Hemorrhagic Cardiac Tamponade—An Unusual Threat in the COVID-19 Recovery Phase
Abstract
:1. Introduction
2. Case Series
2.1. Case 1
2.2. Case 2
2.3. Case 3
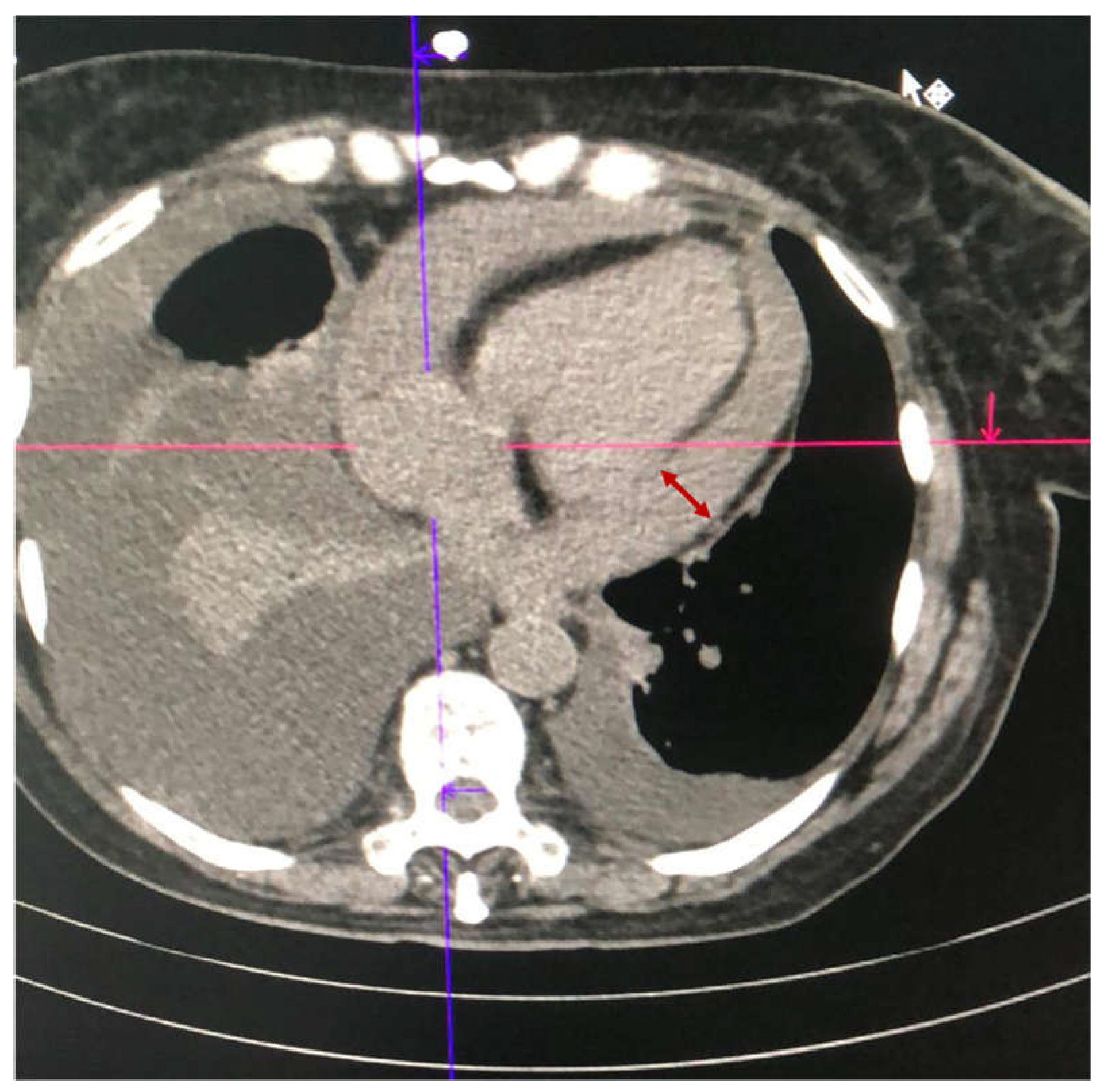
2.4. Case 4
3. Discussion
Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Laboratory Data on Admission | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Leucocytes (×103/μL) | 13.4 | 4.9 | 11.8 | 28.8 |
| Hemoglobin (g/dL) | 10.6 | 10.8 | 10.3 | 13.3 |
| Hematocrit (%) | 34.3 | 33.9 | 32.2 | 40.7 |
| Thrombocytes (×103/μL) | 229 | 258 | 363 | 304 |
| AST (U/L) | 27 | 22 | 45 | 93 |
| ALT (U/L) | 25 | 19 | 20 | 95 |
| Urea (mg/dL) | 120.9 | 23 | 31 | 111 |
| Creatinine (mg/dL) | 2.29 | 0.79 | 0.82 | 3.83 |
| Fibrinogen (U/L) | NA * | NA | 566 | 1492 |
| PCR (mg/L) | NA | 8.23 | 25 | 18 |
| Peak high-sensitive cardiac Troponin (ng/mL) | 23 | 18 | 52 | 110 |
| Albumin | NA | NA | 3.46 | 3.74 |
| INR | 2.41 | 1 | 0.97 | 0.8 |
| aPTT (s) | 25 | 27.3 | 34.5 | 30 |
| Biochemical Analysis | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| glucose (mg/dL) | 119 | 56 | 127 | 118 |
| LDH (U/L) | 92 | 682 | NA * | 557 |
| albumin (g/dL) | 1 | 2.13 | 3.01 | 2.81 |
| proteins (g/dL) cholesterol (mg/dL) | 2 50 | 3.47 117 | 4.88 NA | 4.55 59 |
| Bacterial detection | Absence of bacterial growth | Absence of bacterial growth | Absence of bacterial growth | Absence of bacterial growth |
| Mycobacterium tuberculosis PCR | absent | NA | NA | absent |
| Other PCR detection ** | absent | absent | NA | absent |
References
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Zaheer, S.; Kumar, N.; Singla, T.; Ranga, S. Covid19, beyond just the lungs: A review of multisystemic involvement by Covid19. Pathol. Res. Pract. 2021, 224, 153384. [Google Scholar] [CrossRef] [PubMed]
- Hakmi, H.; Sohail, A.; Brathwaite, C.; Ray, B.; Abrol, S. Cardiac tamponade in COVID-19 patients: Management and outcomes. J. Card. Surg. 2020, 35, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Furqan, M.M.; Verma, B.R.; Cremer, P.C.; Imazio, M.; Klein, A.L. Pericardial Diseases in COVID19: A Contemporary Review. Curr. Cardiol. Rep. 2021, 23, 90. [Google Scholar] [CrossRef]
- Carubbi, F.; Alunno, A.; Leone, S.; Di Gregorio, N.; Mancini, B.; Viscido, A.; Del Pinto, R.; Cicogna, S.; Grassi, D.; Ferri, C. Pericarditis after SARS-CoV-2 Infection: Another Pebble in the Mosaic of Long COVID? Viruses 2021, 13, 1997. [Google Scholar] [CrossRef]
- Kermani-Alghoraishi, M.; Pouramini, A.; Kafi, F.; Khosravi, A. Coronavirus Disease 2019 (COVID-19) and Severe Pericardial Effusion: From Pathogenesis to Management: A Case Report Based Systematic Review. Curr. Probl. Cardiol. 2022, 47, 100933. [Google Scholar] [CrossRef]
- Palmisano, A.; Gambardella, M.; D’Angelo, T.; Vignale, D.; Ascione, R.; Gatti, M.; Peretto, G.; Federico, F.; Shah, A.; Esposito, A. Advanced cardiac imaging in the spectrum of COVID-19 related cardiovascular involvement. Clin. Imaging 2022, 90, 78–89. [Google Scholar] [CrossRef]
- Ghantous, E.; Szekely, Y.; Lichter, Y.; Levi, E.; Taieb, P.; Banai, A.; Sapir, O.; Granot, Y.; Lupu, L.; Hochstadt, A.; et al. Pericardial Involvement in Patients Hospitalized With COVID-19: Prevalence, Associates, and Clinical Implications. J. Am. Heart Assoc. 2022, 11, e024363. [Google Scholar] [CrossRef]
- Diaz-Arocutipa, C.; Saucedo-Chinchay, J.; Imazio, M. Pericarditis in patients with COVID-19: A systematic review. J. Cardiovasc. Med. 2021, 22, 693–700. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.; Wu, F.; Guo, D.; Chen, L.; Fang, Z.; Li, C. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Investig. Radiol. 2020, 55, 327–331. [Google Scholar] [CrossRef]
- Derveni, V.; Kaniaris, E.; Toumpanakis, D.; Potamianou, E.; Ioannidou, I.; Theodoulou, D.; Kyriakoudi, A.; Kyriakopoulou, M.; Pontikis, K.; Daganou, M. Acute life-threatening cardiac tamponade in a mechanically ventilated patient with COVID-19 pneumonia. IDCases 2020, 21, e00898. [Google Scholar] [CrossRef] [PubMed]
- Sauer, F.; Dagrenat, C.; Couppie, P.; Jochum, G.; Leddet, P. Pericardial effusion in patients with COVID-19: Case series. Eur. Heart J. Case Rep. 2020, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Bassanelli, G. SARS-CoV-2 detection in the pericardial fluid of a patient with cardiac tamponade. Eur. J. Intern. Med. 2020, 76, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef] [PubMed]
- Carod-Artal, F.J. Post-COVID-19 syndrome: Epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev. Neurol. 2021, 72, 384–396. [Google Scholar] [CrossRef]
- Amenta, E.M.; Spallone, A.; Rodriguez-Barradas, M.C.; El Sahly, H.M.; Atmar, R.L.; Kulkarni, P.A. Postacute COVID-19: An Overview and Approach to Classification. Open Forum Infect. Dis. 2020, 7, ofaa509. [Google Scholar] [CrossRef]
- CERAVOLO, M.G.; ARIENTI, C.; de SIRE, A.; ANDRENELLI, E.; NEGRINI, F.; LAZZARINI, S.G.; PATRINI, M.; NEGRINI, S. Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 rapid living systematic review. Eur. J. Phys. Rehabil. Med. 2020, 56, 642–651. [Google Scholar] [CrossRef]
- Brito, D.; Meester, S.; Yanamala, N.; Patel, H.B.; Balcik, B.J.; Casaclang-Verzosa, G.; Seetharam, K.; Riveros, D.; Beto, R.J., 2nd; Balla, S.; et al. High Prevalence of Pericardial Involvement in College Student Athletes Recovering From COVID-19. JACC Cardiovasc. Imaging 2021, 14, 541–555. [Google Scholar] [CrossRef]
- Radovanovic, M.; Petrovic, M.; Barsoum, M.K.; Nordstrom, C.W.; Calvin, A.D.; Dumic, I.; Jevtic, D.; Hanna, R.D. Influenza Myopericarditis and Pericarditis: A Literature Review. J. Clin. Med. 2022, 11, 4123. [Google Scholar] [CrossRef]
- Gamaza-Chulián, S.; León-Jiménez, J.; Recuerda-Núñez, M.; Camacho-Freire, S.; Gutiérrez-Barrios, A.; Vargas-Machuca, J.C. Cardiac troponin-T in acute pericarditis. J. Cardiovasc. Med. 2014, 15, 68–72. [Google Scholar] [CrossRef]
- Kandah, F.; Dhruva, P.; Ruiz, J.; Martinez, A.; Kogler, W. Coxsackievirus B infection presenting as a hemorrhagic pericardial effusion causing tamponade. J. Geriatr. Cardiol. 2020, 17, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Hama Amin, B.J.; Kakamad, F.H.; Hamasaeed, A.G.; Salih, A.M.; Hamasaeed, M.G.; Ali, R.K.; Salih, B.K.H. Post COVID-19 hemorrhagic pericardial effusion; A case report with literature review. Ann. Med. Surg. 2022, 74, 103300. [Google Scholar] [CrossRef] [PubMed]
- Del Nonno, F.; Frustaci, A.; Verardo, R.; Chimenti, C.; Nicastri, E.; Antinori, A.; Petrosillo, N.; Lalle, E.; Agrati, C.; Ippolito, G. Virus-Negative Myopericarditis in Human Coronavirus Infection: Report From an Autopsy Series. Circ. Heart Fail. 2020, 13, E007636. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, R.; Cobilinschi, C.; Cotae, A.-M.; Darie, R.; Tincu, R.; Constantinescu, S.; Avram, O.; Moldovan, H.; Grintescu, I.M.; Mirea, L. Hemorrhagic Cardiac Tamponade—An Unusual Threat in the COVID-19 Recovery Phase. Medicina 2023, 59, 3. https://doi.org/10.3390/medicina59010003
Ungureanu R, Cobilinschi C, Cotae A-M, Darie R, Tincu R, Constantinescu S, Avram O, Moldovan H, Grintescu IM, Mirea L. Hemorrhagic Cardiac Tamponade—An Unusual Threat in the COVID-19 Recovery Phase. Medicina. 2023; 59(1):3. https://doi.org/10.3390/medicina59010003
Chicago/Turabian StyleUngureanu, Raluca, Cristian Cobilinschi, Ana-Maria Cotae, Raluca Darie, Radu Tincu, Sorin Constantinescu, Oana Avram, Horatiu Moldovan, Ioana Marina Grintescu, and Liliana Mirea. 2023. "Hemorrhagic Cardiac Tamponade—An Unusual Threat in the COVID-19 Recovery Phase" Medicina 59, no. 1: 3. https://doi.org/10.3390/medicina59010003
APA StyleUngureanu, R., Cobilinschi, C., Cotae, A.-M., Darie, R., Tincu, R., Constantinescu, S., Avram, O., Moldovan, H., Grintescu, I. M., & Mirea, L. (2023). Hemorrhagic Cardiac Tamponade—An Unusual Threat in the COVID-19 Recovery Phase. Medicina, 59(1), 3. https://doi.org/10.3390/medicina59010003








