The Anatomical Pathogenesis of Stress Urinary Incontinence in Women
Abstract
:1. Introduction
2. Anatomical Factors of the Urethra Itself
2.1. The Sealing Effect of the Urethral Mucosa Is Weakened
2.2. Dysfunction or Defect of the Urethral Sphincter
2.3. Decreased Elasticity of the Urethral Wall
2.4. Shortened Length of the Functional Urethra
3. Anatomical Factors Affecting the Urethra (Figure 2)
3.1. Weak Supporting Structure of the Bladder Neck
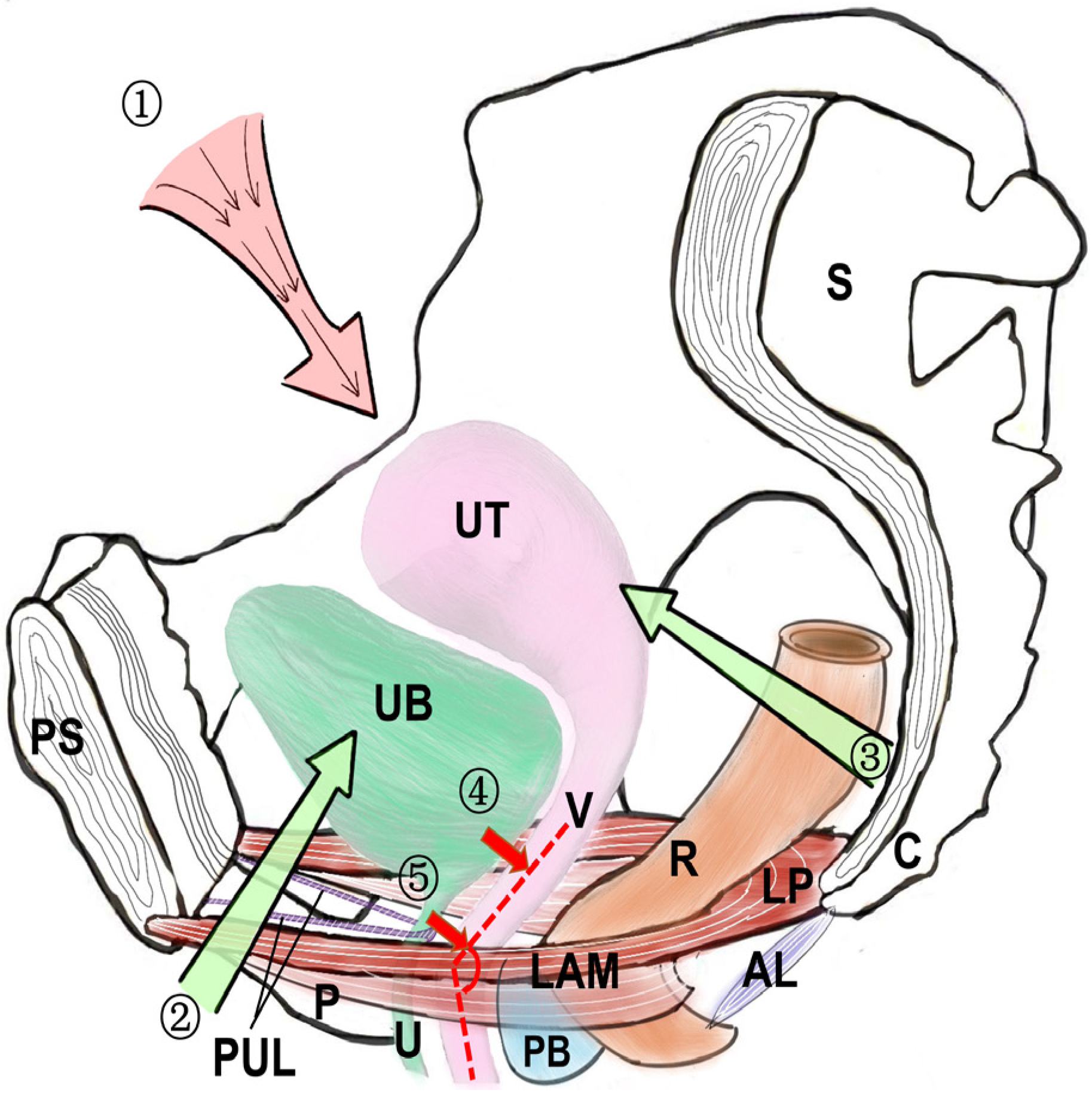
3.2. Defective Nature or Prolapse of the Anterior Vaginal Wall Support
3.3. The Continuity and Integrity of the Pelvic Fascia and Pelvic Fascial Tendon Arch (ATFP) Are Impaired
3.4. Weak Pubic Urethral Ligaments
3.5. Levator Ani Muscle Weakness or Dysfunction
4. Anatomical Factors of the Pelvic Floor Nerves
Pelvic Floor Neuromuscular Injury
5. The Key Anatomical Pathogenesis and Operation Improvement
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Lang, J.; Liu, C.; Han, S.; Huang, J.; Li, X. The epidemiological study of women with urinary incontinence and risk factors for stress urinary incontinence in China. Menopause 2009, 16, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Liong, M.L.; Leong, W.S.; Lau, Y.K.; Khan, N.A.K.; Yuen, K.H. The Impact of Stress Urinary Incontinence on Individual Components of Quality of Life in Malaysian Women. Urology 2018, 112, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.A.; Dumm, W.M. Urinary incontinence in women, without manifest injury to the bladder. Int. Urogynecol. J. Pelvic Floor Dysfunct. 1998, 9, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Enhorning, G. Simultaneous recording of intravesical and intra-urethral pressure. A study on urethral closure in normal and stress incontinent women. Acta Chir. Scand. 1961, 276, 1–68. [Google Scholar]
- Petros, P.E.; Ulmsten, U.I. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet. Et Gynecol. Scand. 1990, 153 (Suppl. S153), 7–31. [Google Scholar] [CrossRef]
- Petros, P.E.; Woodman, P.J. The Integral Theory of continence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2008, 19, 35–40. [Google Scholar] [CrossRef]
- DeLancey, J.O. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am. J. Obstet. Gynecol. 1994, 170, 1713–1720; discussion 1720–1723. [Google Scholar] [CrossRef]
- Arrue Gabilondo, M.; Ginto, L.; Zubikarai, M.; Galán, C.; Saro, J.; Diez-Itza, I. Risk factors associated with stress urinary incontinence 12 years after first delivery. Int. Urogynecol. J. 2021, 32, 3061–3067. [Google Scholar] [CrossRef]
- Wu, J.M. Stress Incontinence in Women. N. Engl. J. Med. 2021, 384, 2428–2436. [Google Scholar] [CrossRef]
- Falah-Hassani, K.; Reeves, J.; Shiri, R.; Hickling, D.; McLean, L. The pathophysiology of stress urinary incontinence: A systematic review and meta-analysis. Int. Urogynecol. J. 2021, 32, 501–552. [Google Scholar] [CrossRef]
- Mistry, M.A.; Klarskov, N.; DeLancey, J.O.; Lose, G. A structured review on the female urethral anatomy and innervation with an emphasis on the role of the urethral longitudinal smooth muscle. Int. Urogynecol. J. 2020, 31, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Adamiak-Godlewska, A.; Tarkowski, R.; Winkler, I.; Romanek-Piva, K.; Skorupska, K.; Jakimiuk, A.J.; Rechberger, T. Stress urinary incontinent women, the influence of age and hormonal status on estrogen receptor alpha and beta gene expression and protein immunoexpression in paraurethral tissues. J. Physiol. Pharmacol. 2018, 69, 53–59. [Google Scholar] [CrossRef]
- Quinn, S.D.; Domoney, C. The effects of hormones on urinary incontinence in postmenopausal women. Climacteric J. Int. Menopause Soc. 2009, 12, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Cody, J.D.; Richardson, K.; Moehrer, B.; Hextall, A.; Glazener, C.M. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst. Rev. 2009, 4, Cd001405. [Google Scholar] [CrossRef]
- Bodner-Adler, B.; Bodner, K.; Kimberger, O.; Halpern, K.; Rieken, M.; Koelbl, H.; Umek, W. Role of serum steroid hormones in women with stress urinary incontinence: A case-control study. BJU Int. 2017, 120, 416–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasser, H.; Tiefenthaler, M.; Steinlechner, M.; Bartsch, G.; Konwalinka, G. Urinary incontinence in the elderly and age-dependent apoptosis of rhabdosphincter cells. Lancet 1999, 354, 918–919. [Google Scholar] [CrossRef]
- Attari, A.; DeLancey, J.O.; Ashton-Miller, J.A. On Structure-Function Relationships in the Female Human Urethra: A Finite Element Model Approach. Ann. Biomed. Eng. 2021, 49, 1848–1860. [Google Scholar] [CrossRef]
- Mazloomdoost, D.; Westermann, L.B.; Mutema, G.; Crisp, C.C.; Kleeman, S.D.; Pauls, R.N. Histologic Anatomy of the Anterior Vagina and Urethra. Female Pelvic Med. Reconstr. Surg. 2017, 23, 329–335. [Google Scholar] [CrossRef]
- Wallner, C.; Dabhoiwala, N.F.; DeRuiter, M.C.; Lamers, W.H. The anatomical components of urinary continence. Eur. Urol. 2009, 55, 932–943. [Google Scholar] [CrossRef]
- Karam, I.; Droupy, S.; Abd-Alsamad, I.; Uhl, J.F.; Benoît, G.; Delmas, V. Innervation of the female human urethral sphincter: 3D reconstruction of immunohistochemical studies in the fetus. Eur. Urol. 2005, 47, 627–633; discussion 634. [Google Scholar] [CrossRef]
- Schäfer, W. Some biomechanical aspects of continence function. Scand. J. Urol. Nephrol. 2001, 35, 44–60; discussion 106–125. [Google Scholar] [CrossRef]
- Tunn, R.; Goldammer, K.; Neymeyer, J.; Gauruder-Burmester, A.; Hamm, B.; Beyersdorff, D. MRI morphology of the levator ani muscle, endopelvic fascia, and urethra in women with stress urinary incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 126, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lang, J.H.; Zhu, L.; Liu, Z.F.; Sun, D.W.; Leng, J.H.; Ren, H.T.; Zhao, Y.H.; Guan, H.Z. Study of morphological changes in levator ani muscle of patients with stress urinary incontinence or pelvic organ prolapse. Zhonghua Fu Chan Ke Za Zhi 2004, 39, 519–521. [Google Scholar]
- Hinata, N.; Murakami, G. The urethral rhabdosphincter, levator ani muscle, and perineal membrane: A review. BioMed Res. Int. 2014, 2014, 906921. [Google Scholar] [CrossRef]
- de Leval, J.; Chantraine, A.; Penders, L. The striated sphincter of the urethra. 1: Recall of knowledge on the striated sphincter of the urethra. J. D’urologie 1984, 90, 439–454. [Google Scholar]
- DeLancey, J.O.; Trowbridge, E.R.; Miller, J.M.; Morgan, D.M.; Guire, K.; Fenner, D.E.; Weadock, W.J.; Ashton-Miller, J.A. Stress urinary incontinence: Relative importance of urethral support and urethral closure pressure. J. Urol. 2008, 179, 2286–2290; discussion 2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, D.M.; Umek, W.; Guire, K.; Morgan, H.K.; Garabrant, A.; DeLancey, J.O. Urethral sphincter morphology and function with and without stress incontinence. J. Urol. 2009, 182, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Tsumori, T.; Tsumiyama, W. Sexual and Regional Differences in Myosin Heavy Chain Expression in the Rat External Urethral Sphincter. Anat. Rec. 2017, 300, 2058–2069. [Google Scholar] [CrossRef] [Green Version]
- Eftekhar, T.; Hajibaratali, B.; Ramezanzadeh, F.; Shariat, M. Postpartum evaluation of stress urinary incontinence among primiparas. Int. J. Gynaecol. Obstet. 2006, 94, 114–118. [Google Scholar] [CrossRef]
- Frauscher, F.; Helweg, G.; Strasser, H.; Enna, B.; Klauser, A.; Knapp, R.; Colleselli, K.; Bartsch, G.; Zur Nedden, D. Intraurethral ultrasound: Diagnostic evaluation of the striated urethral sphincter in incontinent females. Eur. Radiol. 1998, 8, 50–53. [Google Scholar] [CrossRef]
- Swift, S. Intrinsic sphincter deficiency: What is it and does it matter anymore? Int. Urogynecol. J. 2013, 24, 183–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cour, F.; Le Normand, L.; Lapray, J.F.; Hermieu, J.F.; Peyrat, L.; Yiou, R.; Donon, L.; Wagner, L.; Vidart, A. Intrinsic sphincter deficiency and female urinary incontinence. Prog. En Urol. J. De L’association Fr. D’urologie Et De La Soc. Fr. D’urologie 2015, 25, 437–454. [Google Scholar] [CrossRef]
- Zinner, N.R.; Sterling, A.M.; Ritter, R.C. Role of inner urethral softness in urinary continence. Urology 1980, 16, 115–117. [Google Scholar] [CrossRef]
- Zinner, N.R.; Sterling, A.M.; Ritter, R.C. Evaluation of inner urethral softness. Part II. Clinical study using new grooved probe device. Urology 1983, 22, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.J. Urethral elasticity and micturition hydrodynamics in females. Med. Biol. Eng. 1969, 7, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Goepel, C.; Hefler, L.; Methfessel, H.D.; Koelbl, H. Periurethral connective tissue status of postmenopausal women with genital prolapse with and without stress incontinence. Acta Obstet. Et Gynecol. Scand. 2003, 82, 659–664. [Google Scholar] [CrossRef]
- Routzong, M.R.; Martin, L.C.; Rostaminia, G.; Abramowitch, S. Urethral support in female urinary continence part 2: A computational, biomechanical analysis of Valsalva. Int. Urogynecol. J. 2022, 33, 551–561. [Google Scholar] [CrossRef]
- de Vries, A.M.; Venema, P.L.; Heesakkers, J. Midurethral support is also necessary for reflex closure of the urethra. Neurourol. Urodyn. 2018, 37, 2965–2972. [Google Scholar] [CrossRef]
- Saaby, M.L. The urethral closure function in continent and stress urinary incontinent women assessed by urethral pressure reflectometry. Dan. Med. J. 2014, 61, B4795. [Google Scholar]
- Pelsang, R.E.; Bonney, W.W. Voiding cystourethrography in female stress incontinence. Am. J. Roentgenol. 1996, 166, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Grob, A.T.M.; Olde Heuvel, J.; Futterer, J.J.; Massop, D.; Veenstra van Nieuwenhoven, A.L.; Simonis, F.F.J.; van der Vaart, C.H. Underestimation of pelvic organ prolapse in the supine straining position, based on magnetic resonance imaging findings. Int. Urogynecol. J. 2019, 30, 1939–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, K.; Yoshimura, Y.; Narushima, M.; Suzuki, S.; Hattori, R. “Central Road” cystoscopic finding: The road to worsened incontinence following laparoscopic sacrocolpopexy. IJU Case Rep. 2020, 3, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xia, Z.; Feng, X.; Luan, M.; Qin, M. Three-Dimensional Transperineal Ultrasonography for Diagnosis of Female Occult Stress Urinary Incontinence. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 8078–8083. [Google Scholar] [CrossRef]
- Thind, P.O.; Lose, G. [Use of micturition cystourethrography in evaluation of urinary incontinence in women]. Ugeskr. Laeger 1990, 152, 2095–2098. [Google Scholar] [PubMed]
- Varpula, M.; Mäkinen, J.; Kiilholma, P. Cough urethrocystography: The best radiological evaluation of female stress urinary incontinence? Eur. J. Radiol. 1989, 9, 191–194. [Google Scholar] [PubMed]
- Kelvin, F.M.; Maglinte, D.D.; Hale, D.; Benson, J.T. Voiding cystourethrography in female stress incontinence. Am. J. Roentgenol. 1996, 167, 1065–1066. [Google Scholar] [CrossRef]
- Bergman, A.; McKenzie, C.; Ballard, C.A.; Richmond, J. Role of cystourethrography in the preoperative evaluation of stress urinary incontinence in women. J. Reprod. Med. 1988, 33, 372–376. [Google Scholar]
- McKinnie, V.; Swift, S.E.; Wang, W.; Woodman, P.; O’Boyle, A.; Kahn, M.; Valley, M.; Bland, D.; Schaffer, J. The effect of pregnancy and mode of delivery on the prevalence of urinary and fecal incontinence. Am. J. Obstet. Gynecol. 2005, 193, 512–517; discussion 517–518. [Google Scholar] [CrossRef]
- Brandt, F.T.; Lorenzato, F.R.; Nóbrega, L.V.; Albuquerque, C.D.; Falcão, R.; Araújo Júnior, A.A. Intra-abdominal pressure measurement during ultrasound assessment of women with stress urinary incontinence: A novel model. Acta Cir. Bras. 2006, 21, 237–241. [Google Scholar] [CrossRef]
- Kuprasertkul, A.; Christie, A.L.; Alhalabi, F.; Zimmern, P. Very long-term follow-up of the anterior vaginal wall suspension procedure for incontinence and/or prolapse repair. World J. Urol. 2021, 39, 533–542. [Google Scholar] [CrossRef]
- Roch, M.; Gaudreault, N.; Cyr, M.P.; Venne, G.; Bureau, N.J.; Morin, M. The Female Pelvic Floor Fascia Anatomy: A Systematic Search and Review. Life 2021, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, M.; Sagsoz, N.; Bozkurt, M.C.; Apaydin, N.; Elhan, A.; Tekdemir, I. Important anatomical structures used in paravaginal defect repair: Cadaveric study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 112, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Norton, P.A. Pelvic floor disorders: The role of fascia and ligaments. Clin. Obstet. Gynecol. 1993, 36, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Mauroy, B.; Goullet, E.; Stefaniak, X.; Bonnal, J.L.; Amara, N. Tendinous arch of the pelvic fascia: Application to the technique of paravaginal colposuspension. Surg. Radiol. Anat. 2000, 22, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Pit, M.J.; De Ruiter, M.C.; Lycklama, A.N.A.A.; Marani, E.; Zwartendijk, J. Anatomy of the arcus tendineus fasciae pelvis in females. Clin. Anat. 2003, 16, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Petros, P.E. The pubourethral ligaments—An anatomical and histological study in the live patient. Int. Urogynecol. J. Pelvic Floor Dysfunct. 1998, 9, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Vazzoler, N.; Soulié, M.; Escourrou, G.; Seguin, P.; Pontonnier, F.; Bécue, J.; Plante, P. Pubourethral ligaments in women: Anatomical and clinical aspects. Surg. Radiol. Anat. 2002, 24, 33–37. [Google Scholar] [CrossRef]
- Kefer, J.C.; Liu, G.; Daneshgari, F. Pubo-urethral ligament injury causes long-term stress urinary incontinence in female rats: An animal model of the integral theory. J. Urol. 2009, 181, 397–400. [Google Scholar] [CrossRef]
- Gowda, S.N.; Bordoni, B. Anatomy, Abdomen and Pelvis, Levator Ani Muscle. In StatPearls; StatPearls Publishing Copyright © 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Grigorescu, B.A.; Lazarou, G.; Olson, T.R.; Downie, S.A.; Powers, K.; Greston, W.M.; Mikhail, M.S. Innervation of the levator ani muscles: Description of the nerve branches to the pubococcygeus, iliococcygeus, and puborectalis muscles. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2008, 19, 107–116. [Google Scholar] [CrossRef]
- Barber, M.D.; Bremer, R.E.; Thor, K.B.; Dolber, P.C.; Kuehl, T.J.; Coates, K.W. Innervation of the female levator ani muscles. Am. J. Obstet. Gynecol. 2002, 187, 64–71. [Google Scholar] [CrossRef]
- Chojnacki, M.; Borowski, D.; Wielgoś, M.; Węgrzyn, P. [Postpartum levator ani muscle injuries. Diagnosis and treatment]. Ginekol. Pol. 2015, 86, 67–71. [Google Scholar] [CrossRef] [PubMed]
- van Delft, K.; Sultan, A.H.; Thakar, R.; Schwertner-Tiepelmann, N.; Kluivers, K. The relationship between postpartum levator ani muscle avulsion and signs and symptoms of pelvic floor dysfunction. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1164–1171; discussion 1172. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, J.L.; Carroll, M.; Muñoz, A.; Handa, V.L. Pelvic floor muscle strength and the incidence of pelvic floor disorders after vaginal and cesarean delivery. Am. J. Obstet. Gynecol. 2020, 222, e61–e62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoker, J.; Rociu, E.; Bosch, J.L.; Messelink, E.J.; van der Hulst, V.P.; Groenendijk, A.G.; Eijkemans, M.J.; Laméris, J.S. High-resolution endovaginal MR imaging in stress urinary incontinence. Eur. Radiol. 2003, 13, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Aurore, V.; Röthlisberger, R.; Boemke, N.; Hlushchuk, R.; Bangerter, H.; Bergmann, M.; Imboden, S.; Mueller, M.D.; Eppler, E.; Djonov, V. Anatomy of the female pelvic nerves: A macroscopic study of the hypogastric plexus and their relations and variations. J. Anat. 2020, 237, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hokanson, J.A.; Keast, J.R. Advancing our understanding of the neural control of the female human urethra. Neurourol. Urodyn. 2022, 41, 35–41. [Google Scholar] [CrossRef] [PubMed]
- de Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.P.; Mevcha, A.; Wilby, D.; Alatsatianos, A.; Hardman, J.C.; Jacques, S.; Wilton, J.C. Continence and micturition: An anatomical basis. Clin. Anat. 2014, 27, 1275–1283. [Google Scholar] [CrossRef]
- Thor, K.B.; de Groat, W.C. Neural control of the female urethral and anal rhabdosphincters and pelvic floor muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R416–R438. [Google Scholar] [CrossRef] [Green Version]
- Beckel, J.M.; Holstege, G. Neuroanatomy of the lower urinary tract. Handb. Exp. Pharmacol. 2011, 202, 99–116. [Google Scholar] [CrossRef]
- Sievert, K.D.; Bakircioglu, M.E.; Tsai, T.; Nunes, L.; Lue, T.F. The effect of labor and/or ovariectomy on rodent continence mechanism--the neuronal changes. World J. Urol. 2004, 22, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Bremer, R.E.; Barber, M.D.; Coates, K.W.; Dolber, P.C.; Thor, K.B. Innervation of the levator ani and coccygeus muscles of the female rat. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 275, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Nyangoh Timoh, K.; Bessede, T.; Zaitouna, M.; Peschaud, F.; Chevallier, J.M.; Fauconnier, A.; Benoit, G.; Moszkowicz, D. Anatomy of the levator ani muscle and implications for obstetrics and gynaecology. Gynecol. Obstet. Fertil. 2015, 43, 84–90. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, X.; Gao, Z.; Li, L.; Lin, H.; Wang, H.; Zhou, H.; Tian, D.; Zhang, Q.; Shen, J. The Anatomical Pathogenesis of Stress Urinary Incontinence in Women. Medicina 2023, 59, 5. https://doi.org/10.3390/medicina59010005
Yang X, Wang X, Gao Z, Li L, Lin H, Wang H, Zhou H, Tian D, Zhang Q, Shen J. The Anatomical Pathogenesis of Stress Urinary Incontinence in Women. Medicina. 2023; 59(1):5. https://doi.org/10.3390/medicina59010005
Chicago/Turabian StyleYang, Xunguo, Xingqi Wang, Zhenhua Gao, Ling Li, Han Lin, Haifeng Wang, Hang Zhou, Daoming Tian, Quan Zhang, and Jihong Shen. 2023. "The Anatomical Pathogenesis of Stress Urinary Incontinence in Women" Medicina 59, no. 1: 5. https://doi.org/10.3390/medicina59010005
APA StyleYang, X., Wang, X., Gao, Z., Li, L., Lin, H., Wang, H., Zhou, H., Tian, D., Zhang, Q., & Shen, J. (2023). The Anatomical Pathogenesis of Stress Urinary Incontinence in Women. Medicina, 59(1), 5. https://doi.org/10.3390/medicina59010005








