Butt, A.; Heron, C.; Hammond, J.; Morgan, J.; Alavi, A.; Bolton, J.; Bland, M. Prevalence of anxiety and depression in osteoarthritis: Use of the Hospital Anxiety and Depression Scale as a screening tool. Clin. Rheumatol. 2010, 29, 1277–1283. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C. Body mass index and risk of knee osteoarthritis: Systematic review and meta-analysis of prospective studies. BMJ Open 2015, 5, e007568. [Google Scholar] [CrossRef]
- Hanna, F.S.; Teichtahl, A.J.; Wluka, A.E.; Wang, Y.; Urquhart, D.M.; English, D.R.; Giles, G.G.; Cicuttini, F.M. Women have increased rates of cartilage loss and progression of cartilage defects at the knee than men: A gender study of adults without clinical knee osteoarthritis. Menopause 2009, 16, 666–670. [Google Scholar] [CrossRef]
- Sieron, D.; Jablonska, I.; Lukoszek, D.; Szyluk, K.; Meusburger, H.; Delimpasis, G.; Kostrzewa, M.; Platzek, I.; Christe, A. Knee Diameter and Cross-Section Area Measurements in MRI as New Promising Methods of Chondromalacia Diagnosis-Pilot Study. Medicina 2022, 58, 1142. [Google Scholar] [CrossRef]
- Matada, M.S.; Holi, M.S.; Raman, R.; Jayaramu Suvarna, S.T. Visualization of Cartilage from Knee Joint Magnetic Resonance Images and Quantitative Assessment to Study the Effect of Age, Gender and Body Mass Index (BMI) in Progressive Osteoarthritis (OA). Curr. Med. Imaging Rev. 2019, 15, 565–572. [Google Scholar] [CrossRef]
- Go, D.J.; Kim, D.H.; Guermazi, A.; Crema, M.D.; Hunter, D.J.; Hwang, H.S.; Kim, H.A. Metabolic obesity and the risk of knee osteoarthritis progression in elderly community residents: A 3-year longitudinal cohort study. Int. J. Rheum. Dis. 2022, 25, 192–200. [Google Scholar] [CrossRef]
- Sacitharan, P.K.; Vincent, T.L. Cellular ageing mechanisms in osteoarthritis. Mamm. Genome 2016, 27, 421–429. [Google Scholar] [CrossRef]
- Blagojevic, M.; Jinks, C.; Jeffery, A.; Jordan, K.P. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthritis Cartilage 2010, 18, 24–33. [Google Scholar] [CrossRef]
- Georgiev, T.; Angelov, A.K. Modifiable risk factors in knee osteoarthritis: Treatment implications. Rheumatol. Int. 2019, 39, 1145–1157. [Google Scholar] [CrossRef]
- Martin, K.R.; Kuh, D.; Harris, T.B.; Guralnik, J.M.; Coggon, D.; Wills, A.K. Body mass index, occupational activity, and leisure-time physical activity: An exploration of risk factors and modifiers for knee osteoarthritis in the 1946 British birth cohort. BMC Musculoskelet. Disord. 2013, 14, 219. [Google Scholar] [CrossRef]
- Link, T.M.; Sell, C.A.; Masi, J.N.; Phan, C.; Newitt, D.; Lu, Y.; Steinbach, L.; Majumdar, S. 3.0 vs 1.5 T MRI in the detection of focal cartilage pathology--ROC analysis in an experimental model. Osteoarthritis Cartilage 2006, 14, 63–70. [Google Scholar] [CrossRef]
- Kuo, R.; Panchal, M.; Tanenbaum, L.; Crues, J.V., 3rd. 3.0 Tesla imaging of the musculoskeletal system. J. Magn. Reson. Imaging 2007, 25, 245–261. [Google Scholar] [CrossRef]
- Reed, M.E.; Villacis, D.C.; Hatch, G.F., 3rd; Burke, W.S.; Colletti, P.M.; Narvy, S.J.; Mirzayan, R.; Vangsness, C.T., Jr. 3.0-Tesla MRI and arthroscopy for assessment of knee articular cartilage lesions. Orthopedics 2013, 36, e1060–e1064. [Google Scholar] [CrossRef]
- Argentieri, E.C.; Burge, A.J.; Potter, H.G. Magnetic Resonance Imaging of Articular Cartilage within the Knee. J. Knee Surg. 2018, 31, 155–165. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhao, F.C. Comparison of 1.5- and 3.0-T magnetic resonance imaging for evaluating lesions of the knee: A systematic review and meta-analysis (PRISMA-compliant article). Medicine 2018, 97, e12401. [Google Scholar] [CrossRef]
- Kijowski, R.; Blankenbaker, D.G.; Davis, K.W.; Shinki, K.; Kaplan, L.D.; De Smet, A.A. Comparison of 1.5- and 3.0-T MR imaging for evaluating the articular cartilage of the knee joint. Radiology 2009, 250, 839–848. [Google Scholar] [CrossRef]
- Slattery, C.; Kweon, C.Y. Classifications in Brief: Outerbridge Classification of Chondral Lesions. Clin. Orthop. Relat. Res. 2018, 476, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, S.E.; Barawi, A.; Wang, H.; Roelofs, A.J.; Kaneva, M.; Guan, Z.; Lydon, H.; Thomas, B.L.; Thorup, A.S.; Fernandez, B.F.; et al. Agrin induces long-term osteochondral regeneration by supporting repair morphogenesis. Sci. Transl. Med. 2020, 12, aax9086. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Song, K.S.; Kim, J.R.; Lee, S.W. Retrospective evaluation of outcomes of bone peg fixation for osteochondral lesion of the talus. Bone Jt. J. 2020, 102, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, F.V.; van Sambeeck, J.D.P.; Olthuis, G.S.; van der Ree, J.; Koeter, S. Patellar height measurements: Insall-Salvati ratio is most reliable method. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.C.; Rhodes, J.A.; Shah, N.; Gaviola, G.C.; Gomoll, A.H.; Smith, S.E. Routine clinical knee MR reports: Comparison of diagnostic performance at 1.5 T and 3.0 T for assessment of the articular cartilage. Skeletal. Radiol. 2017, 46, 1487–1498. [Google Scholar] [CrossRef]
- Wong, S.; Steinbach, L.; Zhao, J.; Stehling, C.; Ma, C.B.; Link, T.M. Comparative study of imaging at 3.0 T versus 1.5 T of the knee. Skeletal. Radiol. 2009, 38, 761–769. [Google Scholar] [CrossRef]
- O’Connor, M.I. Sex Differences in Osteoarthritis of the Hip and Knee. J. Am. Acad. Orthop. Surg. 2007, 15, S22–S25. [Google Scholar] [CrossRef]
- Grotle, M.; Hagen, K.B.; Natvig, B.; Dahl, F.A.; Kvien, T.K. Obesity and osteoarthritis in knee, hip and/or hand: An epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet. Disord. 2008, 9, 132. [Google Scholar] [CrossRef]
- Murphy, L.; Schwartz, T.A.; Helmick, C.G.; Renner, J.B.; Tudor, G.; Koch, G.; Dragomir, A.; Kalsbeek, W.D.; Luta, G.; Jordan, J.M. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008, 59, 1207–1213. [Google Scholar] [CrossRef]
- Stevens, J.E.; Binder-Macleod, S.; Snyder-Mackler, L. Characterization of the human quadriceps muscle in active elders. Arch. Phys. Med. Rehabil. 2001, 82, 973–978. [Google Scholar] [CrossRef]
- Loeser, R.F. The Role of Aging in the Development of Osteoarthritis. Trans. Am. Clin. Climatol. Assoc. 2017, 128, 44–54. [Google Scholar]
- Temple-Wong, M.M.; Ren, S.; Quach, P.; Hansen, B.C.; Chen, A.C.; Hasegawa, A.; D’Lima, D.D.; Koziol, J.; Masuda, K.; Lotz, M.K.; et al. Hyaluronan concentration and size distribution in human knee synovial fluid: Variations with age and cartilage degeneration. Arthritis Res. Ther. 2016, 18, 18. [Google Scholar] [CrossRef]
- Kanthawang, T.; Bodden, J.; Joseph, G.B.; Lane, N.E.; Nevitt, M.; McCulloch, C.E.; Link, T.M. Obese and overweight individuals have greater knee synovial inflammation and associated structural and cartilage compositional degeneration: Data from the osteoarthritis initiative. Skeletal. Radiol. 2021, 50, 217–229. [Google Scholar] [CrossRef]
- Hubert, J.; Beil, F.T.; Rolvien, T.; Butscheidt, S.; Hischke, S.; Puschel, K.; Frosch, S.; Mussawy, H.; Ries, C.; Hawellek, T. Cartilage calcification is associated with histological degeneration of the knee joint: A highly prevalent, age-independent systemic process. Osteoarthritis Cartilage 2020, 28, 1351–1361. [Google Scholar] [CrossRef]
- Gao, Y.H.; Zhao, C.W.; Liu, B.; Dong, N.; Ding, L.; Li, Y.R.; Liu, J.G.; Feng, W.; Qi, X.; Jin, X.H. An update on the association between metabolic syndrome and osteoarthritis and on the potential role of leptin in osteoarthritis. Cytokine 2020, 129, 155043. [Google Scholar] [CrossRef]
- Pereira, D.; Severo, M.; Ramos, E.; Branco, J.; Santos, R.A.; Costa, L.; Lucas, R.; Barros, H. Potential role of age, sex, body mass index and pain to identify patients with knee osteoarthritis. Int. J. Rheum. Dis. 2017, 20, 190–198. [Google Scholar] [CrossRef]



| Grade | Macroscopy | MRI |
|---|---|---|
| Grade 0 | Normal cartilage | Normal cartilage |
| Grade 1 | Rough surface; chondral softening, focal thickening | Inhomogeneous; high signal; surface intact; cartilage swelling |
| Grade 2 | Irregular surface defects; <50% of cartilage thickness | Superficial ulceration, fissuring, fibrillation; <50% of cartilage thickness |
| Grade 3 | Loss of >50% cartilage thickness | Ulceration fissuring, fibrillation; >50% of depth of cartilage |
| Grade 4 | Cartilage loss | Full thickness chondral wear with exposure of subchondral bone |
| Gender (f:m) | 242:239 |
| Knee (right:left) | 241:240 |
| MRI (1.5T:3T) | 163:318 |
| N | Mean | Median | SD | 25–75 P | |
|---|---|---|---|---|---|
| age (years) | 481 | 45.33 | 45 | 21.66 | 27 to 62 |
| BMI (kg/m2) | 481 | 27.21 | 27 | 5.39 | 24 to 30 |
| weight (kg) | 481 | 79.32 | 80 | 17.78 | 69 to 90 |
| height (m) | 481 | 1.70 | 1.70 | 0.11 | 1.63 to 1.78 |
| knee cross-sectional area (cm2) | 481 | 131.63 | 128 | 30.21 | 111 to 146 |
| horizontal knee diameter (cm) | 481 | 13.42 | 13.5 | 1.64 | 12.4 to 14.4 |
| vertical knee diameter (cm) | 481 | 13.25 | 13.1 | 1.46 | 12.3 to 14.1 |
| maximal knee diameter (cm) | 481 | 13.78 | 13.7 | 1.43 | 12.8 to 14.7 |
| Insall Salvati index | 481 | 1.08 | 1.08 | 0.16 | 0.98 to 1.18 |
| maximal Outerbridge grade per knee | 481 | 2.28 | 3 | 1.55 | 1 to 4 |
| maximal lateral Outerbridge grade | 481 | 1.52 | 2 | 1.41 | 0 to 3 |
| maximal medial Outerbridge grade | 481 | 1.70 | 2 | 1.54 | 0 to 3 |
| maximal retropatellar Outerbridge grade | 481 | 1.89 | 2 | 1.46 | 0 to 3 |
| maximal femur diameter (cm) | 122 | 8.29 | 8.4 | 0.71 | 7.7 to 8.9 |
| normalized knee cross-sectional area | 122 | 15.47 | 14.36 | 4.29 | 13.0 to 16.5 |
| normalized maximal knee diameter | 122 | 0.89 | 0.894 | 0.12 | 0.82 to 0.97 |
| Variable | Odds Ratio | 95% CI | Coefficient | Std Error | p-Value |
|---|---|---|---|---|---|
| Gender = m | 0.768 | 0.53 to 1.10 | −0.26 | 0.18 | 0.152 |
| Knee side = right | 1.071 | 0.75 to 1.54 | 0.07 | 0.18 | 0.710 |
| MRI = 1.5 T | 0.833 | 0.57 to 1.22 | −0.18 | 0.19 | 0.348 |
| Constant | 0.25 | 0.21 | 0.241 |
| Variable | AUC | Std. Error | p-Value | Criterion | Sensitivity | 95% CI | Specificity | 95% CI |
|---|---|---|---|---|---|---|---|---|
| age | 0.865 | 0.02 | <0.0001 | >41 years | 82.8 | 77.5–87.3 | 76.4 | 70.4–81.6 |
| Height (m) | 0.538 | 0.03 | 0.1535 | ≤1.66 m | 45.1 | 38.7–51.6 | 65.0 | 58.5–71.0 |
| weight | 0.672 | 0.02 | <0.0001 | >72 Kg | 80.7 | 75.2–85.5 | 49.8 | 43.3–56.3 |
| BMI | 0.739 | 0.02 | <0.0001 | >24.9 | 82.8 | 77.5–87.3 | 52.7 | 46.2–59.2 |
| Insall-Salvati index | 0.508 | 0.03 | 0.7613 | >1.25 | 9.4 | 6.1–13.8 | 83.5 | 78.2–88.0 |
| AREA (cm2) | 0.676 | 0.02 | <0.0001 | >117.2 cm2 | 80.3 | 74.8–85.1 | 49.0 | 42.4–55.5 |
| vertical diameter (cm) | 0.653 | 0.02 | <0.0001 | >12.7 cm | 74.2 | 68.2–79.6 | 52.3 | 45.8–58.8 |
| horizontal diameter (cm) | 0.667 | 0.02 | <0.0001 | >13.1 cm | 73.0 | 66.9–78.4 | 55.7 | 49.1–62.1 |
| max diameter | 0.685 | 0.02 | <0.0001 | >13.3 | 77.1 | 71.3–82.2 | 52.3 | 45.8–58.8 |
| max diameter normalized | 0.724 | 0.05 | <0.0001 | >1.60 | 73.3 | 60.3–83.9 | 67.7 | 54.7–79.1 |
| normalized area | 0.767 | 0.04 | <0.0001 | >14.08 | 81.7 | 69.6–90.5 | 62.9 | 49.7–74.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primetis, E.; Drakopoulos, D.; Sieron, D.; Meusburger, H.; Szyluk, K.; Niemiec, P.; Obmann, V.C.; Peters, A.A.; Huber, A.T.; Ebner, L.; et al. Knee Diameter and Cross-Sectional Area as Biomarkers for Cartilage Knee Degeneration on Magnetic Resonance Images. Medicina 2023, 59, 27. https://doi.org/10.3390/medicina59010027
Primetis E, Drakopoulos D, Sieron D, Meusburger H, Szyluk K, Niemiec P, Obmann VC, Peters AA, Huber AT, Ebner L, et al. Knee Diameter and Cross-Sectional Area as Biomarkers for Cartilage Knee Degeneration on Magnetic Resonance Images. Medicina. 2023; 59(1):27. https://doi.org/10.3390/medicina59010027
Chicago/Turabian StylePrimetis, Elias, Dionysios Drakopoulos, Dominik Sieron, Hugo Meusburger, Karol Szyluk, Paweł Niemiec, Verena C. Obmann, Alan A. Peters, Adrian T. Huber, Lukas Ebner, and et al. 2023. "Knee Diameter and Cross-Sectional Area as Biomarkers for Cartilage Knee Degeneration on Magnetic Resonance Images" Medicina 59, no. 1: 27. https://doi.org/10.3390/medicina59010027
APA StylePrimetis, E., Drakopoulos, D., Sieron, D., Meusburger, H., Szyluk, K., Niemiec, P., Obmann, V. C., Peters, A. A., Huber, A. T., Ebner, L., Delimpasis, G., & Christe, A. (2023). Knee Diameter and Cross-Sectional Area as Biomarkers for Cartilage Knee Degeneration on Magnetic Resonance Images. Medicina, 59(1), 27. https://doi.org/10.3390/medicina59010027








