Abstract
Cardiothoracic surgical critical care medicine (CT-CCM) is a medical discipline centered on the perioperative care of diverse groups of patients. With an aging demographic and an increase in burden of chronic diseases the utilization of cardiothoracic surgical critical care units is likely to escalate in the coming decades. Given these projections, it is important to assess the state of cardiothoracic surgical intensive care, to develop goals and objectives for the future, and to identify knowledge gaps in need of scientific inquiry. This two-part review concentrates on CT-CCM as its own subspeciality of critical care and cardiothoracic surgery and provides aspirational goals for its practitioners and scientists. In part one, a list of guiding principles and a call-to-action agenda geared towards growth and promotion of CT-CCM are offered. In part two, an evaluation of selected scientific data is performed, identifying gaps in CT-CCM knowledge, and recommending direction to future scientific endeavors.
1. Introduction
Cardiothoracic surgical critical care medicine (CT-CCM) is a unique subspecialty that links diverse clusters of medical diseases to their perioperative management. As the burden of diseases requiring surgical intervention increases over the coming decades, CT-CCM expansion will follow in step. Cardiovascular diseases (CVDs) will be the predominant drivers of this growth. CVD prevalence nearly doubled between 1990 and 2019 [1]. With an aging population and improving socioeconomic status of underdeveloped countries, these trends are projected to continue [2]. By 2030, coronary heart disease and heart failure in the U.S. are forecasted to increase in prevalence from 2010 levels by 16.6% and 25%, respectively [2]. Consequently, procedures such as coronary artery bypass grafting (CABG) and therapies for end-stage heart failure such as durable mechanical ventricular device implantations or heart transplantations are expected to escalate [3]. These estimates hold true even when expansion of interventional cardiology is considered [4,5,6,7].
Other pathologic processes are also likely to affect the growth of CT-CCM. Chronic lung diseases such as chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) are also on the rise [8,9]. Along with technological advances in lung preservation and extracorporeal support, this is likely to result in an increased number of intensive care admissions either after lung transplantation or extracorporeal membrane oxygenation (ECMO) cannulation for chronic respiratory failure [10,11]. Moreover, overall ECMO use is growing in adult populations, with the deployment of ECMO therapy for respiratory failure during the coronavirus disease 2019 (COVID-19) pandemic illustrating the unique expertise provided by CT-CCM intensivists [12]. With zoogenic spillovers of viruses becoming more likely with human encroachment on undeveloped wildlife habitats, the next global health emergency also has the potential to result in respiratory or cardiac sequela requiring mechanical organ support [13,14,15].
Consequently, an increase in patients requiring care in cardiothoracic surgical intensive care units (CT-ICUs) is likely, creating new stressors, placing pressure on current cardiothoracic surgical care systems. It will also force physician scientists to discover, innovate, and improve current therapies and treatments in order to address this growth. With these projections in mind, it is important to evaluate the current state of adult cardiothoracic surgical critical care science and to recognize missing data in the field in anticipation of the future rise in CT-ICU utilization. Initially, deficiencies, limitations, and barriers to advancement of the field need to be realized and addressed. Subsequently, knowledge gaps need to be appreciated and tackled.
In this two-part review, we focus on concepts fundamental to the evolution of CT-CCM and patient care. First, we provide a list of guiding principles, aspirational goals, and a call-to-action agenda needed to promote and advance CT-CCM as a medical science. In part two, we assess current scientific data relevant to CT-CCM populations, identify selected knowledge gaps, and offer recommendations on investigations needed to bridge those gaps.
2. Part 1—The Current State of Cardiothoracic Surgical Critical Care Medicine as a Medical Science
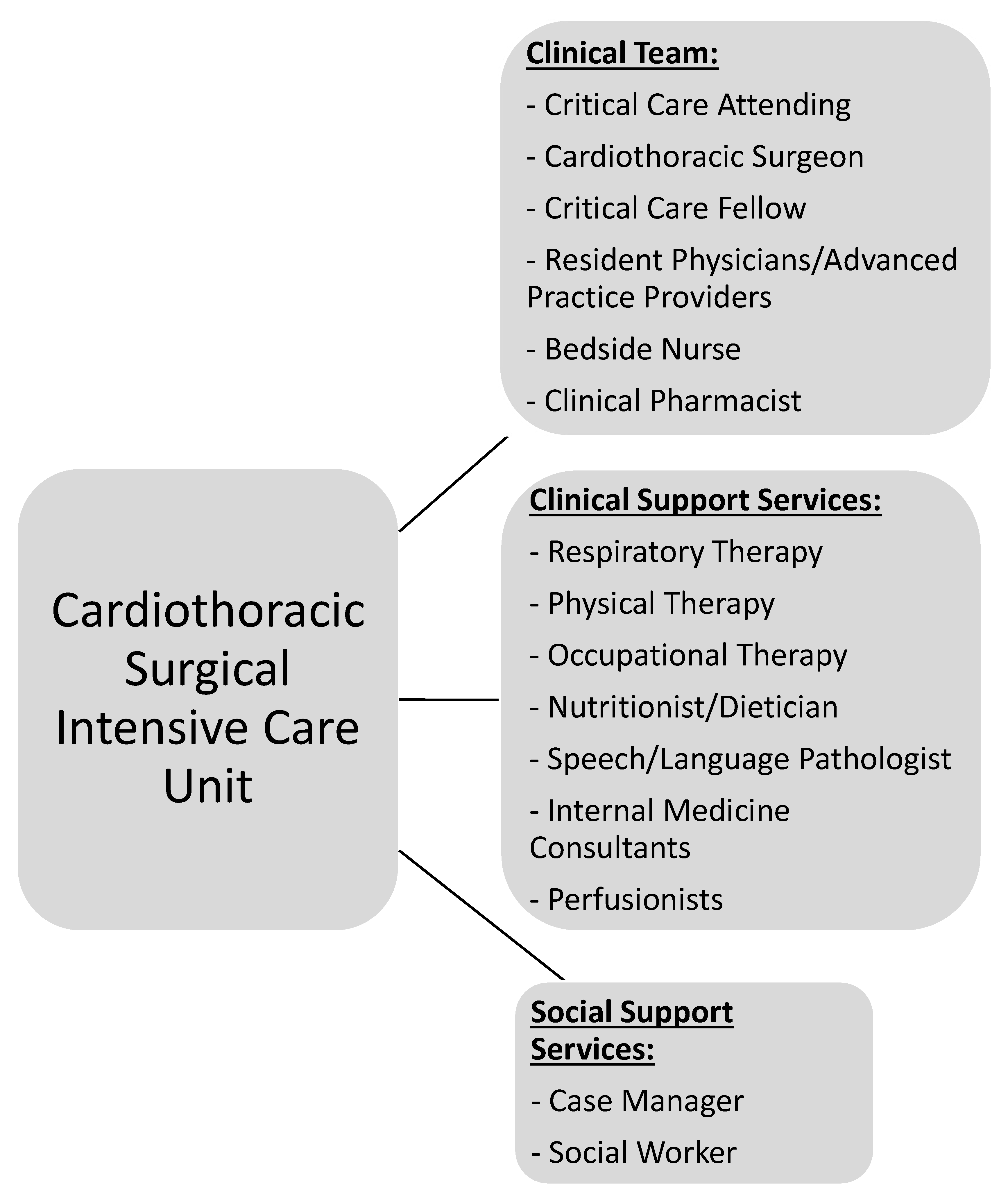
CT-CCM is a multidisciplinary endeavor composed of cardiothoracic surgeons, anesthesiologists, internists, pharmacists, perfusionists, and many more advanced level providers, nurses, and technicians (Figure 1) [2,16]. As such, the research pertaining to the CT-CCM patient populations is dispersed over many different professional societies and published in a variety of specialized journals. These include the American Thoracic Society, The Society of Thoracic Surgeons, Society of Critical Care Anesthesiologists, Society of Cardiovascular Anesthesiologists, American College of Chest Physicians, American Association for Thoracic Surgery, American Society for Artificial Internal Organs, Society of Critical Care Medicine (SCCM), and more, with each organization linked to an exclusive journal publication. Moreover, SCCM, heralded as a hub for all intensive care, seldomly embraces CT-CCM as a unique subset of critical care [17,18,19,20,21,22]. The resulting array of independent societies and the absence of a unified entity responsible for scientific oversight in the field creates compartmentalization of knowledge, hinderance to crosspollination of ideas, and deficiency in strategic planning. Additionally, it diminishes the visibility and advocacy for the field. Consequently, the current model is not conducive to coherent, systematic inquiry necessary for promotion and expansion of CT-CCM knowledge. Table 1 lists additional barriers to knowledge expansion in CT-CCM.

Figure 1.
Representation of the most common network of team members required to be involved in patient care and multidisciplinary rounds in the CT-ICU due to the complex nature of critical illness.

Table 1.
Barriers to Knowledge Expansion in Cardiothoracic Surgical Critical Care Medicine.
The status quo results in insufficient contribution of CT-ICU patients to the sample size of many landmark critical care trials, creating standards of practice that rely heavily on the application of data obtained from non-CT-ICU patient populations [23,24,25,26,27,28]. However, the pathophysiology and patient populations confronted in CT-CCM are unique and require exclusive scientific perspective. For example, determining the appropriate length of antibiotic therapy for a nosocomial pneumonia in patients with severe hypoperfusion related to postoperative heart failure or mechanical circulatory support (MCS) [29] cannot be answered by translating data from other ICU cohorts [30]. Pharmacokinetic features such as altered volume of distribution, or physiologic principles such as tissue penetrance with microcirculatory failure or continuous, versus pulsatile, blood flow, likely affect antibiotic length needed in CT-ICU populations [31,32,33,34,35,36,37]. These unique considerations need to be appreciated when developing standards of care and when designing clinical trials. Application of data from large, mixed population cohorts should be limited, and actively discouraged. However, these shortcomings create opportunities if a growth mindset is adopted; current knowledge gaps are a fertile ground for development of scientific investigations, improved clinical guidelines, and new career paths for medical professionals.
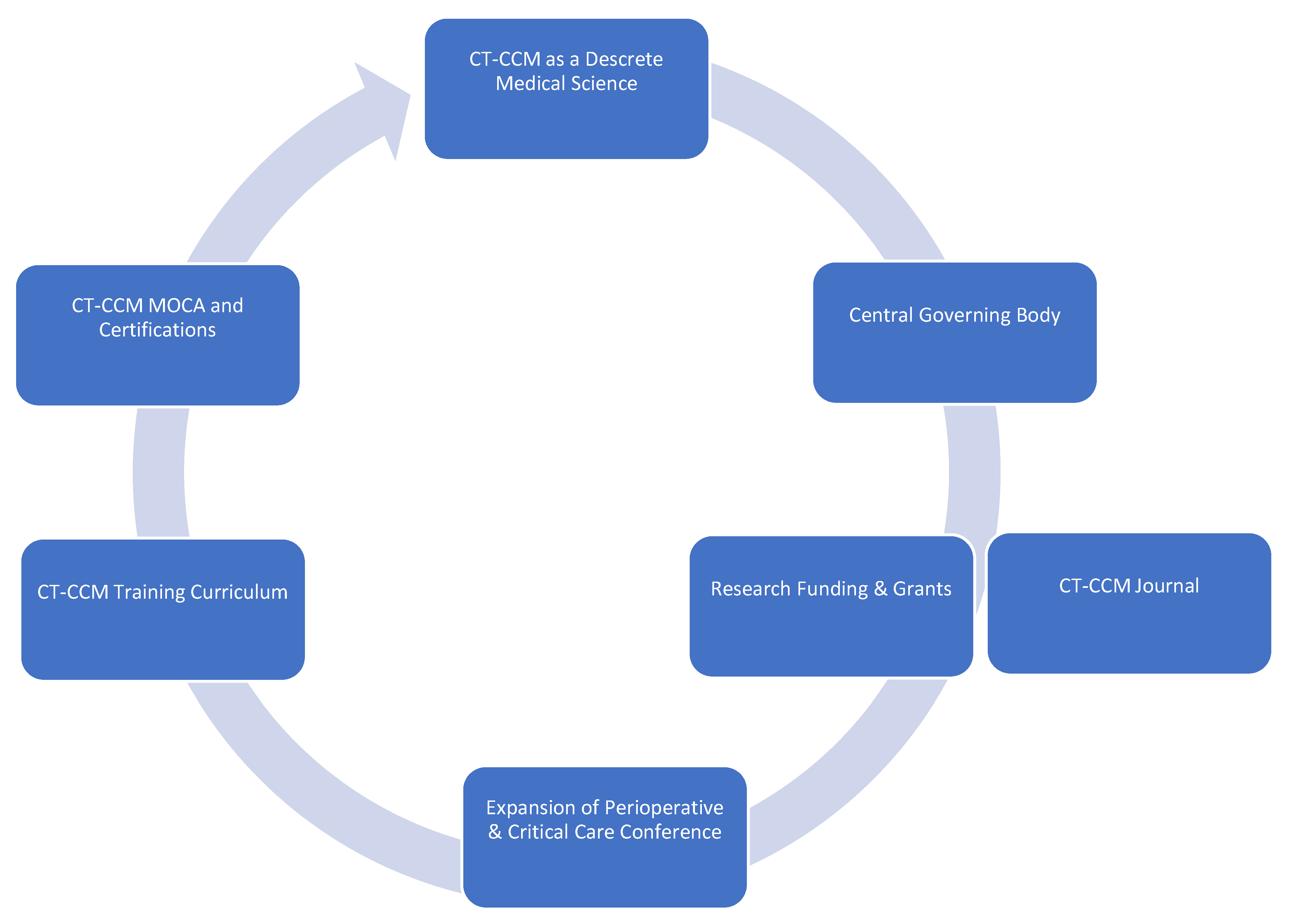
In order to cultivate CT-CCM as a unique science, the discipline requires establishment of foundational principles, as well as goals and objectives guiding future endeavors. Here, we propose major tenets and aspirational goals we find essential to the future of CT-CCM and its growth as a medical science, with major points summarized in Figure 2:

Figure 2.
Framework of major tenets and actionable items of Cardiothoracic Surgical Critical Care Medicine.
Major Tenets of Cardiothoracic Surgical Critical Care Medicine
- Cardiothoracic Surgical Critical Care Medicine is a discrete subspeciality of a medical science.
- CT-ICU patient populations are diverse and medically unique.
- Distinct investigations enlisting CT-ICU cohorts are required to answer basic scientific or clinical questions relating to these populations.
- Data acquired from general medical or surgical ICU studies may not provide evidence easily translatable to CT-CCM. Application of such information should be done with caution.
- Wide knowledge gaps exist in many areas of CT-CCM.
Call-to-Action Agenda
- Formation of a goal setting, centralized governing body, such as CT-CCM specific society.
- Establishment of a scientific journal centered on CT-CCM inquiry.
- Securement of funding and development of grant programs specifically geared towards CT-CCM research.
- Expansion of the Perioperative and Critical Care Conference co-sponsored by the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists to include other stakeholders, such as Society of Critical Care Medicine, the American Association for Thoracic Surgery, the Society of Critical Care Anesthesiologists, and the American Academy of Cardiovascular Perfusion, and more.
- Establishment of a standardized CT-CCM training curriculum, continuing education, and certification.
It is our belief that the development of guiding principles and objectives is necessary for further growth of CT-CCM, and most importantly, for improvement of patient care. Such ambitious trendsetting is advised when innovation is required. Less effort may result in repetition of old patterns, stifling progress. Hence, further expansion of the specialty necessitates bold initiatives. Certainly, inaction is not an option.
3. Part 2 – Selected Gaps in Knowledge and Future Direction of Research
3.1. General Framework and Summary of Important Publications
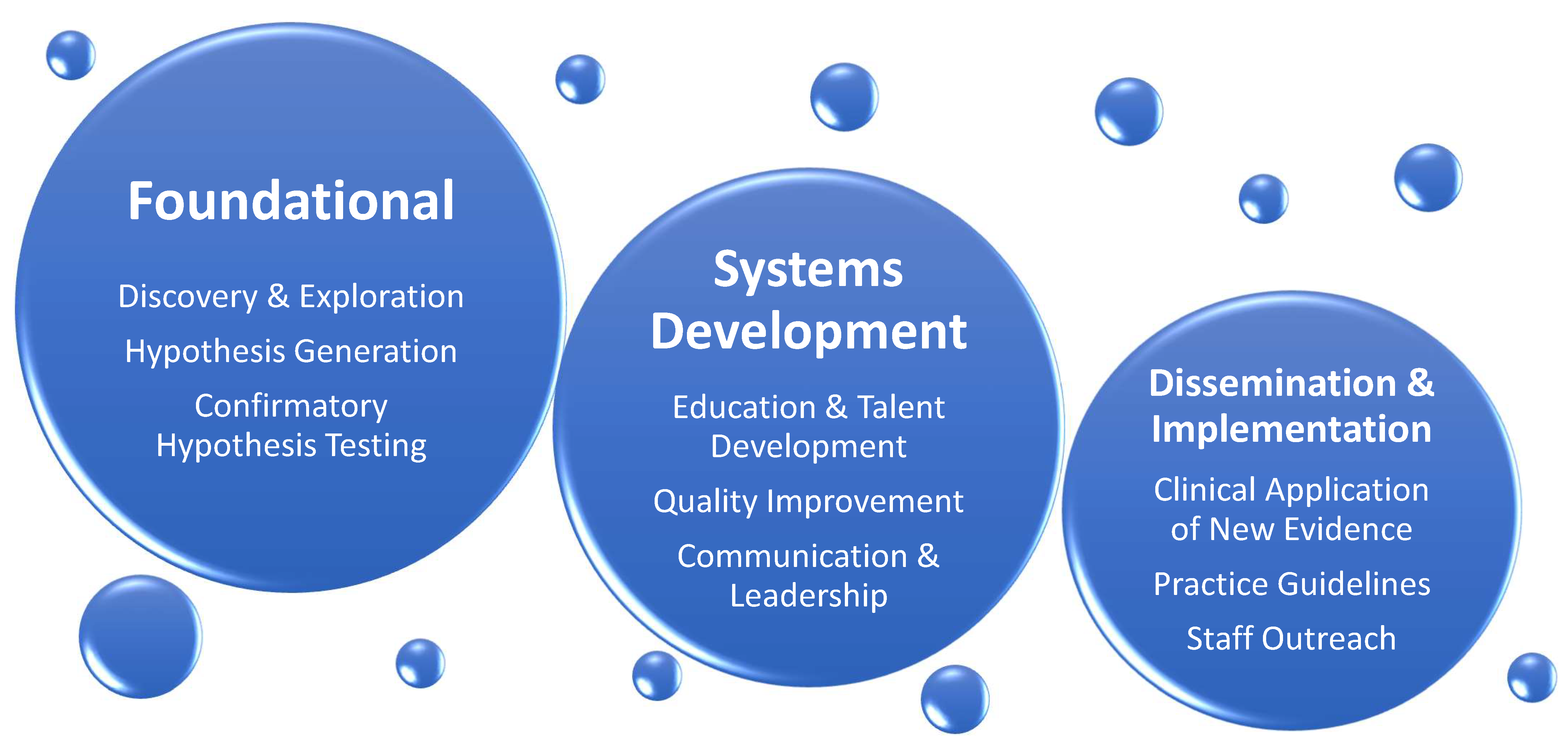
In part two of the review, we identify existing knowledge gaps affecting patients cared in cardiothoracic surgery intensive care unit (CT-ICU) and suggest a direction for further research in the field of CT-CCM. The list of topics is not exhaustive and intends to give sense of breadth and complexity of future work at hand. The discussion is divided into disorder-specific research considerations, followed by considerations for special populations. Figure 3 illustrates general framework of research needed to address CT-CCM knowledge gaps, and Table 2 lists and summarizes publications important to CT-CCM.

Figure 3.
General Categories of Research Needed to Advance Cardiothoracic Surgical Critical Care Medicine. The expansion of knowledge in the field will depend on strong foundational research concentrating on basic science, resulting in new discoveries, hypothesis generation, and confirmatory testing. Concomitantly, inquiry and investigation of educational, quality improvement, and communication and leadership initiatives must occur. Finally, foundational and systems development studies need to be followed by dissemination and implementation research, where knowledge obtained from other two categories can be translated into clinical practice.

Table 2.
List and Summary of Publications Important to Cardiothoracic Surgical Critical Care Medicine.
3.2. Disorder-Specific Considerations
3.2.1. Cardiac Surgery-Associated Acute Kidney Injury (CSA-AKI)
Acute kidney injury is the most common major complication occurring after cardiac surgery with incidence reaching 40% [76]. It remains a significant cause of morbidity and mortality even 10 years after surgery and complete renal function recovery [76,77]. Although heavily researched, more questions than answers remain, including mechanism of injury, prevention, and management [76,77,78].
The pathophysiology of CSA-AKI remains incompletely understood with mechanisms such as ischemia, hypoxemia, reperfusion injury, hypoperfusion, inflammation, neurohumoral activation, extracorporeal circulation, genetic predisposition, and others causally identified [77,78]. These associations have not been verified in animal models, as logistical and cost factors have prevented development of cardiopulmonary bypass (CBP) animal studies [76]. This calls for further scientific pressure to improve the understanding of the processes and mechanisms behind CSA-AKI; an investment into development of a CSA-AKI animal model is prudent.
Many preventative and therapeutic measures have been deployed to combat CSA-AKI. Oxygen delivery, avoidance of hypoperfusion, and cardiac output augmentation continue to be the backbone of CSA-AKI prevention based the on current understanding of pathophysiology [76,79]. For example, goal-directed perfusion with oxygen delivery index >270–300 mL/min/m2 has recently been shown to improve CSA-AKI, but not in patients with high risk for postoperative AKI [79,80,81,82,83,84]. Other maneuvers have also been investigated. The Cochrane review on remote ischemic preconditioning in cardiac and major vascular surgery resulted in moderate to high certainty of no efficacy [85]. Additionally, most pharmacologic strategies have not proven to be helpful in preventing CSA-AKI. Therapeutics such as mannitol, steroids, dopamine, fenoldopam, sodium bicarbonate, theophylline, statins, N-acetylcystine, clonidine and antioxidant supplements have all failed to show renal-protective benefits [76,78]. Similarly, the type of resuscitative fluids used and their effects on kidney health have been debated. The majority of investigations for balanced versus normal saline crystalloid use come from mixed ICU populations, making their application to CT-ICU problematic [86,87]. Moreover, the use of albumin in continues to be controversial, with a mixed bag of results [88]. To date, the most significant positive results on prevention come from PrevAKI trials, where adherence to the Kidney Disease: Improving Global Outcomes (KDIGO) bundle reduced the rate of CSA-AKI in a single center RTC, and rate of moderate and severe AKI in a multicenter trial; the bundle consisted of avoidance of nephrotoxins, optimization of glycemic control, and optimization of volume status and hemodynamics [89,90].
Similarly, the ideal time of initiation of renal replacement therapy (RRT) has been in question [76]. Aside from well-established, life-threatening RRT indications, a single-center ELAIN trial showed benefit of early RRT initiation as compared to delayed initiation in a cohort of nearly 50% of patients recovering from cardiac surgery [76,91]. A retrospective, multicenter observational study in cardiac surgery patients showed similar results [76]. However, due to design flaws and lack of CT-CCM specific RTCs, the answer to early versus delayed RRT remains elusive.
In summary, many questions remain unanswered regarding CSA-AKI. An intense effort is needed to elucidate the mechanisms of renal injury associated with cardiovascular surgery, effective preventative measures, and well-timed and successful therapies.
3.2.2. Delirium
Delirium is the most common neurologic complication encountered in the CT-ICU occurring in 7–50% of patients [92,93,94,95,96,97,98,99]. It is also a risk factor for long-term cognitive dysfunction, functional decline, and mortality, suggesting the continued necessity for research relating to this pathological state [93,94,97]. Further delineation of pathophysiology and mitigation of risk factors of delirium in CT-ICU patient populations are the two areas in need of urgent inquiry important for the development of preventative and therapeutic strategies.
Delirium is a syndrome with a common denominator of dysregulated neuronal function; however, systemic disturbances that produce this clinical entity are diverse [95,96,100]. Consequently, separate evaluation of pathophysiology of delirium in CT-CCM is required because of unique exposures such as profound hypothermia, non-pulsatile flow, or chronic hypoperfusion [96]. A tactic of creation of specific animal models is problematic due to difficulty of demonstrating the presence of delirium in experimental animals [95,96]. In addition, logistics and cost may be prohibitive. However, animal models with adequate face and construct validities would be helpful in identifying cellular and molecular changes [96]. Other innovative measures are also required to elucidate specific mechanisms behind delirium in CT-ICU populations. Further investigations into systems integration failure hypothesis, gut microbiome dysfunction, novel biomarker identification with biobank development, neuroimaging, and electrophysiology are warranted [100,101,102].
Additionally, a pragmatic research methodology is needed to address delirium as it relates to daily clinical practice. Tackling modifiable risk factors is most likely to yield clinically usable results. Focusing on patient-specific risks is especially important. Studies concentrating on optimizing preoperative frailty, physical conditioning, cognitive prehabilitation, nutritional status, hearing impairment, and chronic disease burden in CT-CCM populations are of utmost importance [93,95,99]. Cognitive prehabilitation is especially promising given encouraging results from non-cardiac surgery populations such as the Neurobics trial [103]. A recent feasibility trial of perioperative cognitive training in cardiac surgery established it as a viable target for further investigations [104]. Likewise, chronic disease burdens such as depression, arrhythmias, diabetes mellitus (DM), hypertension (HTN), stroke history, peripheral vascular disease, and obesity have been found to be statistically significant risk factors for post-CABG delirium and additional studies are needed to evaluate optimization of chronic diseases as a preventative measure [99].
Moreover, investigations of modifiable precipitating factors such as postoperative pain control and sedation are crucial. Both uncontrolled pain and excessive opioid administration are significantly related to delirium development [92,93,95,96]. Recent years flourished with new regional anesthetic techniques involving fascial spread of local anesthetics, allowing anesthesiologists to develop blocks covering previously inaccessible dermatomes [105]. Aggressive evaluations of erector spinae, serratus anterior, and transversus thoracis muscle plane blocks with randomized controlled trials (RTCs) in CT-CCM populations are needed to determine their effectiveness for optimal chest wall pain control [106].
Sedation choice is another modifiable risk factor needing further examination. Initial positive reports of dexmedetomidine in cardiac surgery have recently been eclipsed by strong RTCs questioning its benefit [94,98,107,108]. As a result, there continues to be no single sedative agent deemed helpful in delirium treatment or prevention in any realm of critical care. An application of volatile anesthetics for ICU sedation is on the horizon. Inhaled agents have been used for sedation with success in Europe for some time, and now, isoflurane will be evaluated in the U.S. in phase 3 clinical trials INSPiRE-ICU1&2 (NCT05327296) [109,110]. Theorized benefits include decreased opioid use, improved spontaneous breathing, shorter extubation times, and quicker wakeups [111]. INSPiRE-ICU 2 will also evaluate long-term cognitive outcomes. Pending results, CT-CCM cohorts will be another frontier for inhaled anesthetic inquiry. Finally, an ongoing study of lidocaine infusion for COVID-19 ARDS with secondary endpoints of delirium and opioid consumption may further aid clinicians in sedation selection and pain control (NCT04609865) [112].
In summary, investigational strategy into delirium in CT-CCM patient populations should follow a two-pronged response. Basic science research should address mechanisms of delirium in specific clinical scenarios, while pragmatic clinical studies should be designed to help mitigate risk factors of delirium.
3.2.3. Pharmacotherapy
Gaps in knowledge exist in CT-CCM in relation to disease-specific pharmacotherapy. With the appreciation of the magnitude of the missing data, we highlight just a few pieces of a puzzle in the following section.
Patients undergoing CABG are likely the most common patient population seen in the CT-ICU. Frequently, grafts used include saphenous-vein grafts and radial artery grafts. The RADIAL trial compared saphenous vein grafts to radial artery grafts for CABG and demonstrated a significantly lower rate of adverse cardiac events in patients who received radial grafts (hazard ratio 0.67; 95% CI. 0.49–0.90; p = 0.01) [113]. However, the radial artery is more muscular in nature, increasing concern regarding vasospasm leading to myocardial ischemia [114]. As a result, the RADIAL trial had six different regimens to manage to prevent arterial graft spasm that differed in agents (diltiazem, nifedipine, or amlodipine) and duration (6 weeks to indefinite) [113]. Consequently, the quest for antispasmodic medications to help prevent vasospasm is ongoing. Medications that have been studied include nitroglycerin, diltiazem, verapamil, papaverine, and milrinone [115]. However, many of the investigations are limited to single centers with small sample sizes. Due to the lack of conclusive evidence, the ideal agent and duration of its use remain in question [116].
Another area with a paucity of literature is optimal antibiotic management for delayed sternal closure (DSC). Although guidelines give recommendations on agent, dose, and duration of antibiotics for prevention of surgical site infections (SSI), there are no recommendations on antibiotic therapy if primary closure is not performed [117,118]. Feared complications of DSC are the deep sternal wound infection and mediastinitis. Thus, antibiotic prophylaxis is commonly continued, but the regimens and durations vary depending on institution [119,120]. Two recent trials showed that continued administration of prophylactic antibiotics in patients with DSC were not associated with benefits in rates of mediastinitis and deep tissue infections [120,121]. Both studies were limited by their retrospective nature, single center location, and small sample size. Additional studies are needed to determine if prolonged antimicrobial prophylaxis is needed and if so, what the optimal regimen in patients managed with DSC is.
An additional area that presents frequent clinical conundrums in the CT-ICU involves appropriate dosing of pharmacotherapy for patients on extracorporeal membrane oxygenation (ECMO). The available literature has revealed that patients on ECMO have an increased volume of distribution and elimination of certain medications [122]. Drugs with high lipophilicity or protein binding have demonstrated decreased blood concentrations, likely due to drug sequestration in the ECMO circuit, putting the patient at risk for clinical failure [123,124]. Most of the literature investigating optimal dosing are limited to ex vivo data or case reports and case series [122]. Due to the limitations of the literature, the provider is presented with the task of weighing the risk of an adverse drug event and the risk of clinical failure. Where this may be of most concern is the dosing of antibiotics for patients on ECMO as clinical failure could be detrimental. A recent publication comparing serum concentrations of various antibiotics in patients on ECMO versus medical therapy demonstrated a higher rate of failure to reach target concentrations of piperacillin (48.4% vs. 13.0%) and linezolid (34.8% vs. 15.0%) [125]. Previously, the medications that were lipophilic or highly protein-bound raised concern for therapeutic failure. However, neither piperacillin nor linezolid are particularly lipophilic with relatively low protein binding, making it less likely that sequestration in the ECMO circuit is responsible for failure to attain target concentrations. The Surviving Sepsis Campaign [126] recommends therapeutic drug monitoring (TDM) in critically ill patients due to alterations in volume of distribution and elimination. For patients on ECMO, TDM becomes imperative as the addition of ECMO adds another complexity to dosing considerations in an already critically ill patient. However, many institutions do not have the ability to provide TDM for many of these medications. Future studies should focus on the use of TDM for patients on ECMO and the effect of alternative dosing regimens in obtaining therapeutic goals.
In conclusion, significant knowledge gaps exist in relation to pharmactotherapy specific to CT-ICU patient populations. Provided examples serve as a sample of a work ahead. However, other important pharmaceutical concepts deserve to be accounted for in this review. These include:
- -
- Therapy for vasoplegia after CBP;
- -
- Vasopressor of choice for hypotension;
- -
- Inotrope of choice based on pathology;
- -
- Utility of a calcium sensitizer;
- -
- Antibiotic therapy duration for hospital-acquired infections in cardiogenic shock;
- -
- Pathology and mechanical support specific anticoagulation regimens and reversal agents;
- -
- Nalaxone and spinal cord protection;
- -
- Multimodal analgesics.
3.2.4. Transfusion and Blood Conservation
One in five cardiothoracic surgery patients will receive blood [127]. Unlike many fields, optimal red cell transfusion thresholds are relatively well-defined in CT surgery. For the general cardiac surgical population, a threshold of <7.5 g/dL is safe: a 5243-participant study of a <7.5 g/dL versus a <9.5 g/dL trigger for transfusion during and after moderate-to-high-risk cardiothoracic surgery found reduced transfusions and no evidence of harm in the restrictive group [128], with consistent outcomes at six months [129]. It is quite likely that a threshold of 7.0 is comparable to 7.5, but that has not been explicitly studied in large cardiac surgery trials. For non-surgical patients with active ischemia, a threshold between 7–8 g/dL is likely safe but not firmly established: a trial of 668 patients with anemia and myocardial infarction found that a transfusion trigger of <7 g/dL was noninferior to a trigger of 10 g/dL for major adverse cardiac events at 30 days—although the confidence interval for this result may include clinically significant harm [130]. The results of the 3500-patient Myocardial Ischemia and Transfusion (MINT) trial, expected to conclude in 2023, will likely clarify the safety of restrictive transfusions in patients with active myocardial ischemia [131]. Other blood products are not well-studied in the CT-ICU or any field. Triggers for plasma, cryoprecipitate, and platelet transfusion suggested by various cardiothoracic societies are reasonable but largely based on expert opinion or low-quality evidence [132]. Limited new data imply that the traditional emphasis on early platelet transfusion to overcome CPB-associated platelet dysfunction and consumption is misplaced and that fibrinogen repletion is more clinically effective [133,134]. Cold-stored human platelets may offer better efficacy and safety than traditional room-temperature-stored platelets, and the results from an ongoing trial in complex cardiac surgery are eagerly anticipated [135]. Alternatives to transfusion have also been studied: multiple meta-analyses of acute normovolemic hemodilution find that it reduces red blood cell transfusion by around 0.75 units per case and mildly reduces blood loss [136]. However, published trials are highly heterogeneous and the practice remains controversial [137]. Finally, factor concentrates may be reasonable alternatives to transfusion in cardiac surgery: a trial of 827 patients found that using fibrinogen concentrate was non-inferior to cryoprecipitate for post-CPB bleeding associated with hypofibrinogenemia [138], and similar studies are planned to compare 4-factor prothrombin complex concentrates with plasma [139].
Laboratory testing is a critical adjunct to transfusion practice in the CT-ICU. Guidelines from the Society of Cardiovascular Anesthesiologists and other organizations have placed significant emphasis of the benefits of laboratory-guided transfusion algorithms [132], which reduce transfusions and bleeding compared to physician judgement [140]. The relative benefits of specific coagulation testing platforms remain unproven and hotly debated—trials purporting to show the superiority of viscoelastic systems such as thromboelastography (TEG) over conventional coagulation tests have been confounded by the manner in which the tests were performed for the trial, e.g., comparing beside TEG to coagulation tests performed in a distant laboratory [141]. Well-run trials in which logistical confounders were eliminated have failed to find a relative benefit of one testing platform over another [142]. This suggests that testing algorithms should be designed to emphasize whatever platform is most pragmatic at the specific institution, without undue preference for a specific method. One exception to this may be in the case of a patient who has taken antiplatelet agents, where modified viscoelastic assays such as TEG Platelet Mapping may provide some useful information to guide timing and dosage of transfused platelets [143]. In the near future, rapid advances in point-of-care genomic and epigenetic testing may also be used to identify patients with differential responses to antiplatelet and anticoagulant medications, as well as responsiveness to transfusion therapy [144,145].
3.2.5. Paralysis after Aortic Aneurysm Surgery
Patients who receive surgical or endovascular treatment for thoracic aortic aneurysms and/or dissection are often in the CT-ICU for several days to weeks. A contributing factor for longer ICU stays in these patients are post-operative complications. One of the most devastating complications of either open surgical or endovascular repair of aortic aneurysms is spinal cord ischemia and/or paralysis. Although both treatment paradigms have a risk of postoperative paralysis, there are different mechanisms which govern the pathophysiology of open aortic surgery and thoracic endovascular aortic repair (TEVAR) mediated paralysis [146]. A recently published article demonstrated in a large animal model that these two mechanisms are different. Namely, the open surgical approach causes ischemic reperfusion injury versus critical permanent hypoperfusion in endovascular repair. A preventative therapeutic measure to treat these different phenomena will require an animal model that accurately maps the different pathophysiology of ischemic spinal cord injury in open and endovascular repair in humans. However, this new information needs to be confirmed in humans and other animal models.
In our small and large animal models, transient aortic clamping during open surgical repair has been shown to cause primarily grey matter damage via reactive oxygen species mediated blood–spinal cord barrier disruption and leakage of intracellular contents, causing central cord edema and neuronal death. TEVAR, on the other hand, has been shown to cause white matter damage likely due to chronic hypoperfusion of segmental arteries by the stent graft. CT-ICU management of these patients currently consists of spinal cord drains to reduce spinal cord edema and the usage of vasopressors to maintain systemic perfusion pressure. The treatment of paraplegia after spinal cord ischemia requires further mechanistic clarity and basic science research to develop a pharmacologic treatment to either prevent ischemic spinal cord injury perioperatively or reverse it postoperatively. Additionally, these treatments should be tailored to the specific patient, keeping in mind the procedure they underwent and the specific mechanism of spinal cord damage.
Which animal model best replicates the pathophysiology of paraplegia after open and endovascular repair? It is well known that the blood supply to the spinal cord varies across species, which raises the question of which model is the most appropriate to conduct basic science research [147]. Once the ideal animal model is developed to test therapeutics, there is another obstacle which researchers must consider, the mismatch which often occurs between the size of the lesion within the spinal cord observed on imaging and the functional symptoms of the lesion in the spinal cord. Simply put, lesions of similar size can result in different clinical outcomes. Termed the “neuroanatomical functional paradox”, this phenomenon is a persistent barrier to designing animal models of spinal cord injury [148]. This paradox makes it especially difficult to develop therapeutics, because certain regions of the spinal cord have higher levels of “eloquence” and have higher sensitivity to detect the effectiveness of treatment.
On the clinical side, currently the standard of care is to drain the cerebrospinal fluid (CSF) and to manage the systemic perfusion pressure, despite low evidence and small studies demonstrating the efficacy. Even the definition of high and low-risk is not well delineated. This is a hurdle for clinicians who aim to design randomized controlled trials, given the perceived benefit of the spinal drain. Additionally, the complication associated with the spinal drain is significant, requiring clinicians to weigh the risk of prophylactic spinal drains with its benefits. A solution to this problem is a randomized controlled trial which analyzes the efficacy of prophylactic spinal drains versus their complications.
Finally, there is a need for biorepositories using biological fluids (e.g., blood, CSF, urine) of patients who develop paralysis after open and endovascular aortic repair. The goal of such an endeavor will be to gain mechanistic clarity and allow researchers from many institutions to contribute to and acquire data from this repository. This can potentially fuel drug discovery targeting specific mechanisms which are involved in the pathogenesis of spinal cord injury and/or paraplegia after open and endovascular aortic repair.
There is a gap in the knowledge in the field of spinal cord perfusion and that addressing the following four points is of paramount importance:
- (1)
- What is the ideal small or large animal model for open and endovascular repair of aortic aneurysms, given the variety of anatomical blood supply to the spinal cord across species?
- (2)
- Given the neuro-radiological-anatomical functional paradox, therapeutic treatment for both disease paradigms is different and there is a need for more preclinical trials targeting the specific mechanism behind the grey- and white-matter lesions.
- (3)
- There is a need for randomized controlled trials testing the efficacy of spinal cord drains perioperatively to prevent paralysis.
- (4)
- There is a need for a repository containing the biological fluids of non-paralyzed patients as well as patients who develop paralysis after aortic interventions to gain mechanistic insight which will guide pharmaceutical discovery in this field.
3.2.6. Cardiac Surgical Unit—Advanced Life Support
Cardiac Surgical Unit—Advanced Life Support (CSU-ALS) was conceived as a more focused resuscitation protocol for the specific needs of post-sternotomy patients, as compared with standard ACLS. The impetus for the protocol was the recognition that external cardiac massage is best avoided or minimized in post-sternotomy patients as they are more exposed to injury from this method of artificial perfusion. In addition, the most common causes of cardiac arrest after cardiac surgery (malignant arrhythmias, bradycardia, cardiac tamponade, bleeding) are immediately reversible with appropriate recognition and treatment [149]. The goal of rapid re-sternotomy if initial resuscitation attempts fail is another hallmark of the protocol. The rationale for this is multifaceted. First, several common causes of arrest are either alleviated with re-sternotomy (cardiac tamponade) or more readily treatable (bleeding source recognition, epicardial pacing wire dislodgement, arrhythmias treatable by internal cardioversion). Second, the recognition of the superiority of internal cardiac massage to external massage with respect to perfusion pressures and rate of ROSC is another benefit of rapid re-sternotomy. Finally, the right ventricular injury from the posterior sternum during chest compressions has been described, calling for development of less injurious maneuvers [150,151].
The CSU-ALS protocol gained significant validation when a panel of experts from the Society for Thoracic Surgeons (STS) endorsed it in 2017 [149]. This prompted a wide-spread desire to implement CSU-ALS in cardiothoracic surgical ICUs world-wide. As a novel approach to resuscitation in this unique patient population, this presents significant opportunity for research and improvement on every aspect of the protocol. Given the prevalence of cardiac disease and need for surgical intervention via sternotomy, any improvements in this protocol have the potential to result in dramatic improvements in outcomes for a large patient population. In addition, with its relatively recent endorsement by the STS, there is a significant need for wider awareness and training in hospitals in the practice of the CSU-ALS protocol. Finally, strong scientific effort is necessary to further study CSU-ALS approach. Inviting American Heart Association to join future endeavors would help to achieve both, wider awareness, and research development.
4. Special Populations
4.1. Extracorporeal Membrane Oxygenation for Respiratory Failure
ECMO utilization for respiratory failure refractory to medical management has expanded [152,153,154], yet many questions remain regarding potential benefits, including appropriate implementation and management [155]. First, limited randomized work exists elucidating selection of patients with respiratory failure that may benefit from ECMO. Patient selection continues to focus on disease severity assessed by hypoxia, respiratory acidosis and mortality risk [156,157]. However, risks and benefits of both ECMO and conservative management are patient-specific [156,157]. Comparison of survival scores for ARDS with the use of ECMO (the RESP score [158]), and without ECMO (the Murray score [159]) can be insightful, yet additional considerations should include relative contraindications [156] and consideration of pre-morbid and active clinical characteristics. These include age, body mass index, disease comorbidities, right ventricular function, [160] ventilatory compliance, [161] clinical timing and more. Additional research is required to offer highly patient-specific guidance towards appropriate implementation of ECMO.
The appropriate timing of ECMO initiation also remains unclear and may impact outcomes. Early initiation may improve oxygen delivery while minimizing ventilator-induced lung injury (VILI). Later initiation, however, may offer time to respond to conventional therapy and avoidance of ECMO-specific risks. “Early” initiation of ECMO starts within 3–6 h of initiation of intensive therapy [156,157], and is advocated for by the Extracorporeal Life Support Organization (ELSO). Presently, this is supported by limited randomized [157,162] and observational work [163]. Nonetheless, well-designed and powered trials comparing modern-conservative management (traditional measures with later “rescue” ECMO) to early ECMO institution are required [162].
Conversely, the tail-end of acceptable ECMO initiation is generally considered 7–10 days, noting that VILI progression and/or other end-organ injury may limit potential benefits. Nonetheless, reports do suggest that later initiation may be acceptable as a rescue strategy [164]. A strict cutoff, therefore, is ill-defined and trials have poorly dictated the benefits, or lack thereof, of “late” ECMO initiation. More guidance is urgently needed, highlighted by the COVID-19 pandemic, where initial hypoxia with reasonable ventilatory compliance is common, but late deterioration and worsened ventilatory parameters may have prompted late consideration of ECMO [165].
Moreover, knowledge gaps in the appropriate management of ECMO exist. First, the ideal ventilatory strategy while on ECMO is yet to be defined. Certainly, adamant avoidance of VILI is critical in order to maintain benefits of ECMO utilization [166]. Evidence is needed to dictate optimal PEEP and FIO2 strategies, ventilatory approaches to minimizes self-induced lung injury and consideration for prone positioning [166,167]. Next, research is needed to define the optimal anticoagulant drug, dose, and monitoring strategy to prevent bleeding and thrombotic events for patients on ECMO [168,169,170,171]. Some patients may even benefit from the exclusion of anticoagulation altogether [172]. Next, selecting an optimal cannulation approach, including dual versus single cannulation strategies with or without right ventricle support, remain challenging [173,174]. These are further emphasized since some approaches could enable unique “wearable” membrane oxygenators, one potential future direction of ECMO support [175]. Other approaches, such as extracorporeal carbon dioxide removal (ECOR), offer some unique benefits in the face of significant weakness and remain without clear guidelines or consensus [176]. Even integration of dialysis circuits into ECMO remains actively debated [177].
Lastly, other practice guidelines and general critical care considerations must be applied to patients on ECMO. This includes strategies towards reducing nosocomial infections in the ICU, including unique considerations for patients on ECMO [178], and the ethics of end-of-life care while on significant organ support [179].
4.2. Extracorporeal Cardiopulmonary Resuscitation (E-CPR)
Another area that warrants expertise and experience to provide rapid deployment of an immense resource pool is E-CPR. Compared to conventional CPR (C-CPR), E-CPR can markedly improve cardiac output to normal or near-normal levels. E-CPR can therefore serve as an expedient and effective bridge to workup, intervention and/or decision that, compared to conventional CPR, may improve mortality and neurologic outcomes by limiting low-flow states related to C-CPR compression quality and ischemia-reperfusion injury (IRI) related to C-CPR pauses in compressions [180,181]. Furthermore, E-CPR, once initiated, can maintain blood flow without compression-induced chest and cardiothoracic trauma.
Importantly, patient benefits have yet to be fully elucidated; studies remain underpowered and have not shown convincing, statistically significant improvements in survival and neurologic outcomes [182]. This is critical as E-CPR requires extensive, rapid resource deployment with heavy infrastructure and cost considerations [183,184]. Furthermore, appropriate implementation considerations exist, including the approach to transport during C-CPR, appropriate timing of initiation after C-CPR begins [182,185], and initial anticoagulation strategies. The latter issue is particularly nuanced as approximately 50% of patients suffering from arrest where E-CPR is utilized meet disseminated intravascular coagulation criteria at the time of cannulation [186] yet many require intense anticoagulation to support percutaneous coronary intervention.
4.3. Enhanced Recovery after Surgery (ERAS)
ERAS protocols are multidisciplinary and multimodal initiatives designed to shorten a patient’s entire perioperative journey through reduction in complications and a faster return to normal activity [187,188]. Various surgical subspecialties have validated evidenced-based ERAS protocols. However, such consistent and reproducible protocols for cardiac surgery are lacking in the literature. There are a multitude of challenges and limitations in developing such protocols, with the most frequently cited being an understanding that cardiac surgery patients have more complex pathologies and comorbidities compared to other sub-specialty surgery populations and undergo more diverse and invasive surgical approaches. This recognized complexity led to the recent formation of the ERAS Cardiac Society, whose guidelines and manuscript summarize key elements from prior studies marked with the class of evidence and level of recommendation to advise future practice [189].
Despite the promising results of such emerging guidance, ERAS protocols are not only a matter of application but require a willingness to change and break with long-established patterns. Some of the new patterns identified within cardiac surgery involve the changing patient population as already previously identified. Using coronary artery bypass grafting (CABG) as an example, the mean age of patients undergoing such surgery has increased from 58.3 to 68.5 years, with 38 percent of patients being 70 years or older with an average ejection fraction of 35 percent [190]. Since the beginning of the century, observational, risk-adjusted, and propensity-matched studies have further documented an increased mortality after CABG in women compared with men for all age-adjusted groups [191]. Future ERAS protocols for cardiac surgery should therefore consider the specific needs of the growing elderly population and women as they traverse their perioperative journey to decrease their proven incurred risk of heightened morbidity and mortality.
The goal of cardiac surgery in elderly patients has been described as focused on the improvement of quality of life rather than in prolonging life expectancy [192]. Influencing variables for such a focus have been shown to be related to a patient’s individual pre-operative nutritional and functional status. Three specific areas of interest in ERAS protocols with only minimal to moderate quality evidence and research include preoperative measurement of albumin for risk stratification, preoperative correction of nutritional deficiency, and prehabilitation. Hypoalbuminemia, for which the elderly are already at risk, has been shown to be a prognostic indicator of increased time on a ventilator, length of hospital stay, infection, and recovery from acute kidney injury [187,193,194,195]. Additionally, there are currently no high-powered trials looking at the benefits of early nutritional therapy for elderly cardiac patients who are considered high risk, while ERAS protocols for colorectal surgery have shown benefits of pre-supplementation to include a reduction in prevalence of infection post-operatively [196]. Low albumin and other nutritional markers are associated with greater morbidity and mortality in cardiac surgery patients, but there is minimal quality support for their strong recommendation and application in ERAS protocols, with further knowledge gaps existing regarding their specific importance in the elderly population. Regarding prehabilitation, three non-cardiac surgery studies have demonstrated benefits in functional capacity and reduction in postoperative complications in the context of ERAS [197,198,199]. Still, such interventions prior to cardiac surgery have yet to be examined to enable advancement of this area of ERAS research as well.
An additional unique circumstance that can influence recovery after surgery in the elderly population involves caregivers. Caregivers of elderly patients have an expertise that stems from their lived experience with the daily impact of cardiac disease and resulting therapeutic burdens [200]. This places them with a strategic position to provide insights into the development of clinical practice guidelines. Therefore, it would be beneficial to include such perspectives in the development of ERAS protocols for elderly patients after cardiac surgery in the future.
Another vulnerable population undergoing cardiac surgery is women. Cardiovascular disease is the leading cause of morbidity and mortality for women in the United States and worldwide [201]. Differences in surgical outcomes between men and women are multifactorial. Frequent explanations in the literature as to why women suffer greater cardiac morbidity include that they often present more acutely, have a delayed diagnosis, or angiographically lack significant coronary artery disease early in their disease process [202]. Even despite this lower predisposition to develop visible atherosclerosis and aortopathy, women have been shown to be at least three times more likely to rupture or dissect a thoracic aneurysm [203]. A multicenter retrospective study showed that women had worse outcomes for both elective and emergent cardiac valve surgery with prolonged length of CT-ICU stay and higher rates of respiratory failure [204]. Additionally, in the well-known EXCEL trial, women who were older with more comorbidities at the time of revascularization had increased procedural and post-operative complications [205]. The increased risk for women undergoing cardiac surgery necessitates their specific inclusion and acknowledgement of specific risk in ERAS protocols. There has also been significant attention brought to referral, diagnostic, and research bias within the medical community with disastrous implications that are not limited to cardiac surgery [191]. In a multicenter review by the American College of Cardiology, there was continued underrepresentation of women in cardiovascular research ranging from basic science studies to difficulties in enrolling women in cardiac surgery trials [191]. Due to women’s increased risk and lack of adequate management data, sex-based ERAS protocols and algorithms should also be part of further research in cardiac surgery.
4.4. Lung Transplantation
Since the performance of the first lung transplant procedure over fifty years ago, continued advancements in the field have resulted in not only more transplants being performed but also in expanded opportunities for patients who may not have previously qualified. Indeed, even older patients with several comorbidities who would not have been candidates a decade ago are now receiving and benefiting from lung transplantation [206,207,208]. However, as their complexity has increased, there has been a comparable need to be able to manage these comorbidities, which in turn has required an increasing investment from critical care services. Not only has this challenged the intensivist, but also respiratory therapists, nutritionists, physical therapists, and others who contribute to the multi-disciplinary structure of the modern intensive care unit.
In an earlier era of lung transplantation, patients with advanced lung disease already committed to mechanical ventilation were universally deemed not to be candidates for transplant. Following some successes with offering transplant to younger patients, dependency on mechanical ventilation is no longer considered an absolute contraindication for the procedure [209,210,211]. Indeed, even more aggressive respiratory interventions such as ECMO can now be employed in support of potential lung transplant candidates. The latter circumstance has been exemplified during the recent SARS- CoV-2 pandemic when many patients requiring emergency ‘rescue’ lung transplant needed temporary ECMO support as a ‘bridge’ to that procedure [212,213,214]. Additionally, the initiation of ECMO using a dual lumen cannula placed via the internal jugular vein has allowed for selected candidates to receive needed physical therapy and ambulate prior to transplantation [215,216]. In addition to pulmonary support via ECMO, other end-organ support such as invasive RRT and ventricular assist devices may be utilized in candidates where dual organ transplantation is being considered (i.e., heart–lung or lung–kidney) [217,218,219,220]. As these therapies must be employed in the ICU setting, the modern intensivist must be facile with their use.
Once successful lung transplantation has occurred, recipients return to the ICU for immediate post-operative management where the challenges to the intensive care team continue. These include complex ventilator management, hemodynamic support and fluid resuscitation, the initiation of systemic immunosuppression with its potential to endow significant infections, and possibly the need for continued or additional mechanical organ support (i.e., cardiac and renal) [221,222,223]. Among these, appropriate ventilator management is particularly important as the goal is to liberate the recipient from this support as soon as is feasible. Heightened expertise in this regard is typically necessary as the lung allografts of new transplant recipients can be complicated by ischemia reperfusion injury (i.e., primary graft dysfunction), pleural fluid collections (including hemothorax), diaphragm dysfunction, stenosis of the vascular anastomoses, and complications of the bronchial surgical anastomoses [224,225,226,227,228,229]. Additionally, continued ECMO support may be necessary until the lung allograft(s) mature following the implantation surgery.
In some instances, recipients may have a prolonged need for post-surgical ventilator support, and in these situations the expertise of extended members of the ICU team becomes especially important. As many of these recipients may have entered the transplant surgery with some decrement in overall physical conditioning and reduced muscle mass, the ICU nutritionist must be particularly attentive to their early post-transplant nutritional needs. This is necessary to prevent further compromise of respiratory muscle mass and resulting dysfunction. To further assist recovering muscle function, appropriate physical therapy must also be initiated in the ICU setting, often with the recipient still requiring mechanical ventilation [230,231]. Once liberated from the ventilator, recipients must be assessed for any speech and swallowing difficulties; the latter being particularly important as aspiration of swallowed matter (food or medications) can adversely affect the new lung allograft(s).
Lastly, in addition to the critical care management of lung transplant candidates and recipients, the intensivist has recently assumed an increasing role in the management of potential organ donors. Indeed, studies have suggested that the continued involvement of an intensivist and delivery of critical care services following declaration of neurologic death typically results in an overall increase in the procurement of not only lungs suitable for transplantation, but other organs as well [232]. For the intensivist, this requires a change in the overall approach to the potential deceased organ donor by shifting treatment away from one that is designed to support cerebral perfusion to one that prioritizes individual organs. One example of this would be a move away from the use of hyperventilation and osmotic diuretics for the treatment of cerebral edema in favor of a more liberal fluid resuscitation strategy aimed at supporting renal and hepatic function. Furthermore, as there is a significant endocrinopathy associated with the onset of neurologic death, hormonal resuscitation of the potential donor (as can be provided by the intensivist) has been shown to also enhance the suitability of all organs for transplantation [233,234]. Recently, the Society of Critical Care Medicine has published guidelines for the management of potential organ donors and has encouraged continued involvement of intensivists in this endeavor [235].
5. Ethical Considerations
The critical care patients encountered in the CT-ICUs present a unique challenge that pushes the bounds of medical ethics in modern hospital systems. These specific populations are often admitted following surgical procedures and possess some of the highest morbidity and mortality encountered in medicine. This often creates ethical conundrums. First, patients frequently undergo these procedures without prior planning for the possibility of being unable to make future decisions on their own. Second, families compound undue burden and stress as they are often unaware of patient’s wishes [236]. Moreover, medical teams, frequently inexperienced in appropriate management of difficult social or end-of-life situations, promote physician-driven decisions [237,238]. Furthermore, publicly reported quality metrics such as 30-day mortality after general cardiothoracic surgery and 1-year mortality after transplantation can be at odds with patient/family goals [239,240,241]. Finally, timing of therapy cessation, defining “futility”, and withdrawal of care while on extensive mechanical support can also be encountered. These problems often result in significant ethical dilemmas, especially when continuation of therapies might not have been chosen by the patient in the first place.
Patient autonomy is of paramount importance; therefore, patients or their families have the right to refuse therapies at any point. This can be incredibly painful to the team who has invested time, effort, and care into the case. However, the concept of reciprocal obligation of the patient to continue treatment until deemed futile by the medical team after the health system has invested significant resources for their care is paternalistic. Ultimately, in a perfect world without limits on the availability of medical care, we would fully support patients to the extent that they desire, everyone would always be capable of expressing their wishes, and all patients would have an educated family with excellent coping mechanisms to handle end of life decisions making, with all practitioners endlessly skilled in communicating with every family. Unfortunately, this world does not exist, and medical teams should act in accordance with patients’ wishes, maintaining their autonomy.
When working through philosophical and ethical problems, a consensus decision between the patient, support persons, and care team is the best and the most practical answer. One solution is for medical teams to add conflict management to their clinical skills during medical training. Active commitment to learning communication and conflict resolution, along with an ethical lens for our clinical care, can benefit practitioners, patients, and medical systems [242,243]. Finally, increasing the utilization of palliative medicine specialists can aid both families and medical teams significantly [244,245].
In summary, further scientific work is needed to guide education of future physicians in communication skills, conflict resolution, and clinical application of ethical principles. Additionally, clinically active care teams should investigate how investment of time and effort to train their members in these areas benefits their patients with quality improvement projects. Finally, we recommend a development of close relationships with palliative medicine colleagues and encourage their frequent involvement. Research is needed to further elucidate the value of palliative team involvement with all patients coming through CT-ICUs.
6. Conclusions
The demand for cardiothoracic surgical critical care will continue to expand as the burden of chronic diseases increases and the population ages. In result, utilization of CT-ICUs will escalate. This calls for reevaluation of care provided in these ICUs in preparation for the surge. Given the diversity of disciplines practicing CT-CCM, expertise specific to the field is dispersed over many different specialties, societies, and journals. Resultant compartmentalization of scientific inquiry without centralized governing body hinders growth, innovation, and patient care. Significant knowledge gaps exist in CT-CCM with significant portion of current CT-ICU care standards relying on data obtained from medical or general surgical patient cohorts. To improve the current model, CT-CCM needs to be recognized as a its own subspecialty and science. Additionally, subspeciality specific society, a journal publication, comprehensive annual meeting of all the stakeholders, and unified research agenda are necessary to promote its expansion. The future of cardiothoracic surgical critical care depends on realization of its distinction, unification of its professionals, realization of its knowledge gaps, and initiation of research required to answer the most pressing questions.
Author Contributions
R.K.: conceived the paper, designed the manuscript, designed the figures and tables, performed the literature search on delirium and CS-AKI, wrote Delirium and CS-AKI sections of the manuscript, revised the Ethical Considerations, revised the manuscript drafts, and prepared it for final submission. J.L.: designed the manuscript, reviewed the literature, and wrote the CALS section of the manuscript. B.J.K.: designed the manuscript, reviewed the literature, designed Figure 1, and wrote the ERAS section of the manuscript. M.T.L.: designed the manuscript, designed Figure 2, reviewed the literature, and wrote the ECLS section of the manuscript, revised the first draft of the manuscript, and commented on the manuscript idea. A.S.H.: reviewed the literature and wrote the Transfusion Medicine section of the manuscript. D.R.N.: reviewed the literature, and wrote the Lung Transplantation section of the manuscript. A.H.: helped write the Paralysis After Aortic Aneurysm Surgery section of the manuscript. A.T.: designed the manuscript, reviewed the literature, and wrote the Ethical Considerations section of the manuscript. A.R.: designed the manuscript, reviewed the literature, and wrote the Pharmacotherapy section of the manuscript. H.A.: designed the manuscript, reviewed the literature, and wrote the Paralysis After Aortic Aneurysm Surgery section of the manuscript. A.M.B.: reviewed the final draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Institutional Review Board Statement
Not a clinical trial and no subjects, hence, no IRB necessary. Not applicable.
Informed Consent Statement
Not applicable. No human subjects.
Data Availability Statement
No data sets, or research data. Not applicable.
Acknowledgments
We thank Ravi Tripathi for divisional support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Moffatt-Bruce, S.; Crestanello, J.; Way, D.P.; Williams, T.E., Jr. Providing cardiothoracic services in 2035: Signs of trouble ahead. J. Thorac. Cardiovasc. Surg. 2018, 155, 824–829. [Google Scholar] [CrossRef] [PubMed]
- John, A.S.; Jackson, J.L.; Moons, P.; Uzark, K.; Mackie, A.S.; Timmins, S.; Lopez, K.N.; Kovacs, A.H.; Gurvitz, M.; American Heart Association Adults With congenital Heart Disease Committee of the Council on Lifelong congenital Heart, D; et al. Advances in Managing Transition to Adulthood for Adolescents With Congenital Heart Disease: A Practical Approach to Transition Program Design: A Scientific Statement From the American Heart Association. J. Am. Heart Assoc. 2022, 11, e025278. [Google Scholar] [CrossRef]
- Kilic, A. The future of left ventricular assist devices. J. Thorac. Dis. 2015, 7, 2188–2193. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Tang, G.H.L.; Nguyen, S.; Forcillo, J.; George, I.; Kaneko, T.; Thourani, V.H.; Bavaria, J.E.; Cheung, A.W.; Reardon, M.J.; et al. The train has left: Can surgeons still get a ticket to treat structural heart disease? J. Thorac. Cardiovasc. Surg. 2019, 157, 2369–2376 e2362. [Google Scholar] [CrossRef]
- Welt, F.G.P. CABG versus PCI-End of the Debate? N. Engl. J. Med. 2022, 386, 185–187. [Google Scholar] [CrossRef]
- Khakban, A.; Sin, D.D.; FitzGerald, J.M.; McManus, B.M.; Ng, R.; Hollander, Z.; Sadatsafavi, M. The projected epidemic of chronic obstructive pulmonary disease hospitalizations over the next 15 years. A population-based perspective. Am. J. Respir. Crit. Care Med. 2017, 195, 287–291. [Google Scholar] [CrossRef]
- Sauleda, J.; Núñez, B.; Sala, E.; Soriano, J.B. Idiopathic pulmonary fibrosis: Epidemiology, natural history, phenotypes. Med. Sci. 2018, 6, 110. [Google Scholar] [CrossRef]
- Nathan, S.D. The future of lung transplantation. Chest 2015, 147, 309–316. [Google Scholar] [CrossRef]
- Azzi, M.; Aboab, J.; Alviset, S.; Ushmorova, D.; Ferreira, L.; Ioos, V.; Memain, N.; Issoufaly, T.; Lermuzeaux, M.; Laine, L.; et al. Extracorporeal CO2 removal in acute exacerbation of COPD unresponsive to non-invasive ventilation. BMJ Open Respir. Res. 2021, 8, e001089. [Google Scholar] [CrossRef] [PubMed]
- Sauer, C.M.; Yuh, D.D.; Bonde, P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015, 61, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Tollefson, J. Why deforestation and extinctions make pandemics more likely. Nature 2020, 584, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Sleepwalking into the next pandemic. Nat. Med. 2022, 28, 1325. [CrossRef]
- Vetter, T.R. The Next Viral Pandemic: Not a Question of If, But When and How. Anesthesia Analg. 2022, 135, 900–902. [Google Scholar] [CrossRef]
- Katz, N.M. The emerging specialty of cardiothoracic surgical critical care: The leadership role of cardiothoracic surgeons on the multidisciplinary team. J. Thorac. Cardiovasc. Surg. 2007, 134, 1109–1111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buchman, T.G. A Celebration of the Society of Critical Care Medicine at the Half-Century Mark. Crit. Care Med. 2021, 49, 167–168. [Google Scholar] [CrossRef]
- Bion, J.; Brown, C.; Gomersall, C.; Boulanger, C.; Isherwood, P.; Schulman, D. Society of Critical Care Medicine 50th Anniversary Review Series: Critical Care Education. Crit. Care Med. 2021, 49, 1241–1253. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Wunsch, H.; Rowan, K.; Singer, M.; Rubenfeld, G.D.; Angus, D.C. Reflections on critical care’s past, present, and future. Crit. Care Med. 2021, 49, 1855–1865. [Google Scholar] [CrossRef]
- Biban, P.; Marlow, N.; Te Pas, A.B.; Fanaroff, A.A.; Jobe, A.H. Advances in Neonatal Critical Care: Pushing at the Boundaries and Connecting to Long-Term Outcomes. Crit. Care Med. 2021, 49, 2003–2016. [Google Scholar] [CrossRef]
- Chang, C.W.; Provencio, J.J.; Shah, S. Neurological Critical Care: The Evolution of Cerebrovascular Critical Care. Crit. Care Med. 2021, 49, 881–900. [Google Scholar] [CrossRef] [PubMed]
- Checchia, P.A.; Brown, K.L.; Wernovsky, G.; Penny, D.J.; Bronicki, R.A. The evolution of pediatric cardiac critical care. Crit. Care Med. 2021, 49, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Pro, C.I.; Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Tan, J.C.; Harvey, S.E.; Bell, D.; et al. Protocolised Management In Sepsis (ProMISe): A multicentre randomised controlled trial of the clinical effectiveness and cost-effectiveness of early, goal-directed, protocolised resuscitation for emerging septic shock. Health Technol. Assess 2015, 19, 1–150. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Pun, B.T.; Herr, D.L.; Maze, M.; Girard, T.D.; Miller, R.R.; Shintani, A.K.; Thompson, J.L.; Jackson, J.C.; Deppen, S.A.; et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA 2007, 298, 2644–2653. [Google Scholar] [CrossRef]
- Moss, M.; Ulysse, C.A.; Angus, D.C.; National Heart, L.; Blood Institute, P.C.T.N. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. Reply. N. Engl. J. Med. 2019, 381, 787–788. [Google Scholar] [CrossRef]
- Finfer, S.; Micallef, S.; Hammond, N.; Navarra, L.; Bellomo, R.; Billot, L.; Delaney, A.; Gallagher, M.; Gattas, D.; Li, Q.; et al. Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults. N. Engl. J Med 2022, 386, 815–826. [Google Scholar] [CrossRef]
- Chastre, J.; Wolff, M.; Fagon, J.Y.; Chevret, S.; Thomas, F.; Wermert, D.; Clementi, E.; Gonzalez, J.; Jusserand, D.; Asfar, P.; et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: A randomized trial. JAMA 2003, 290, 2588–2598. [Google Scholar] [CrossRef]
- Veiga, R.P.; Paiva, J.-A. Pharmacokinetics–pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 1–34. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Raffaeli, G.; Pokorna, P.; Allegaert, K.; Mosca, F.; Cavallaro, G.; Wildschut, E.D.; Tibboel, D. Drug disposition and pharmacotherapy in neonatal ECMO: From fragmented data to integrated knowledge. Front. Pediatr. 2019, 7, 360. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Bellomo, R.; Cotta, M.O.; Koch, B.C.; Lyster, H.; Ostermann, M.; Roger, C.; Shekar, K.; Watt, K.; Abdul-Aziz, M.H. Machines that help machines to help patients: Optimising antimicrobial dosing in patients receiving extracorporeal membrane oxygenation and renal replacement therapy using dosing software. Intensive Care Med. 2022, 48, 1338–1351. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.A.; Sieg, A.C. Evaluation of altered drug pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, E.J.; McDonnell, B.J. The unique physiology of left ventricular assist device patients–keep your finger on the pulse! Exp. Physiol. 2020, 105, 747–748. [Google Scholar] [CrossRef]
- Hall, S.F.; Athans, V.; Wanek, M.R.; Wang, L.; Estep, J.D.; Williams, B. Evaluation of a hospital-wide vancomycin-dosing nomogram in patients with continuous-flow left ventricular assist devices. Int. J. Artif. Organs 2021, 44, 411–417. [Google Scholar] [CrossRef]
- Jennings, D.L.; Makowski, C.T.; Chambers, R.M.; Lanfear, D.E. Dosing of vancomycin in patients with continuous-flow left ventricular assist devices: A clinical pharmacokinetic analysis. Int. J. Artif. Organs. 2014, 37, 270–274. [Google Scholar] [CrossRef]
- Purohit, S.N.; Cornwell III, W.K.; Pal, J.D.; Lindenfeld, J.; Ambardekar, A.V. Living without a pulse: The vascular implications of continuous-flow left ventricular assist devices. Circ. Heart Fail. 2018, 11, e004670. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J. Pro: Cardiothoracic anesthesiologists should run postcardiac surgical intensive care units. J. Cardiothorac. Vasc. Anesth. 2004, 18, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J. Con: Cardiothoracic anesthesiologists should not run postcardiac surgical intensive care units. J. Cardiothorac. Vasc. Anesth. 2004, 18, 525–526. [Google Scholar] [CrossRef]
- Katz, N.M. The evolution of cardiothoracic critical care. J. Thorac. Cardiovasc. Surg. 2011, 141, 3–6. [Google Scholar] [CrossRef][Green Version]
- Whitson, B.A.; D’Cunha, J. The thoracic surgical intensivist: The best critical care doctor for our thoracic surgical patients. In Seminars in Thoracic and Cardiovascular Surgery; WB Saunders: Philadelphia, PA, USA, 2011; pp. 12–13. [Google Scholar]
- Sherif, H.M. Cardiothoracic surgical critical care: Principles, goals and direction. Int. J. Surg. 2012, 3, 111–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sherif, H.M. Developing a curriculum for cardiothoracic surgical critical care: Impetus and goals. J. Thorac. Cardiovasc. Surg. 2012, 143, 804–808. [Google Scholar] [CrossRef][Green Version]
- Katz, N.M. It is time for certification in cardiothoracic critical care. J. Thorac. Cardiovasc. Surg. 2013, 145, 1446–1447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Calhoon, J.H.; Shemin, R.J.; Allen, M.S.; Baumgartner, W.A. The American board of thoracic surgery: Update. Ann. Thorac. Surg. 2013, 95, 1517–1519. [Google Scholar] [CrossRef]
- Baumgartner, W.A.; Calhoon, J.H.; Shemin, R.J.; Allen, M.S. Critical care: American Board of Thoracic Surgery update. J. Thorac. Cardiovasc. Surg. 2013, 145, 1448–1449. [Google Scholar] [CrossRef] [PubMed]
- Sherif, H.M.; Cohn, L.H. Certification in cardiothoracic surgical critical care. J. Thorac. Cardiovasc. Surg. 2014, 147, 1454–1455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katz, N.M. Meeting the expanded challenges of the cardiothoracic intensive care unit. J. Thorac. Cardiovasc. Surg. 2015, 150, 777–778. [Google Scholar] [CrossRef][Green Version]
- Mehta, Y. Is cardiac anaesthesiologist the best person to look after cardiac critical care? Ann. Card. Anaesth. 2015, 18, 4. [Google Scholar] [CrossRef]
- Sherif, H.M. Cardiothoracic surgical critical care certification: A future of distinction. J. Thorac. Cardiovasc. Surg. 2016, 152, 34–36. [Google Scholar] [CrossRef]
- Sherif, H.M. Cardiothoracic surgical critical care surgeons: Many of the few. J. Thorac. Cardiovasc. Surg. 2016, 152, 642–643. [Google Scholar] [CrossRef]
- Andersen, N.D. Certification in cardiothoracic surgical critical care: A distinction for some or for all? J Thorac Cardiovasc Surg 2016, 152, 37–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whitson, B.A. Cardiothoracic surgical critical care is critical to cardiothoracic surgery. J. Thorac. Cardiovasc. Surg. 2016, 152, 938–939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chan, E.G.; D’Cunha, J. Redefining our cardiothoracic surgical intensive care units: Change is good. J. Thorac. Cardiovasc. Surg. 2016, 152, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.C.; Ivascu, N.S.; Pearl, R.G. Cardiothoracic Critical Care: A New Specialty. ASA Monit. 2017, 81, 28–30. [Google Scholar]
- Bartels, K.; Dieleman, S.J. Cardiothoracic Anesthesia and Critical Care: An Ever-Changing (and Evolving) Field. Anesthesiol. Clin. 2019, 37, xv–xvii. [Google Scholar] [CrossRef]
- Shelton, K.T.; Wiener-Kronish, J.P. Evolving role of anesthesiology intensivists in cardiothoracic critical care. Anesthesiology 2020, 133, 1120–1126. [Google Scholar] [CrossRef]
- Savino, J.S.; Hanson, C.W., 3rd; Gardner, T.J. Cardiothoracic intensive care: Operation and administration. Semin. Thorac. Cardiovasc. Surg. 2000, 12, 362–370. [Google Scholar] [CrossRef]
- Stamou, S.C.; Camp, S.L.; Stiegel, R.M.; Reames, M.K.; Skipper, E.; Watts, L.T.; Nussbaum, M.; Robicsek, F.; Lobdell, K.W. Quality improvement program decreases mortality after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2008, 136, 494–499 e498. [Google Scholar] [CrossRef]
- Stamou, S.C.; Camp, S.L.; Reames, M.K.; Skipper, E.; Stiegel, R.M.; Nussbaum, M.; Geller, R.; Robicsek, F.; Lobdell, K.W. Continuous quality improvement program and major morbidity after cardiac surgery. Am. J. Cardiol. 2008, 102, 772–777. [Google Scholar] [CrossRef]
- Camp, S.L.; Stamou, S.C.; Stiegel, R.M.; Reames, M.K.; Skipper, E.R.; Madjarov, J.; Velardo, B.; Geller, H.; Nussbaum, M.; Geller, R.; et al. Quality improvement program increases early tracheal extubation rate and decreases pulmonary complications and resource utilization after cardiac surgery. J. Card. Surg. 2009, 24, 414–423. [Google Scholar] [CrossRef]
- Whitman, G.J.; Haddad, M.; Hirose, H.; Allen, J.G.; Lusardi, M.; Murphy, M.A. Cardiothoracic surgeon management of postoperative cardiac critical care. Arch. Surg. 2011, 146, 1253–1260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, K.; Singal, R.; Manji, R.A.; Zarychanski, R.; Bell, D.D.; Freed, D.H.; Arora, R.C.; Group, C.H.R.i.M.I. The benefits of 24/7 in-house intensivist coverage for prolonged-stay cardiac surgery patients. J. Thorac. Cardiovasc. Surg. 2014, 148, 290–297. e296. [Google Scholar] [CrossRef][Green Version]
- Benoit, M.A.; Bagshaw, S.M.; Norris, C.M.; Zibdawi, M.; Chin, W.D.; Ross, D.B.; van Diepen, S. Postoperative Complications and Outcomes Associated With a Transition to 24/7 Intensivist Management of Cardiac Surgery Patients. Crit. Care Med. 2017, 45, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Huard, P.; Kalavrouziotis, D.; Lipes, J.; Simon, M.; Tardif, M.-A.; Blackburn, S.; Langevin, S.; Sia, Y.T.; Mohammadi, S. Does the full-time presence of an intensivist lead to better outcomes in the cardiac surgical intensive care unit? J. Thorac. Cardiovasc. Surg. 2020, 159, 1363–1375. e1367. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Kang, P.J.; Kim, J.B.; Jung, S.H.; Choo, S.J.; Chung, C.H.; Lee, J.W. Influence of a high-intensity staffing model in a cardiac surgery intensive care unit on postoperative clinical outcomes. J. Thorac. Cardiovasc. Surg. 2020, 159, 1382–1389. [Google Scholar] [CrossRef]
- Arora, R.C.; Chatterjee, S.; Shake, J.G.; Hirose, H.; Engelman, D.T.; Rabin, J.; Firstenberg, M.; Moosdorf, R.G.; Geller, C.M.; Hiebert, B. Survey of contemporary cardiac surgery intensive care unit models in the United States. Ann. Thorac. Surg. 2020, 109, 702–710. [Google Scholar] [CrossRef]
- Lee, L.S.; Clark, A.J.; Namburi, N.; Naum, C.C.; Timsina, L.R.; Corvera, J.S.; Beckman, D.J.; Everett, J.E.; Hess, P.J. The presence of a dedicated cardiac surgical intensive care service impacts clinical outcomes in adult cardiac surgery patients. J. Card. Surg. 2020, 35, 787–793. [Google Scholar] [CrossRef]
- Kennedy-Metz, L.R.; Barbeito, A.; Dias, R.D.; Zenati, M.A. Importance of high-performing teams in the cardiovascular intensive care unit. J. Thorac. Cardiovasc. Surg. 2022, 163, 1096–1104. [Google Scholar] [CrossRef]
- Shaefi, S.; Pannu, A.; Mueller, A.L.; Flynn, B.; Evans, A.; Jabaley, C.S.; Mladinov, D.; Wall, M.; Siddiqui, S.; Douin, D.J. Nationwide Clinical Practice Patterns of Anesthesiology Critical Care Physicians—A Survey to Members of the Society of Critical Care Anesthesiologists. Anesth. Analg. 2022, 10-1213. [Google Scholar] [CrossRef]
- Tung, A. Critical care of the cardiac patient. Anesth. Clin 2013, 31, 421–432. [Google Scholar] [CrossRef]
- Lobdell, K.W.; Haden, D.W.; Mistry, K.P. Cardiothoracic Critical Care. Surg. Clin. N. Am. 2017, 97, 811–834. [Google Scholar] [CrossRef] [PubMed]
- Aneman, A.; Brechot, N.; Brodie, D.; Colreavy, F.; Fraser, J.; Gomersall, C.; McCanny, P.; Moller-Sorensen, P.H.; Takala, J.; Valchanov, K.; et al. Advances in critical care management of patients undergoing cardiac surgery. Intensiv. Care Med. 2018, 44, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Birriel, B.; D’Angelo, K. End-of-Life Care in Cardiothoracic Surgery. Crit. Care Nurs. Clin. N. Am. 2019, 31, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Heiden, B.T.; Tetteh, E.; Robbins, K.J.; Tabak, R.G.; Nava, R.G.; Marklin, G.F.; Kreisel, D.; Meyers, B.F.; Kozower, B.D.; McKay, V.R.; et al. Dissemination and Implementation Science in Cardiothoracic Surgery: A Review and Case Study. Ann. Thorac. Surg. 2022, 114, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bellomo, R. Cardiac surgery-associated acute kidney injury: Risk factors, pathophysiology and treatment. Nat. Rev. Nephrol. 2017, 13, 697–711. [Google Scholar] [CrossRef]
- Hobson, C.E.; Yavas, S.; Segal, M.S.; Schold, J.D.; Tribble, C.G.; Layon, A.J.; Bihorac, A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009, 119, 2444–2453. [Google Scholar] [CrossRef] [PubMed]
- Albright, R.C. Acute kidney injury (AKI) in cardiac surgery. In Cardiopulmonary Bypass, 2nd ed.; Perrino, J.A.C., Falter, F., Ghosh, S., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 203–213. [Google Scholar]
- Mukaida, H.; Matsushita, S.; Yamamoto, T.; Minami, Y.; Sato, G.; Asai, T.; Amano, A. Oxygen delivery-guided perfusion for the prevention of acute kidney injury: A randomized controlled trial. J. Thorac. Cardiovasc. Surg. 2021. [Google Scholar] [CrossRef]
- Ranucci, M.; Johnson, I.; Willcox, T.; Baker, R.A.; Boer, C.; Baumann, A.; Justison, G.A.; De Somer, F.; Exton, P.; Agarwal, S. Goal-directed perfusion to reduce acute kidney injury: A randomized trial. J. Thorac. Cardiovasc. Surg. 2018, 156, 1918–1927.e1912. [Google Scholar] [CrossRef]
- Rasmussen, S.R.; Kandler, K.; Nielsen, R.V.; Cornelius Jakobsen, P.; Knudsen, N.N.; Ranucci, M.; Christian Nilsson, J.; Ravn, H.B. Duration of critically low oxygen delivery is associated with acute kidney injury after cardiac surgery. Acta Anaesthesiol. Scand. 2019, 63, 1290–1297. [Google Scholar] [CrossRef]
- Carrasco-Serrano, E.; Jorge-Monjas, P.; Muñoz-Moreno, M.F.; Gómez-Sánchez, E.; Priede-Vimbela, J.M.; Bardají-Carrillo, M.; Cubero-Gallego, H.; Tamayo, E.; Ortega-Loubon, C. Impact of Oxygen Delivery on the Development of Acute Kidney Injury in Patients Undergoing Valve Heart Surgery. J. Clin. Med. 2022, 11, 3046. [Google Scholar] [CrossRef]
- Oshita, T.; Hiraoka, A.; Nakajima, K.; Muraki, R.; Arimichi, M.; Chikazawa, G.; Yoshitaka, H.; Sakaguchi, T. A better predictor of acute kidney injury after cardiac surgery: The largest area under the curve below the oxygen delivery threshold during cardiopulmonary bypass. J. Am. Heart Assoc. 2020, 9, e015566. [Google Scholar] [CrossRef] [PubMed]
- Broadwin, M.; Palmeri, M.; Kelting, T.; Groom, R.; Robich, M.; Lucas, F.L.; Kramer, R. Goal Directed Perfusion Is Not Associated with a Decrease in Acute Kidney Injury in Patients Predicted to Be at High Risk for Acute Renal Failure after Cardiac Surgery. J. Extra-Corpor. Technol. 2022, 54, 128–134. [Google Scholar] [PubMed]
- Menting, T.P.; Wever, K.E.; Ozdemir-van Brunschot, D.M.; Van der Vliet, D.J.; Rovers, M.M.; Warle, M.C. Ischaemic preconditioning for the reduction of renal ischaemia reperfusion injury. Cochrane Database Syst. Rev. 2017, 3, Cd010777. [Google Scholar] [CrossRef]
- Krajewski, M.; Raghunathan, K.; Paluszkiewicz, S.; Schermer, C.; Shaw, A. Meta-analysis of high-versus low-chloride content in perioperative and critical care fluid resuscitation. J. Br. Surg. 2015, 102, 24–36. [Google Scholar] [CrossRef]
- Zayed, Y.Z.; Aburahma, A.M.; Barbarawi, M.O.; Hamid, K.; Banifadel, M.; Rashdan, L.; Bachuwa, G.I. Balanced crystalloids versus isotonic saline in critically ill patients: Systematic review and meta-analysis. J. Intensiv. Care 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Otero, T.M.; Aljure, O.D.; Yu, S. Postoperative resuscitation with hypertonic saline or hyperoncotic albumin in patients following cardiac surgery: A review of the literature. J. Card. Surg. 2021, 36, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Meersch, M.; Schmidt, C.; Hoffmeier, A.; Van Aken, H.; Wempe, C.; Gerss, J.; Zarbock, A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med. 2017, 43, 1551–1561. [Google Scholar] [CrossRef]
- Zarbock, A.; Küllmar, M.; Ostermann, M.; Lucchese, G.; Baig, K.; Cennamo, A.; Rajani, R.; McCorkell, S.; Arndt, C.; Wulf, H. Prevention of Cardiac Surgery–Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers: The PrevAKI-Multicenter Randomized Controlled Trial. Anesth. Analg. 2021, 133, 292–302. [Google Scholar] [CrossRef]
- Zarbock, A.; Kellum, J.A.; Schmidt, C.; Van Aken, H.; Wempe, C.; Pavenstädt, H.; Boanta, A.; Gerß, J.; Meersch, M. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA 2016, 315, 2190–2199. [Google Scholar] [CrossRef]
- McPherson, J.A.; Wagner, C.E.; Boehm, L.M.; Hall, J.D.; Johnson, D.C.; Miller, L.R.; Burns, K.M.; Thompson, J.L.; Shintani, A.K.; Ely, E.W. Delirium in the cardiovascular intensive care unit: Exploring modifiable risk factors. Crit. Care Med. 2013, 41, 405. [Google Scholar] [CrossRef]
- Sanson, G.; Khlopenyuk, Y.; Milocco, S.; Sartori, M.; Dreas, L.; Fabiani, A. Delirium after cardiac surgery. Incidence, phenotypes, predisposing and precipitating risk factors, and effects. Heart Lung 2018, 47, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Turan, A.; Duncan, A.; Leung, S.; Karimi, N.; Fang, J.; Mao, G.; Hargrave, J.; Gillinov, M.; Trombetta, C.; Ayad, S. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): A randomised placebo-controlled trial. Lancet 2020, 396, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.R. Acute brain failure: Pathophysiology, diagnosis, management, and sequelae of delirium. Crit. Care Clin. 2017, 33, 461–519. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.E.; Mart, M.F.; Cunningham, C.; Shehabi, Y.; Girard, T.D.; MacLullich, A.M.; Slooter, A.J.; Ely, E. Delirium. Nat. Rev. Dis. Prim. 2020, 6, 1–26. [Google Scholar] [CrossRef]
- Greaves, D.; Psaltis, P.J.; Ross, T.J.; Davis, D.; Smith, A.E.; Boord, M.S.; Keage, H.A. Cognitive outcomes following coronary artery bypass grafting: A systematic review and meta-analysis of 91,829 patients. Int. J. Cardiol. 2019, 289, 43–49. [Google Scholar] [CrossRef]
- Cook, D.J.; Bruggink, S.M. Cerebral morbidity in adult cardiac surgery. In Cardiopulmonary Bypass, 2nd ed.; Perrino, J.A.C., Falter, F., Ghosh, S., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 186–202. [Google Scholar]
- Greaves, D.; Psaltis, P.J.; Davis, D.H.; Ross, T.J.; Ghezzi, E.S.; Lampit, A.; Smith, A.E.; Keage, H.A. Risk factors for delirium and cognitive decline following coronary artery bypass grafting surgery: A systematic review and meta-analysis. J. Am. Heart Assoc. 2020, 9, e017275. [Google Scholar] [CrossRef]
- Maldonado, J.R. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int. J. Geriatr. Psychiatry 2018, 33, 1428–1457. [Google Scholar] [CrossRef]
- Migirov, A.; Chahar, P.; Maheshwari, K. Postoperative delirium and neurocognitive disorders. Curr. Opin. Crit. Care 2021, 27, 686–693. [Google Scholar] [CrossRef]
- Oh, E.S.; Akeju, O.; Avidan, M.S.; Cunningham, C.; Hayden, K.M.; Jones, R.N.; Khachaturian, A.S.; Khan, B.A.; Marcantonio, E.R.; Needham, D.M.; et al. A roadmap to advance delirium research: Recommendations from the NIDUS Scientific Think Tank. Alzheimers Dement 2020, 16, 726–733. [Google Scholar] [CrossRef]
- Humeidan, M.L.; Reyes, J.-P.C.; Mavarez-Martinez, A.; Roeth, C.; Nguyen, C.M.; Sheridan, E.; Zuleta-Alarcon, A.; Otey, A.; Abdel-Rasoul, M.; Bergese, S.D. Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing major noncardiac surgery: The neurobics randomized clinical trial. JAMA Surg. 2021, 156, 148–156. [Google Scholar] [CrossRef]
- O’Gara, B.P.; Mueller, A.; Gasangwa, D.V.I.; Patxot, M.; Shaefi, S.; Khabbaz, K.; Banner-Goodspeed, V.; Pascal-Leone, A.; Marcantonio, E.R.; Subramaniam, B. Prevention of early postoperative decline: A randomized, controlled feasibility trial of perioperative cognitive training. Anesth. Analg. 2020, 130, 586. [Google Scholar] [CrossRef] [PubMed]
- Kelava, M.; Alfirevic, A.; Bustamante, S.; Hargrave, J.; Marciniak, D. Regional anesthesia in cardiac surgery: An overview of fascial plane chest wall blocks. Anesth. Analg. 2020, 131, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, P.; Labaste, F.; Gobin, J.; Marcheix, B.; Minville, V. Bilateral Parasternal Block and Bilateral Erector Spinae Plane Block Reduce Opioid Consumption in During Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1249–1250. [Google Scholar] [CrossRef]
- Hughes, C.G.; Mailloux, P.T.; Devlin, J.W.; Swan, J.T.; Sanders, R.D.; Anzueto, A.; Jackson, J.C.; Hoskins, A.S.; Pun, B.T.; Orun, O.M. Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N. Engl. J. Med. 2021, 384, 1424–1436. [Google Scholar] [CrossRef]
- Smith, W.; Whitlock, E.L. Cardiac surgery, ICU sedation, and delirium: Is dexmedetomidine the silver bullet. Curr. Opin. Anesthsiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.; Oomen, G.; Carey, P. A technical review of the history, development and performance of the anaesthetic conserving device “AnaConDa” for delivering volatile anaesthetic in intensive and post-operative critical care. J. Clin. Monit. Comput. 2018, 32, 595–604. [Google Scholar] [CrossRef]
- Beitler, J. Efficacy and Safety of Inhaled Isoflurane Delivered Via the Sedaconda ACD-S Compared to Intravenous Propofol for Sedation of Mechanically Ventilated Intensive Care Unit Adult Patients (INSPiRE-ICU2) (INSPiRE-ICU2). Available online: https://clinicaltrials.gov/ct2/show/study/NCT05327296 (accessed on 21 September 2022).
- Meiser, A.; Volk, T.; Wallenborn, J.; Guenther, U.; Becher, T.; Bracht, H.; Schwarzkopf, K.; Knafelj, R.; Faltlhauser, A.; Thal, S.C. Inhaled isoflurane via the anaesthetic conserving device versus propofol for sedation of invasively ventilated patients in intensive care units in Germany and Slovenia: An open-label, phase 3, randomised controlled, non-inferiority trial. Lancet Respir. Med. 2021, 9, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Lefebvre, F.; Harlay, M.-L.; Glady, L.; Becker, G.; Muller, C.; Aberkane, O.; Tawk, M.; Julians, M.; Romoli, A. Impact of intravenous lidocaine on clinical outcomes of patients with ARDS during COVID-19 pandemia (LidoCovid): A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Benedetto, U.; Fremes, S.; Biondi-Zoccai, G.; Sedrakyan, A.; Puskas, J.D.; Angelini, G.D.; Buxton, B.; Frati, G.; Hare, D.L. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N. Engl. J. Med. 2018, 378, 2069–2077. [Google Scholar] [CrossRef]
- Verma, S.; Szmitko, P.E.; Weisel, R.D.; Bonneau, D.; Latter, D.; Errett, L.; LeClerc, Y.; Fremes, S.E. Should radial arteries be used routinely for coronary artery bypass grafting? Circulation 2004, 110, e40–e46. [Google Scholar] [CrossRef]
- Schwann, T.A.; Gaudino, M.; Baldawi, M.; Tranbaugh, R.; Schwann, A.N.; Habib, R.H. Optimal management of radial artery grafts in CABG: Patient and target vessel selection and anti-spasm therapy. J. Card. Surg. 2018, 33, 205–212. [Google Scholar] [CrossRef]
- Gaudino, M.; Luciani, N.; Nasso, G.; Salica, A.; Canosa, C.; Possati, G. Is postoperative calcium channel blocker therapy needed in patients with radial artery grafts? J. Thorac. Cardiovasc. Surg. 2005, 129, 532–535. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg. Infect. 2013, 14, 73–156. [Google Scholar] [CrossRef] [PubMed]
- Engelman, R.; Shahian, D.; Shemin, R.; Guy, T.S.; Bratzler, D.; Edwards, F.; Jacobs, M.; Fernando, H.; Bridges, C. The Society of Thoracic Surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part II: Antibiotic choice. Ann. Thorac. Surg. 2007, 83, 1569–1576. [Google Scholar] [CrossRef]
- Silvetti, S.; Landoni, G.; Castagnola, E.; Nuri, H.; Pomé, G.; Moscatelli, A. Antibiotic management for delayed sternal closure following pediatric cardiac surgery: A systematic review of recent literature. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, J.L.; Wanek, M.R.; Udeh, C.I.; Neuner, E.A.; Fraser, T.G.; Attia, T.; Roselli, E.E. Evaluation of prophylactic antibiotic use for delayed sternal closure after cardiothoracic operation. Ann. Thorac. Surg. 2018, 105, 1365–1369. [Google Scholar] [CrossRef]
- Li, M.; Mazzeffi, M.A.; Gammie, J.S.; Banoub, M.; Pazhani, Y.; Herr, D.; Madathil, R.; Pousatis, S.; Bathula, A. Characterization of postoperative infection risk in cardiac surgery patients with delayed sternal closure. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Burcham, P.K.; Rozycki, A.J.; Abel, E.E. Considerations for analgosedation and antithrombotic management during extracorporeal life support. Ann. Transl. Med. 2017, 5, 69. [Google Scholar] [CrossRef]
- Shekar, K.; Roberts, J.A.; Mcdonald, C.I.; Fisquet, S.; Barnett, A.G.; Mullany, D.V.; Ghassabian, S.; Wallis, S.C.; Fung, Y.L.; Smith, M.T. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit. Care 2012, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Roberts, J.A.; Mcdonald, C.I.; Ghassabian, S.; Anstey, C.; Wallis, S.C.; Mullany, D.V.; Fung, Y.L.; Fraser, J.F. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: Results from an ex vivo study. Crit. Care 2015, 19, 1–8. [Google Scholar] [CrossRef]
- Kühn, D.; Metz, C.; Seiler, F.; Wehrfritz, H.; Roth, S.; Alqudrah, M.; Becker, A.; Bracht, H.; Wagenpfeil, S.; Hoffmann, M. Antibiotic therapeutic drug monitoring in intensive care patients treated with different modalities of extracorporeal membrane oxygenation (ECMO) and renal replacement therapy: A prospective, observational single-center study. Crit. Care 2020, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, E.; Kolte, D.; Kennedy, K.F.; Beale, C.E.; Abbott, J.D.; Ehsan, A.; Gurm, H.S.; Carson, J.L.; Mamdani, S.; Aronow, H.D. Institutional Red Blood Cell Transfusion Rates Are Correlated Following Endovascular and Surgical Cardiovascular Procedures: Evidence That Local Culture Influences Transfusion Decisions. J. Am. Heart Assoc. 2020, 9, e016232. [Google Scholar] [CrossRef] [PubMed]
- Mazer, C.D.; Whitlock, R.P.; Fergusson, D.A.; Hall, J.; Belley-Cote, E.; Connolly, K.; Khanykin, B.; Gregory, A.J.; de Médicis, É.; McGuinness, S.; et al. Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. N. Engl. J. Med. 2017, 377, 2133–2144. [Google Scholar] [CrossRef]
- Mazer, C.D.; Whitlock, R.P.; Fergusson, D.A.; Belley-Cote, E.; Connolly, K.; Khanykin, B.; Gregory, A.J.; de Médicis, É.; Carrier, F.M.; McGuinness, S.; et al. Six-Month Outcomes after Restrictive or Liberal Transfusion for Cardiac Surgery. N. Engl. J. Med. 2018, 379, 1224–1233. [Google Scholar] [CrossRef]
- Ducrocq, G.; Gonzalez-Juanatey, J.R.; Puymirat, E.; Lemesle, G.; Cachanado, M.; Durand-Zaleski, I.; Arnaiz, J.A.; Martínez-Sellés, M.; Silvain, J.; Ariza-Solé, A.; et al. Effect of a Restrictive vs Liberal Blood Transfusion Strategy on Major Cardiovascular Events Among Patients With Acute Myocardial Infarction and Anemia: The REALITY Randomized Clinical Trial. JAMA 2021, 325, 552–560. [Google Scholar] [CrossRef]
- Carson, J.L.; Brooks, M.M.; Abbott, J.D.; Chaitman, B.; Kelsey, S.F.; Triulzi, D.J.; Srinivas, V.; Menegus, M.A.; Marroquin, O.C.; Rao, S.V.; et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am. Heart J. 2013, 165, 964–971.e961. [Google Scholar] [CrossRef]
- Raphael, J.; Mazer, C.D.; Subramani, S.; Schroeder, A.; Abdalla, M.; Ferreira, R.; Roman, P.E.; Patel, N.; Welsby, I.; Greilich, P.E.; et al. Society of Cardiovascular Anesthesiologists Clinical Practice Improvement Advisory for Management of Perioperative Bleeding and Hemostasis in Cardiac Surgery Patients. Anesth. Analg. 2019, 129, 1209–1221. [Google Scholar] [CrossRef]
- Tanaka, K.A.; Egan, K.; Szlam, F.; Ogawa, S.; Roback, J.D.; Sreeram, G.; Guyton, R.A.; Chen, E.P. Transfusion and hematologic variables after fibrinogen or platelet transfusion in valve replacement surgery: Preliminary data of purified lyophilized human fibrinogen concentrate versus conventional transfusion. Transfusion 2014, 54, 109–118. [Google Scholar] [CrossRef]
- Rahe-Meyer, N.; Solomon, C.; Hanke, A.; Schmidt, D.S.; Knoerzer, D.; Hochleitner, G.; Sørensen, B.; Hagl, C.; Pichlmaier, M. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery: A randomized, placebo-controlled trial. Anesthesiology 2013, 118, 40–50. [Google Scholar] [CrossRef]
- Krachey, E.; Viele, K.; Spinella, P.C.; Steiner, M.E.; Zantek, N.D.; Lewis, R.J. The design of an adaptive clinical trial to evaluate the efficacy of platelets stored at low temperature in surgical patients. J. Trauma Acute Care Surg. 2018, 84, S41–S46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, C.; Wang, Y.; Yu, L.; Yan, M. Preoperative Acute Normovolemic Hemodilution for Minimizing Allogeneic Blood Transfusion: A Meta-Analysis. Anesth. Analg. 2015, 121, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Barile, L.; Fominskiy, E.; Di Tomasso, N.; Alpìzar Castro, L.E.; Landoni, G.; De Luca, M.; Bignami, E.; Sala, A.; Zangrillo, A.; Monaco, F. Acute Normovolemic Hemodilution Reduces Allogeneic Red Blood Cell Transfusion in Cardiac Surgery: A Systematic Review and Meta-analysis of Randomized Trials. Anesth. Analg. 2017, 124, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Callum, J.; Farkouh, M.E.; Scales, D.C.; Heddle, N.M.; Crowther, M.; Rao, V.; Hucke, H.P.; Carroll, J.; Grewal, D.; Brar, S.; et al. Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery: The FIBRES Randomized Clinical Trial. JAMA 2019, 322, 1966–1976. [Google Scholar] [CrossRef] [PubMed]
- Karkouti, K.; Bartoszko, J.; Grewal, D.; Bingley, C.; Armali, C.; Carroll, J.; Hucke, H.P.; Kron, A.; McCluskey, S.A.; Rao, V.; et al. Comparison of 4-Factor Prothrombin Complex Concentrate With Frozen Plasma for Management of Hemorrhage During and After Cardiac Surgery: A Randomized Pilot Trial. JAMA Netw. Open 2021, 4, e213936. [Google Scholar] [CrossRef] [PubMed]
- Avidan, M.S.; Alcock, E.L.; Da Fonseca, J.; Ponte, J.; Desai, J.B.; Despotis, G.J.; Hunt, B.J. Comparison of structured use of routine laboratory tests or near-patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br. J. Anaesth. 2004, 92, 178–186. [Google Scholar] [CrossRef]
- Gonzalez, E.; Moore, E.E.; Moore, H.B.; Chapman, M.P.; Chin, T.L.; Ghasabyan, A.; Wohlauer, M.V.; Barnett, C.C.; Bensard, D.D.; Biffl, W.L.; et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016, 263, 1051–1059. [Google Scholar] [CrossRef]
- Baksaas-Aasen, K.; Gall, L.S.; Stensballe, J.; Juffermans, N.P.; Curry, N.; Maegele, M.; Brooks, A.; Rourke, C.; Gillespie, S.; Murphy, J.; et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): A randomized, controlled trial. Intensive Care Med. 2021, 47, 49–59. [Google Scholar] [CrossRef]
- Chowdhury, M.; Shore-Lesserson, L.; Mais, A.M.; Leyvi, G. Thromboelastograph with Platelet Mapping(TM) predicts postoperative chest tube drainage in patients undergoing coronary artery bypass grafting. J. Cardiothorac. Vasc. Anesth. 2014, 28, 217–223. [Google Scholar] [CrossRef]
- de Siqueira, G.M.V.; Pereira-Dos-Santos, F.M.; Silva-Rocha, R.; Guazzaroni, M.E. Nanopore Sequencing Provides Rapid and Reliable Insight Into Microbial Profiles of Intensive Care Units. Front. Public Health 2021, 9, 710985. [Google Scholar] [CrossRef]
- Baccarelli, A.A.; Byun, H.M. Platelet mitochondrial DNA methylation: A potential new marker of cardiovascular disease. Clin. Epigenet. 2015, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.; Tili, E.; Nuovo, G.; Kelani, H.; Ramadan, M.E.; Williams, J.; Binzel, K.; Rajan, J.; Mast, D.; Efanov, A.A.; et al. Endovascular repair and open repair surgery of thoraco-abdominal aortic aneurysms cause drastically different types of spinal cord injury. Sci. Rep. 2021, 11, 7834. [Google Scholar] [CrossRef] [PubMed]
- Aldskogius, H. Animal Models of Spinal Cord Repair; Springer: Berlin/Heidelberg, Germany, 2013; 1 online resource (XII, 336 p. 349 illus., 336 illus. in color). [Google Scholar]
- Fouad, K.; Popovich, P.G.; Kopp, M.A.; Schwab, J.M. The neuroanatomical-functional paradox in spinal cord injury. Nat. Rev. Neurol. 2021, 17, 53–62. [Google Scholar] [CrossRef]
- Dunning, J.; Levine, A.; Ley, J.; Strang, T.; Lizotte Jr, D.E.; Lamarche, Y.; Bartley, T.; Zellinger, M.; Katz, N.; Arora, R.C. The society of thoracic surgeons expert consensus for the resuscitation of patients who arrest after cardiac surgery. Ann. Thorac. Surg. 2017, 103, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, H.; Gust, R.; Bottiger, B.W. Cardiopulmonary resuscitation after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 1995, 9, 352. [Google Scholar] [CrossRef]
- Kempen, P.M.; Allgood, R. Right ventricular rupture during closed-chest cardiopulmonary resuscitation after pneumonectomy with pericardiotomy: A case report. Crit. Care Med. 1999, 27, 1378–1379. [Google Scholar] [CrossRef]
- Combes, A.; Peek, G.J.; Hajage, D.; Hardy, P.; Abrams, D.; Schmidt, M.; Dechartres, A.; Elbourne, D. ECMO for severe ARDS: Systematic review and individual patient data meta-analysis. Intensiv. Care Med. 2020, 46, 2048–2057. [Google Scholar] [CrossRef]
- Shrestha, D.B.; Sedhai, Y.R.; Budhathoki, P.; Gaire, S.; Subedi, P.; Maharjan, S.; Yuan, M.; Asija, A.; Memon, W. Extracorporeal Membrane Oxygenation (ECMO) Dependent Acute Respiratory Distress Syndrome (ARDS): A Systematic Review and Meta-Analysis. Cureus 2022, 14, e25696. [Google Scholar] [CrossRef]
- Urner, M.; Barnett, A.G.; Bassi, G.L.; Brodie, D.; Dalton, H.J.; Ferguson, N.D.; Heinsar, S.; Hodgson, C.L.; Peek, G.; Shekar, K.; et al. Venovenous extracorporeal membrane oxygenation in patients with acute covid-19 associated respiratory failure: Comparative effectiveness study. BMJ 2022, 377, e068723. [Google Scholar] [CrossRef]
- Akoumianaki, E.; Jonkman, A.; Sklar, M.C.; Georgopoulos, D.; Brochard, L. A rational approach on the use of extracorporeal membrane oxygenation in severe hypoxemia: Advanced technology is not a panacea. Ann. Intensive Care 2021, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Tonna, J.E.; Abrams, D.; Brodie, D.; Greenwood, J.C.; RUBIO Mateo-Sidron, J.A.; Usman, A.; Fan, E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021, 67, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Schmidt, M.; Bailey, M.; Sheldrake, J.; Hodgson, C.; Aubron, C.; Rycus, P.T.; Scheinkestel, C.; Cooper, D.J.; Brodie, D.; Pellegrino, V.; et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am. J. Respir. Crit. Care Med. 2014, 189, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.F.; Matthay, M.A.; Luce, J.M.; Flick, M.R. An expanded definition of the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1988, 138, 720–723. [Google Scholar] [CrossRef]
- Zochios, V.; Parhar, K.; Tunnicliffe, W.; Roscoe, A.; Gao, F. The Right Ventricle in ARDS. Chest 2017, 152, 181–193. [Google Scholar] [CrossRef]
- Amato, M.B.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.; Mercat, A.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef]
- Bartlett, R.H. Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome: EOLIA and Beyond. Crit. Care Med. 2019, 47, 114–117. [Google Scholar] [CrossRef]
- Steimer, D.A.; Hernandez, O.; Mason, D.P.; Schwartz, G.S. Timing of ECMO Initiation Impacts Survival in Influenza-Associated ARDS. Thorac. Cardiovasc. Surg. 2019, 67, 212–215. [Google Scholar] [CrossRef]
- Olivier, P.-Y.; Ottavy, G.; Hoff, J.; Auchabie, J.; Darreau, C.; Pierrot, M. Prolonged time from intubation to cannulation in VV-ECMO for COVID-19: Does it really matter? Crit. Care 2021, 25, 385. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329–2330. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Schmidt, M.; Pham, T.; Beitler, J.R.; Fan, E.; Goligher, E.C.; McNamee, J.J.; Patroniti, N.; Wilcox, M.E.; Combes, A.; et al. Mechanical Ventilation for Acute Respiratory Distress Syndrome during Extracorporeal Life Support. Research and Practice. Am. J. Respir. Crit. Care Med. 2020, 201, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.C.; Kao, K.C. Mechanical Ventilation during Extracorporeal Membrane Oxygenation in Acute Respiratory Distress Syndrome: A Narrative Review. J. Clin. Med. 2021, 10, 4953. [Google Scholar] [CrossRef]
- Liu, L.; Liu, F.; Tan, J.; Zhao, L. Bivalirudin versus heparin in adult and pediatric patients with extracorporeal membrane oxygenation therapy: A systematic review and meta-analysis. Pharm. Res 2022, 177, 106089. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Gorantla, V.; Thomas, S.E. Venous thromboembolic events in the setting of extracorporeal membrane oxygenation support in adults: A systematic review. Thromb. Res. 2022, 212, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Kalbhenn, J.; Zieger, B. Bleeding During Veno-Venous ECMO: Prevention and Treatment. Front. Med. 2022, 9, 879579. [Google Scholar] [CrossRef]
- McMichael, A.B.V.; Ryerson, L.M.; Ratano, D.; Fan, E.; Faraoni, D.; Annich, G.M. 2021 ELSO Adult and Pediatric Anticoagulation Guidelines. ASAIO J. 2022, 68, 303–310. [Google Scholar] [CrossRef]
- Fina, D.; Matteucci, M.; Jiritano, F.; Meani, P.; Kowalewski, M.; Ballotta, A.; Ranucci, M.; Lorusso, R. Extracorporeal membrane oxygenation without systemic anticoagulation: A case-series in challenging conditions. J. Thorac. Dis. 2020, 12, 2113–2119. [Google Scholar] [CrossRef]
- Pavlushkov, E.; Berman, M.; Valchanov, K. Cannulation techniques for extracorporeal life support. Ann. Transl. Med. 2017, 5, 70. [Google Scholar] [CrossRef]
- Shah, A.; Dave, S.; Goerlich, C.E.; Kaczorowski, D.J. Hybrid and parallel extracorporeal membrane oxygenation circuits. JTCVS Tech. 2021, 8, 77–85. [Google Scholar] [CrossRef]
- Bartlett, R.H. ECMO: The next ten years. Egypt. J. Crit. Care Med. 2016, 4, 7–10. [Google Scholar] [CrossRef]
- Giraud, R.; Banfi, C.; Assouline, B.; De Charrière, A.; Cecconi, M.; Bendjelid, K. The use of extracorporeal CO2 removal in acute respiratory failure. Ann. Intensiv. Care 2021, 11, 43. [Google Scholar] [CrossRef]
- Chen, H.; Yu, R.G.; Yin, N.N.; Zhou, J.X. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: A systematic review. Crit. Care 2014, 18, 675. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Franchineau, G.; Combes, A. Recent advances in venovenous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Curr. Opin. Crit. Care 2019, 25, 71–76. [Google Scholar] [CrossRef]
- Smith, A.; Morgan, C.; Ledot, S.; Doyle, J.; Xu, T.; Shedden, L.; Passariello, M.; Patel, B.; Doyle, A.M.; Price, S.; et al. Veno-venous extracorporeal membrane oxygenation for the acute respiratory distress syndrome: A bridge too far? Acta Cardiol. 2021, 76, 455–458. [Google Scholar] [CrossRef]
- Dennis, M.; Lal, S.; Forrest, P.; Nichol, A.; Lamhaut, L.; Totaro, R.J.; Burns, B.; Sandroni, C. In-Depth Extracorporeal Cardiopulmonary Resuscitation in Adult Out-of-Hospital Cardiac Arrest. J. Am. Heart Assoc. 2020, 9, e016521. [Google Scholar] [CrossRef]
- Richardson, A.S.C.; Tonna, J.E.; Nanjayya, V.; Nixon, P.; Abrams, D.C.; Raman, L.; Bernard, S.; Finney, S.J.; Grunau, B.; Youngquist, S.T.; et al. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement From the Extracorporeal Life Support Organization. ASAIO J. 2021, 67, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Belohlavek, J.; Smalcova, J.; Rob, D.; Franek, O.; Smid, O.; Pokorna, M.; Horák, J.; Mrazek, V.; Kovarnik, T.; Zemanek, D.; et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2022, 327, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Massion, P.B.; Joachim, S.; Morimont, P.; Dulière, G.L.; Betz, R.; Benoit, A.; Amabili, P.; Lagny, M.; Lizin, J.; Massaro, A.; et al. Feasibility of extracorporeal membrane oxygenation cardiopulmonary resuscitation by low volume centers in Belgium. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12484. [Google Scholar] [CrossRef]
- Addison, D.; Cheng, E.; Forrest, P.; Livingstone, A.; Morton, R.L.; Dennis, M. Cost-effectiveness of extracorporeal cardiopulmonary resuscitation for adult out-of-hospital cardiac arrest: A systematic review. Resuscitation 2022, 178, 19–25. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef]
- Ruggeri, L.; Franco, A.; Alba, A.C.; Lembo, R.; Frassoni, S.; Scandroglio, A.M.; Calabrò, M.G.; Zangrillo, A.; Pappalardo, F. Coagulation Derangements in Patients With Refractory Cardiac Arrest Treated With Extracorporeal Cardiopulmonary Resuscitation. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Mene-Afejuku, T.O.; Moisa, E.-A.; Akinlonu, A.; Dumancas, C.; Veranyan, S.; Perez, J.A.; Salazar, P.; Chaudhari, S.; Pekler, G.; Mushiyev, S. The relevance of serum albumin among elderly patients with acute decompensated heart failure. J. Geriatr. Cardiol. 2019, 16, 522. [Google Scholar]
- Gebauer, A.; Petersen, J.; Konertz, J.; Brickwedel, J.; Schulte-Uentrop, L.; Reichenspurner, H.; Girdauskas, E. Enhanced Recovery After Cardiac Surgery: Where Do We Stand? Curr. Anesthesiol. Rep. 2021, 11, 501–506. [Google Scholar] [CrossRef]
- Engelman, D.T.; Ali, W.B.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H. Guidelines for perioperative care in cardiac surgery: Enhanced recovery after surgery society recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef]
- Acinapura, A.; Jacobowitz, I.; Kramer, M.; Adkins, M.; Zisbrod, Z.; Cunningham Jr, J. Demographic changes in coronary artery bypass surgery and its effect on mortality and morbidity. Eur. J. Cardio-Thorac. Surg. 1990, 4, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chandramouli, C.; Allocco, B.; Gong, E.; Lam, C.S.; Yan, L.L. Women’s participation in cardiovascular clinical trials from 2010 to 2017. Circulation 2020, 141, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.N.; Miranda, L.M.; Barros, P.M.; Fragata, J.I. Quality of life after elective cardiac surgery in elderly patients. Interact. CardioVascular Thorac. Surg. 2019, 28, 199–205. [Google Scholar] [CrossRef]
- Kudsk, K.A.; Tolley, E.A.; DeWitt, R.C.; Janu, P.G.; Blackwell, A.P.; Yeary, S.; King, B.K. Preoperative albumin and surgical site identify surgical risk for major postoperative complications. J. Parenter. Enter. Nutr. 2003, 27, 1–9. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, W.J.; Kim, J.Y.; Chin, J.H.; Choi, D.K.; Sim, J.Y.; Choo, S.J.; Chung, C.H.; Lee, J.W.; Choi, I.C. Effect of exogenous albumin on the incidence of postoperative acute kidney injury in patients undergoing off-pump coronary artery bypass surgery with a preoperative albumin level of less than 4.0 g/dl. Anesthesiology 2016, 124, 1001–1011. [Google Scholar] [CrossRef]
- Karas, P.L.; Goh, S.L.; Dhital, K. Is low serum albumin associated with postoperative complications in patients undergoing cardiac surgery? Interact. Cardiovasc. Thorac. Surg. 2015, 21, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Waitzberg, D.L.; Saito, H.; Plank, L.D.; Jamieson, G.G.; Jagannath, P.; Hwang, T.-L.; Mijares, J.M.; Bihari, D. Postsurgical infections are reduced with specialized nutrition support. World J. Surg. 2006, 30, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.M.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: A randomized blinded controlled trial. Ann. Surg. 2018, 267, 50–56. [Google Scholar] [CrossRef]
- Li, C.; Carli, F.; Lee, L.; Charlebois, P.; Stein, B.; Liberman, A.S.; Kaneva, P.; Augustin, B.; Wongyingsinn, M.; Gamsa, A. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: A pilot study. Surg. Endosc. 2013, 27, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Li, C.; Lee, L.; Awasthi, R.; Augustin, B.; Gamsa, A.; Liberman, A.S.; Stein, B.; Charlebois, P.; Feldman, L.S. Prehabilitation versus rehabilitation: A randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014, 121, 937–947. [Google Scholar] [CrossRef]
- Oravec, N.; Arora, R.C.; Bjorklund, B.; Gregora, A.; Monnin, C.; Duhamel, T.A.; Kent, D.E.; Schultz, A.S.; Chudyk, A.M. Expanding enhanced recovery protocols for cardiac surgery to include the patient voice: A scoping review protocol. Syst. Rev. 2021, 10, 1–10. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N. Heart disease and stroke statistics—2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar]
- Hessian, R.; Jabagi, H.; Ngu, J.M.; Rubens, F.D. Coronary surgery in women and the challenges we face. Can. J. Cardiol. 2018, 34, 413–421. [Google Scholar] [CrossRef]
- Cho, L.; Kibbe, M.R.; Bakaeen, F.; Aggarwal, N.R.; Davis, M.B.; Karmalou, T.; Lawton, J.S.; Ouzounian, M.; Preventza, O.; Russo, A.M. Cardiac surgery in women in the current era: What are the gaps in care? Circulation 2021, 144, 1172–1185. [Google Scholar] [CrossRef]
- Volgman, A.S.; Bairey Merz, C.N.; Aggarwal, N.T.; Bittner, V.; Bunch, T.J.; Gorelick, P.B.; Maki, P.; Patel, H.N.; Poppas, A.; Ruskin, J. Sex differences in cardiovascular disease and cognitive impairment: Another health disparity for women? J. Am. Heart Assoc. 2019, 8, e013154. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.-C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown III, W.M.; Lembo, N.J. Five-year outcomes after PCI or CABG for left main coronary disease. N. Engl. J. Med. 2019, 381, 1820–1830. [Google Scholar] [CrossRef] [PubMed]
- Hayanga, A.J.; Aboagye, J.K.; Hayanga, H.E.; Morrell, M.; Huffman, L.; Shigemura, N.; Bhama, J.K.; Bermudez, C.A. Contemporary analysis of early outcomes after lung transplantation in the elderly using a national registry. J. Heart Lung Transplant. 2015, 34, 182–188. [Google Scholar] [CrossRef]
- Benissan-Messan, D.Z.; Ganapathi, A.M.; Guo, M.; Henn, M.C.; Keller, B.C.; Howsare, M.; Rosenheck, J.P.; Kirkby, S.E.; Mokadam, N.A.; Nunley, D. Lung transplantation in the septuagenarian can be successfully performed though long-term results impacted by diseases of aging. Clin. Transplant. 2022, 36, e14593. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.; Ravichandran, B.; Madathil, R.; McLaughlin, P.; Cipriano, S.; Timofte, I. Outcomes Associated with Septuagenarian Lung Transplant Recipients. J. Heart Lung Transplant. 2020, 39, S320–S321. [Google Scholar] [CrossRef]
- Flume, P.A.; Egan, T.M.; Westerman, J.H.; Paradowski, L.J.; Yankaskas, J.R.; Detterbeck, F.C.; Mill, M.R. Lung transplantation for mechanically ventilated patients. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 1994, 13, 15–21; discussion 22. [Google Scholar]
- Vermeijden, J.W.; Zijlstra, J.G.; Erasmus, M.E.; van der Bij, W.; Verschuuren, E.A. Lung transplantation for ventilator-dependent respiratory failure. J. Heart Lung Transplant. 2009, 28, 347–351. [Google Scholar] [CrossRef]
- Bartz, R.R.; Love, R.B.; Leverson, G.E.; Will, L.R.; Welter, D.L.; Meyer, K.C. Pre-transplant mechanical ventilation and outcome in patients with cystic fibrosis. J. Heart Lung Transplant. 2003, 22, 433–438. [Google Scholar] [CrossRef]
- Bharat, A.; Machuca, T.N.; Querrey, M.; Kurihara, C.; Garza-Castillon Jr, R.; Kim, S.; Manerikar, A.; Pelaez, A.; Pipkin, M.; Shahmohammadi, A. Early outcomes after lung transplantation for severe COVID-19: A series of the first consecutive cases from four countries. Lancet Respir. Med. 2021, 9, 487–497. [Google Scholar] [CrossRef]
- Magnusson, J.M.; Silverborn, M.; Broome, M.; Riise, G.C.; Dellgren, G. Long-term Extracorporeal Membrane Oxygenation Bridge to Lung Transplantation After COVID-19. Ann. Thorac. Surg. 2022, 113, e5–e8. [Google Scholar] [CrossRef]
- Mohanka, M.R.; Joerns, J.; Lawrence, A.; Bollineni, S.; Kaza, V.; Cheruku, S.; Leveno, M.; Chen, C.; Terada, L.S.; Kershaw, C.D. ECMO Long Haulers: A Distinct Phenotype of COVID-19–Associated ARDS with Implications for Lung Transplant Candidacy. Transplantation 2022, 106, e202–e211. [Google Scholar] [CrossRef]
- Harano, T.; Chan, E.G.; Furukawa, M.; Dos Santos, P.R.; Morrell, M.R.; Sappington, P.L.; Sanchez, P.G. Oxygenated right ventricular assist device with a percutaneous dual-lumen cannula as a bridge to lung transplantation. J. Thorac. Dis. 2022, 14, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; D’Ovidio, F.; Garan, A.R.; Brodie, D.; Sonett, J.R.; Farr, M.A.; Arcasoy, S.M.; Takeda, K. Minimally invasive central venoarterial extracorporeal membrane oxygenation for long-term ambulatory support as a bridge to heart–lung transplant. J. Artif. Organs 2020, 23, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Pak, C.; Oh, D.K.; Kim, H.C.; Kang, P.J.; Lee, G.D.; Choi, S.H.; Jung, S.-H.; Hong, S.-B. Right ventricular assist device with extracorporeal membrane oxygenation for bridging right ventricular heart failure to lung transplantation: A single-center case series and literature review. J. Cardiothorac. Vasc. Anesth. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kinaschuk, K.; Bozso, S.J.; Halloran, K.; Kapasi, A.; Jackson, K.; Nagendran, J. Mechanical circulatory support as a bridge to lung transplantation: A single Canadian institution review. Can. Respir. J. 2017, 2017, 5947978. [Google Scholar] [CrossRef]
- Rana, R.; Ghandehari, S.; Falk, J.; Simsir, S.; Ghaly, A.; Cheng, W.; Cohen, J.; Peng, A.; Czer, L.; Schwarz, E. Successful combined heart-bilateral lung-kidney transplantation from a same donor to treat severe hypertrophic cardiomyopathy with secondary pulmonary hypertension and renal failure: Case report and review of the literature. Transplant. Proc. 2011, 43, 2820–2826. [Google Scholar] [CrossRef]
- Borro, J.M.; Rama, P.; Rey, T.; Fernández-Rivera, C. Long-term success of combined kidney–lung transplantation in a patient with cystic fibrosis. Arch. Bronconeumol. 2013, 49, 272–274. [Google Scholar] [CrossRef]
- Mason, D.P.; Boffa, D.J.; Murthy, S.C.; Gildea, T.R.; Budev, M.M.; Mehta, A.C.; McNeill, A.M.; Smedira, N.G.; Feng, J.; Rice, T.W. Extended use of extracorporeal membrane oxygenation after lung transplantation. J. Thorac. Cardiovasc. Surg. 2006, 132, 954–960. [Google Scholar] [CrossRef]
- Boffini, M.; Simonato, E.; Ricci, D.; Scalini, F.; Marro, M.; Pidello, S.; Attisani, M.; Solidoro, P.; Lausi, P.O.; Fanelli, V. Extracorporeal membrane oxygenation after lung transplantation: Risk factors and outcomes analysis. Ann. Cardiothorac. Surg. 2019, 8, 54. [Google Scholar] [CrossRef]
- Mason, D.P.; Solovera-Rozas, M.; Feng, J.; Rajeswaran, J.; Thuita, L.; Murthy, S.C.; Budev, M.M.; Mehta, A.C.; Haug III, M.; McNeill, A.M. Dialysis after lung transplantation: Prevalence, risk factors and outcome. J. Heart Lung Transplant. 2007, 26, 1155–1162. [Google Scholar] [CrossRef]
- Crothers, E.; Kennedy, D.S.; Emmanuel, S.; Molan, N.; Scott, S.; Rogers, K.; Glanville, A.R.; Ntoumenopoulos, G. Incidence of early diaphragmatic dysfunction after lung transplantation: Results of a prospective observational study. Clin. Transplant. 2021, 35, e14409. [Google Scholar] [CrossRef]
- Diamond, J.M.; Lee, J.C.; Kawut, S.M.; Shah, R.J.; Localio, A.R.; Bellamy, S.L.; Lederer, D.J.; Cantu, E.; Kohl, B.A.; Lama, V.N.; et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2013, 187, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Yserbyt, J.; Dooms, C.; Vos, R.; Dupont, L.J.; Van Raemdonck, D.E.; Verleden, G.M. Anastomotic airway complications after lung transplantation: Risk factors, treatment modalities and outcome—A single-centre experience. Eur. J. Cardio-Thorac. Surg. 2016, 49, e1–e8. [Google Scholar] [CrossRef]
- Santacruz, J.F.; Mehta, A.C. Airway complications and management after lung transplantation: Ischemia, dehiscence, and stenosis. Proc. Am. Thorac. Soc. 2009, 6, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Siddiqui, H.U.; Thuita, L.; Rappaport, J.; Bribriesco, A.C.; McCurry, K.R.; Yun, J.; Unai, S.; Budev, M.; Murthy, S.C. Natural history of pleural complications after lung transplantation. Ann. Thorac. Surg. 2021, 111, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.; Roldan, J.; Roman, A.; Bravo, C.; Monforte, V.; Pallissa, E.; Gic, I.; Sole, J.; Morell, F. Acute and chronic pleural complications in lung transplantation. J. Heart Lung Transplant. 2003, 22, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Hodgin, K.E.; Nordon-Craft, A.; McFann, K.K.; Mealer, M.L.; Moss, M. Physical therapy utilization in intensive care units: Results from a national survey. Crit. Care Med. 2009, 37, 561. [Google Scholar] [CrossRef]
- Taito, S.; Shime, N.; Ota, K.; Yasuda, H. Early mobilization of mechanically ventilated patients in the intensive care unit. J. Intensive Care 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Singbartl, K.; Murugan, R.; Kaynar, A.; Crippen, D.; Tisherman, S.; Shutterly, K.; Stuart, S.; Simmons, R.; Darby, J. Intensivist-led management of brain-dead donors is associated with an increase in organ recovery for transplantation. Am. J. Transplant. 2011, 11, 1517–1521. [Google Scholar] [CrossRef]
- Rosendale, J.D.; Kauffman, H.M.; McBride, M.A.; Chabalewski, F.L.; Zaroff, J.G.; Garrity, E.R.; Delmonico, F.L.; Rosengard, B.R. Aggressive pharmacologic donor management results in more transplanted organs1. Transplantation 2003, 75, 482–487. [Google Scholar] [CrossRef]
- Turco, L.M.; Glorsky, S.L.; Winfield, R.D. Hormone replacement therapy in brain-dead organ donors: A comprehensive review with an emphasis on traumatic brain injury. J. Trauma Acute Care Surg. 2019, 86, 702–709. [Google Scholar] [CrossRef]
- Kotloff, R.M.; Blosser, S.; Fulda, G.J.; Malinoski, D.; Ahya, V.N.; Angel, L.; Byrnes, M.C.; DeVita, M.A.; Grissom, T.E.; Halpern, S.D. Management of the potential organ donor in the ICU: Society of critical care medicine/American college of chest physicians/association of organ procurement organizations consensus statement. Crit. Care Med. 2015, 43, 1291–1325. [Google Scholar] [CrossRef] [PubMed]
- Tramm, R.; Ilic, D.; Murphy, K.; Sheldrake, J.; Pellegrino, V.; Hodgson, C. Experience and needs of family members of patients treated with extracorporeal membrane oxygenation. J. Clin. Nurs. 2017, 26, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Akkermans, A.; Lamerichs, J.; Schultz, M.; Cherpanath, T.; van Woensel, J.; van Heerde, M.; van Kaam, A.; van de Loo, M.; Stiggelbout, A.; Smets, E. How doctors actually (do not) involve families in decisions to continue or discontinue life-sustaining treatment in neonatal, pediatric, and adult intensive care: A qualitative study. Palliat. Med. 2021, 35, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Gregoretti, C.; Cortegiani, A. Palliative care in intensive care units: Why, where, what, who, when, how. BMC Anesthesiol. 2018, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, M.L.; Brasel, K.J.; Mosenthal, A.C. Beyond 30-day mortality: Aligning surgical quality with outcomes that patients value. JAMA Surg. 2014, 149, 631–632. [Google Scholar] [CrossRef] [PubMed]
- Walkey, A.J.; Barnato, A.E.; Wiener, R.S.; Nallamothu, B.K. Accounting for patient preferences regarding life-sustaining treatment in evaluations of medical effectiveness and quality. Am. J. Respir. Crit. Care Med. 2017, 196, 958–963. [Google Scholar] [CrossRef]
- Turnbull, A.E.; Sahetya, S.K.; Biddison, E.; Hartog, C.S.; Rubenfeld, G.D.; Benoit, D.D.; Guidet, B.; Gerritsen, R.T.; Tonelli, M.R.; Curtis, J.R. Competing and conflicting interests in the care of critically ill patients. Intensive Care Med. 2018, 44, 1628–1637. [Google Scholar] [CrossRef]
- Kayser, J.B.; Kaplan, L.J. Conflict management in the ICU. Crit. Care Med. 2020, 48, 1349–1357. [Google Scholar] [CrossRef]
- Spijkers, A.S.; Akkermans, A.; Smets, E.; Schultz, M.J.; Cherpanath, T.G.; van Woensel, J.; van Heerde, M.; van Kaam, A.H.; van de Loo, M.; Willems, D.L. How doctors manage conflicts with families of critically ill patients during conversations about end-of-life decisions in neonatal, pediatric, and adult intensive care. Intensive Care Med. 2022, 48, 910–922. [Google Scholar] [CrossRef]
- Kim, J.M.; Godfrey, S.; O’Neill, D.; Sinha, S.S.; Kochar, A.; Kapur, N.K.; Katz, J.N.; Warraich, H.J. Integrating palliative care into the modern cardiac intensive care unit: A review. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 442–449. [Google Scholar] [CrossRef]
- Yefimova, M.; Aslakson, R.A.; Yang, L.; Garcia, A.; Boothroyd, D.; Gale, R.C.; Giannitrapani, K.; Morris, A.M.; Johanning, J.M.; Shreve, S. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020, 155, 138–146. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).