Abstract
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerular disease worldwide today. The NLRP3 inflammasome is a polyprotein complex and an important participant in inflammation. Accumulating studies have shown that the NLRP3 inflammasome participates in a variety of kidney diseases, including IgAN. This review focuses on the role of the NLRP3 inflammasome in IgAN and summarizes multiple involved pathways, which may provide novel treatments for IgAN treatment.
1. Introduction
Immunoglobulin A nephropathy (IgAN) is the most common variety of primary glomerular disease worldwide today, and the deposition of IgA immune complexes (IgA-ICs) within glomeruli is the most outstanding characteristic [1,2,3]. The deposition of immune complexes can activate mesangial cell proliferation and induce cytokine secretion, resulting in inflammation and ultimately leading to kidney damage [3,4]. Studies have shown that approximately one third of IgAN patients progress to end-stage renal disease (ESRD) within 20 years [1,5].
The nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) compose a group of pattern recognition receptors (PRRs) and participate in inducing host innate immune responses to cellular injury [6]. The NLR family pyrin domain-containing 3 (NLRP3) is one of the best understood members and the core protein of the NLRP3 inflammasome [6,7]. The NLRP3 inflammasome is an approximately 700 kD polyprotein complex and an important participant in inflammation, which consists of NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and the protease caspase-1 [6,7,8]. Active caspase-1 cleaves the cytokines pro-interleukin-1β (pro-IL-1β) and pro-interleukin-18 (pro-IL-18) into their mature and biologically active forms IL-1β and IL-18, inducing inflammation and tissue damage [9].
NLRP3 inflammasome activation is a two-step process, consisting of priming and activation. A priming signal is required for its activation, such as ligands for Toll-like receptors (TLRs), NLRs or cytokine receptors, which trigger the transcription of nuclear factor-kappa B (NF-κB) [9,10]. NF-κB promotes the expression of NLRP3 and pro-IL-1β, but does not upregulate pro-IL-18, ASC or pro-caspase-1 [9,11]. Inflammasome can be activated via both exogenous pathogen-associated molecular patterns (PAMPs) and endogenous damage-associated molecular patterns (DAMPs) [10]. It happens when exposed to stimulus such as reactive oxygen species (ROS), mitochondrial dysfunction, lysosomal damage, ionic flux, pathogen-associated RNA and bacterial or fungal toxins [9,10,12]. NLRP3 inflammasome activation happens not only in immune cells, such as macrophages and dendritic cells, but also in kidney cells, such as podocytes, mesangial cells, renal tubular epithelium, etc. [7,8,13].
Based on the above findings, accumulating studies have shown that the NLRP3 inflammasome participates in a variety of kidney diseases, including diabetic nephropathy (DN), obesity-related kidney disease, acute kidney injury (AKI), crystal-related nephropathy, lupus nephritis (LN) and IgAN [7,8,9,10,12,14,15]. Previous studies have demonstrated that IgA-ICs can initiate the activation of the NLRP3 inflammasome in IgAN macrophages and podocytes [13,16]. Additionally, the markers of NLRP3 inflammasome activation, IL-18 and IL-1β, were elevated in IgAN patients [16,17,18]. Studies in IgAN mouse models have indicated that NLRP3 inflammasome-related pathways may be strongly associated with the progression of IgAN [17,19]. Another study found that colorectal neoplasia differentially expressed (CRNDE) exacerbates IgAN progression by promoting NLRP3 inflammasome activation in macrophages, and the inhibition of CRNDE promoted NLRP3 degradation [20]. These studies revealed that the inhibition of the NLRP3 inflammasome may be a beneficial strategy for the treatment of IgAN. Therefore, this review focuses on the role of the NLRP3 inflammasome in IgAN and identifying novel treatments for IgAN patients.
2. The NLRP3 Inflammasome and Related Pathways
2.1. The NLRP3 Inflammasome and NF-κB Pathway
Previous studies have demonstrated that NF-κB plays a pivotal role in the pathogenesis of inflammation, and NF-κB expression is correlated with the poor prognosis of IgAN patients [21,22]. Varieties of endogenous or exogenous stimuli could trigger the transcription of NF-κB, which is the main signal inducing the activation of the NLRP3 inflammasome [10,23].
Studies have illustrated that activation of the NF-κB/NLRP3 pathway might participate in the pathogenesis of inflammation in IgAN, and inhibiting NLRP3 activation can alleviate the inflammation [4,17,19,24,25,26,27]. For example, He L. et al. found that triptolide could down-regulate serum levels of IL-1β and IL-18 and may exert an anti-inflammatory effect by suppressing NLRP3 and TLR4 expression on IgAN rats [27]. Another study on rats found that artemisinin and hydroxychloroquine combination therapy exert protective effects on IgAN by inhibiting NF-κB signaling and NLRP3 inflammasome activation [25]. A recent study also discovered that IgAN mice benefited from compound K (a major absorbable intestinal bacterial metabolite of ginsenosides) and Icariin (a major constituent of flavonoids isolated from plants of the genus Epimedium) by inhibiting the NF-κB/NLRP3 pathway, respectively [17,26].
In summary, these findings indicate that the NF-κB/NLRP3 pathway is essential in the pathogenesis of IgAN, and the inhibition of its activation may be an effective therapeutic method.
2.2. The NLRP3 Inflammasome and Autophagy
Autophagy, a vital intracellular process that degrades dysfunctional proteins and organelles (e.g., mitochondria) via lysosome-mediated degradation, clears damaged intracellular pathogens and regulates the diverse immune system such as antigen presentation [28,29,30]. Autophagy has now been identified as an important regulator of the NLRP3 inflammasome [30,31,32,33]. Previous studies have shown that inflammatory signals lead to an induction of autophagy, which plays a negative role in the activation of the NLRP3 inflammasome and promotes cell survival and restores tissue homeostasis after damage in autoimmune diseases, including IgAN [16,26,28].
Accumulating evidence has indicated that the regulation of inflammasomes and autophagy may be the key for the treatment of multiple diseases, including kidney disease [30,31,32,33]. Qu et al. showed that cisplatin may induce kidney injury by inhibiting autophagy and activating NLRP3 inflammasomes [34]. Additionally, in their later study, they found that astragaloside IV could alleviate cisplatin-induced AKI by inducing autophagy and limiting the expression of the NLRP3 inflammasome [35]. Recent reviews also outlined that autophagy inhibits inflammatory responses induced in AKI through the inhibition of inflammasome activation, suggesting that the enhancement of autophagy, such as the use of autophagy activators, might be a potential target for the treatment of AKI [33,36].
The relation between NLRP3 and autophagy also plays a vital role in the development of IgAN. In mouse models of progressive IgAN, researchers showed that resveratrol inhibits the NLRP3 inflammasome activation by augmenting autophagy and preserving mitochondrial integrity [37]. Additionally, in cultured macrophages, Tris dibenzylideneacetone dipalladium (Tris DBA), a small-molecule palladium complex, was found to inhibit the activation of the NLRP3 inflammasome and regulate the autophagy/NLRP3 inflammasome axis through SIRT1 and SIRT3 [16]. In addition, a recent study in Taiwan found that compound K inhibited the activation of the renal NLRP3 inflammasome in treated IgAN mice, and increased induction of autophagy in IgA-IC-primed macrophages, revealing the protective mechanisms of autophagy in IgAN [26]. In their later study in vitro and vivo, LCC18, a benzamide-linked small molecule, was found to improve renal function and reduce proteinuria in IgAN by blocking the priming of the NLRP3 inflammasome and inhibiting its activation through autophagy induction, further confirming the positive effect of autophagy in IgAN [38].
Collectively, these results suggested that inhibiting NLRP3 activation through autophagy induction may be a potential novel therapeutic approach for IgAN.
2.3. The NLRP3 Inflammasome and Mitochondrial Reactive Oxygen Species
Previous studies have indicated that the most typical mechanism for activating the NLRP3 inflammasome is the production of ROS, especially mitochondrial ROS (mtROS) [10,39,40,41]. Mitochondrial dysfunction has long been considered a necessary factor in triggering NLRP3-mediated inflammation, and overproduction of mtROS is a key factor in NLRP3 inflammasome activation [39,41]. Excessive mtROS production induces thioredoxin (TRX) separation from thioredoxin-interacting protein (TXNIP), and then the latter binds to NLRP3 and activates the NLRP3 inflammasome [39,42].
A growing number of studies have revealed the role of blocking mtROS in kidney diseases, such as ischemic and cisplatin-induced AKI, DN, etc. [39,43,44,45,46,47]. A previous study found that Mito TEMPO, a mitochondria-targeted antioxidant, can inhibit mtROS overproduction and NLRP3 inflammasome activation, and it verified that the NLRP3 inflammasome can be activated via the mROS-TXNIP-NLRP3 signal pathway, providing a potential therapeutic target for ischemic AKI [43]. Han et al. also found that oral administration of the mitochondria-targeted antioxidant MitoQ reduced mtROS levels, thereby inhibiting the TXNIP/NLRP3/IL-1β signaling pathway, leading to the alleviation of kidney injury in DN mice [39].
The ROS signaling pathway has also been shown to be involved in IgAN [19,48]. It has been well-recognized that albuminuria is a risk factor of IgAN, and albuminuria triggers mitochondrial dysfunction and mtROS generation, resulting in renal tubular inflammation through mtROS-meditated activation of the NLRP3 inflammasome [24,49]. A previous study found that IgA ICs could induce the activation of the NLRP3 inflammasome through ROS in macrophages [48]. Yang et al. found in induced accelerated progressive IgAN mice that antroquinonol (a pure active compound from Antrodia camphorata mycelium) promoted the Nrf2 antioxidant pathway, inhibited NLRP3 inflammasome activation and significantly improved renal function [50]. Additionally, in IgA-IC-primed macrophages, they discovered that antroquinonol inhibited NLRP3 inflammasome activation by reducing ROS production [50]. Hua et al. also found that osthole inhibited ROS production, activation of NF-κB and the NLRP3 inflammasome, exerting its reno-protective effects on the progression of IgAN both in vitro and in vivo [19]. Based on these findings, ROS inhibition may be a potential choice to inhibit NLRP3 activation and reduce inflammation in IgAN.
2.4. The NLRP3 Inflammasome and Exosomes
Exosomes are small extracellular vesicles (30–150 nm) secreted by all healthy and abnormal cells and are abundant in all bodily fluids [51,52]. Exosomes contain specific protein, lipid, RNA and DNA compositions that are derived from the endocytosis membrane and can transmit signals to recipient cells, playing a key role in intercellular communications [51,53,54]. Exosomes play significant roles in inflammation and immune response, and they are considered promising biomarkers for diagnosis and therapy in various diseases, including kidney diseases such as LN, AKI, DN and IgAN [51,55,56,57,58,59,60].
Emerging evidence has revealed the relationship between exosomes and the NLRP3 inflammasome [61,62,63,64]. Recent studies have shown that exosomes can influence the course of NLRP3 inflammasome-associated diseases by secreting different substances that affect key molecules in the canonical pathway [61,62]. Dai et al. discovered that exosomes relieve myocardial ischemia/reperfusion injury by inactivating the TLR4/NF-κB/NLRP3 inflammasome signaling pathway in a neonatal rat model induced by ischemia/reperfusion [63]. In another rat model, Tang et al. found that exosomal miR-320b can directly target NLRP3 and inhibit pyroptosis, thereby protecting the myocardium from ischemia/reperfusion injury by inhibiting pyroptosis [65].
Recent research also focused on the mechanism by which exosomes mediate inflammation in IgAN [4,25]. Bai et al. found that artemisinin and hydroxychloroquine combination therapy could significantly promote the secretion of exosomes in the renal tissue of IgAN rats and inhibit the expressions of NF-κB signal and NLRP3 inflammasome-related protein [25]. Subsequently, Li et al. found that Zhen-wu-tang (a well-known traditional Chinese formula) regulated exosome secretion, which influenced the NF-KB/NLRP3 signaling pathway in the human mesangial cell proliferation model, and it could also reinforce the secretion of exosomes in an IgAN rat model [4]. These results have provided new evidence that enhancing the secretion of exosomes to inhibit the NF-κB/NLRP3 signaling pathway is a promising approach for IgAN treatment.
Moreover, a recent study in IgAN patients found that supplementation of probiotics can significantly improve gut dysbiosis and ameliorate IgAN by inhibiting the NLRP3/ASC/Caspase-1 signaling pathway [66]. A summary of the main publications about treatments related to pathways between IgAN and the NLRP3 inflammasome is shown in Table 1. Figure 1 illustrates the related pathways between IgAN and the NLRP3 inflammasome and the plausible mechanism of treatments.

Table 1.
Studies about treatments related to pathways between IgAN and NLRP3 inflammasome.
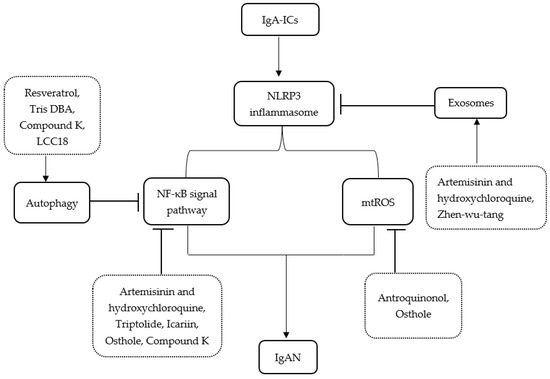
Figure 1.
Schematic representation for the related pathways between IgAN and the NLRP3 inflammasome and the plausible mechanism of treatments. IgA-ICs, IgA immune complexes; IgAN, IgA nephropathy; Tris DBA, Tris dibenzylideneacetone dipalladium; ROS, reactive oxygen species.
Other studies have also shown that the renin–angiotensin–aldosterone system (RAAS) and endoplasmic reticulum stress (ERS) can regulate the NLRP3 inflammasome and play an important part in the development of renal diseases, including DN, obesity-related kidney disease and AKI [8,67,68,69,70]. The relationship between the NLRP3 inflammasome and RAAS and ERS is expected to be found in IgAN.
3. Conclusions
In this review, we summarized information regarding multiple pathways between IgAN and the NLRP3 inflammasome, including the NF-κB/NLRP3 pathway, autophagy, mtROS production and exosomes. These studies suggest that NLRP3 could be a promising therapeutic target for the design of a novel therapeutic treatment for IgAN. In the future, these pathways need to be completely understood and are worthy of further investigation in humans.
Author Contributions
Conceptualization, K.L. and J.Y.; resources, X.W. and L.Z.; writing—original draft preparation, X.W. and L.Z.; writing—review and editing, X.W. and K.L.; visualization and supervision, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, J.K.; Kim, J.H.; Lee, S.C.; Kang, E.W.; Chang, T.I.; Moon, S.J.; Yoon, S.Y.; Yoo, T.H.; Kang, S.W.; Choi, K.H. Clinical Features and Outcomes of IgA Nephropathy with Nephrotic Syndrome. Clin. J. Am. Soc. Nephrol. 2012, 7, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Rifai, A. IgA nephropathy: Immune mechanisms beyond IgA mesangial deposition. Kidney Int. 2007, 72, 239–241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zachova, K.; Kosztyu, P.; Zadrazil, J.; Matousovic, K.; Vondrak, K.; Hubacek, P.; Julian, B.A.; Moldoveanu, Z.; Novak, Z.; Kostovcikova, K.; et al. Role of Epstein-Barr Virus in Pathogenesis and Racial Distribution of IgA Nephropathy. Front. Immunol. 2020, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, R.; Pang, Y.; Li, J.; Cao, Y.; Fu, H.; Fang, G.; Chen, Q.; Liu, B.; Wu, J.; et al. Zhen-Wu-Tang Protects IgA Nephropathy in Rats by Regulating Exosomes to Inhibit NF-κB/NLRP3 Pathway. Front. Pharmacol. 2020, 11, 1080. [Google Scholar] [CrossRef]
- Wyatt, R.J.; Julian, B.A. IgA nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef]
- Vilaysane, A.; Chun, J.; Seamone, M.E.; Wang, W.; Chin, R.; Hirota, S.; Li, Y.; Clark, S.A.; Tschopp, J.; Trpkov, K.; et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 2010, 21, 1732–1744. [Google Scholar] [CrossRef]
- Qiu, Y.-Y.; Tang, L.-Q. Roles of the NLRP3 inflammasome in the pathogenesis of diabetic nephropathy. Pharmacol. Res. 2016, 114, 251–264. [Google Scholar] [CrossRef]
- Ke, B.; Shen, W.; Fang, X.; Wu, Q.; Wu, Q. The NLPR3 inflammasome and obesity-related kidney disease. J. Cell. Mol. Med. 2018, 22, 16–24. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Anton-Pampols, P.; Diaz-Requena, C.; Martinez-Valenzuela, L.; Gomez-Preciado, F.; Fulladosa, X.; Vidal-Alabro, A.; Torras, J.; Lloberas, N.; Draibe, J. The Role of Inflammasomes in Glomerulonephritis. Int. J. Mol. Sci. 2022, 23, 4208. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; Macdonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Kim, S.-M.; Kim, K.-P.; Lee, S.-H.; Moon, J.-Y. The Role of Inflammasome-Dependent and Inflammasome-Independent NLRP3 in the Kidney. Cells 2019, 8, 1389. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Pei, G.-Q.; Tang, Y.; Tan, L.; Qin, W. IgA1 deposition may induce NLRP3 expression and macrophage transdifferentiation of podocyte in IgA nephropathy. J. Transl. Med. 2019, 17, 406. [Google Scholar] [CrossRef]
- Mulay, S.R.; Anders, H.-J. Crystal nephropathies: Mechanisms of crystal-induced kidney injury. Nat. Rev. Nephrol. 2017, 13, 226–240. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Lima, C.A.D.; Vajgel, G.; Sandrin-Garcia, P. The Role of NLRP3 Inflammasome in Lupus Nephritis. Int. J. Mol. Sci. 2021, 22, 12476. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Hua, K.-F.; Yang, S.-R.; Tsai, Y.-S.; Yang, S.-M.; Hsieh, C.-Y.; Wu, C.-C.; Chang, J.-F.; Arbiser, J.L.; Chang, C.-T.; et al. Tris DBA ameliorates IgA nephropathy by blunting the activating signal of NLRP3 inflammasome through SIRT1- and SIRT3-mediated autophagy induction. J. Cell. Mol. Med. 2020, 24, 13609–13622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.-Z.; Li, Y.-S.; Zhang, L.; Hao, L.-R. Icariin ameliorates IgA nephropathy by inhibition of nuclear factor kappa b/Nlrp3 pathway. FEBS Open Bio 2017, 7, 54–63. [Google Scholar] [CrossRef]
- Shi, B.; Ni, Z.; Cao, L.; Zhou, M.; Mou, S.; Wang, Q.; Zhang, M.; Fang, W.; Yan, Y.; Qian, J. Serum IL-18 is closely associated with renal tubulointerstitial injury and predicts renal prognosis in IgA nephropathy. Mediat. Inflamm. 2012, 2012, 728417. [Google Scholar] [CrossRef]
- Hua, K.-F.; Yang, S.-M.; Kao, T.-Y.; Chang, J.-M.; Chen, H.-L.; Tsai, Y.-J.; Chen, A.; Yang, S.-S.; Chao, L.K.; Ka, S.-M. Osthole mitigates progressive IgA nephropathy by inhibiting reactive oxygen species generation and NF-κB/NLRP3 pathway. PloS ONE 2013, 8, e77794. [Google Scholar] [CrossRef]
- Shen, M.; Pan, X.; Gao, Y.; Ye, H.; Zhang, J.; Chen, Y.; Pan, M.; Huang, W.; Xu, X.; Zhao, Y.; et al. LncRNA CRNDE Exacerbates IgA Nephropathy Progression by Promoting NLRP3 Inflammasome Activation in Macrophages. Immunol. Investig. 2022, 51, 1515–1527. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.-C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.E.B.; Costa, R.S.; Ravinal, R.C.; Ramalho, L.Z.; Dos Reis, M.A.; Coimbra, T.M.; Dantas, M. NF-kB expression in IgA nephropathy outcome. Dis. Markers 2011, 31, 9–15. [Google Scholar] [CrossRef]
- Lorenz, G.; Darisipudi, M.N.; Anders, H.-J. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol. Dial. Transpl. 2014, 29, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zhu, F.; Xu, Z.; Xiong, J. Role of Inflammasomes in Kidney Diseases via Both Canonical and Non-canonical Pathways. Front. Cell Dev. Biol. 2020, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Li, J.; Li, H.; Song, J.; Zhou, Y.; Lu, R.; Liu, B.; Pang, Y.; Zhang, P.; Chen, J.; et al. Renoprotective effects of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via suppressing NF-κB signaling and NLRP3 inflammasome activation by exosomes in rats. Biochem. Pharmacol. 2019, 169, 113619. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Hua, K.-F.; Hsu, W.-H.; Suzuki, Y.; Chu, L.J.; Lee, Y.-C.; Takahata, A.; Lee, S.-L.; Wu, C.-C.; Nikolic-Paterson, D.J.; et al. IgA Nephropathy Benefits from Compound K Treatment by Inhibiting NF-κB/NLRP3 Inflammasome and Enhancing Autophagy and SIRT1. J. Immunol. 2020, 205, 202–212. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Peng, X.; Liu, G.; Tang, C.; Liu, H.; Liu, F.; Zhou, H.; Peng, Y. Anti-inflammatory effects of triptolide on IgA nephropathy in rats. Immunopharmacol. Immunotoxicol. 2015, 37, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.-S.; Shenderov, K.; Huang, N.-N.; Kabat, J.; Abu-Asab, M.; Fitzgerald, K.A.; Sher, A.; Kehrl, J.H. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012, 13, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.K.; Lee, S.-J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef]
- Biasizzo, M.; Kopitar-Jerala, N. Interplay between NLRP3 Inflammasome and Autophagy. Front. Immunol. 2020, 11, 591803. [Google Scholar] [CrossRef]
- Sun, Q.; Fan, J.; Billiar, T.R.; Scott, M.J. Inflammasome and autophagy regulation—A two-way street. Mol. Med. 2017, 23, 188–195. [Google Scholar] [CrossRef]
- Takahama, M.; Akira, S.; Saitoh, T. Autophagy limits activation of the inflammasomes. Immunol. Rev. 2018, 281, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Pan, Q.; Yang, N. Autophagy and Inflammation Regulation in Acute Kidney Injury. Front. Physiol. 2020, 11, 576463. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Gao, H.; Tao, L.; Zhang, Y.; Zhai, J.; Song, Y.; Zhang, S. Autophagy inhibition-enhanced assembly of the NLRP3 inflammasome is associated with cisplatin-induced acute injury to the liver and kidneys in rats. J. Biochem. Mol. Toxicol. 2018, 33, e22208. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Gao, H.; Tao, L.; Zhang, Y.; Zhai, J.; Sun, J.; Song, Y.; Zhang, S. Astragaloside IV protects against cisplatin-induced liver and kidney injury via autophagy-mediated inhibition of NLRP3 in rats. J. Toxicol. Sci. 2019, 44, 167–175. [Google Scholar] [CrossRef]
- Duann, P.; Lianos, E.A.; Ma, J.; Lin, P.-H. Autophagy, Innate Immunity and Tissue Repair in Acute Kidney Injury. Int. J. Mol. Sci. 2016, 17, 662. [Google Scholar] [CrossRef]
- Chang, Y.-P.; Ka, S.-M.; Hsu, W.-H.; Chen, A.; Chao, L.K.; Lin, C.-C.; Hsieh, C.-C.; Chen, M.-C.; Chiu, H.-W.; Ho, C.-L.; et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell. Physiol. 2015, 230, 1567–1579. [Google Scholar] [CrossRef]
- Yang, S.-R.; Hua, K.-F.; Takahata, A.; Wu, C.-Y.; Hsieh, C.-Y.; Chiu, H.-W.; Chen, C.-H.; Mukhopadhyay, D.; Suzuki, Y.; Ka, S.-M.; et al. LCC18, a benzamide-linked small molecule, ameliorates IgA nephropathy in mice. J. Pathol. 2021, 253, 427–441. [Google Scholar] [CrossRef]
- Han, Y.; Xu, X.; Tang, C.; Gao, P.; Chen, X.; Xiong, X.; Yang, M.; Yang, S.; Zhu, X.; Yuan, S.; et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Chen, M.-L.; Zhu, X.-H.; Ran, L.; Lang, H.-D.; Yi, L.; Mi, M.-T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, Y.-R.; Tang, T.-T.; Pan, M.-M.; Xu, S.-C.; Ma, K.-L.; Lv, L.-L.; Liu, H.; Liu, B.-C. mROS-TXNIP axis activates NLRP3 inflammasome to mediate renal injury during ischemic AKI. Int. J. Biochem. Cell. Biol. 2018, 98, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhang, J.; Gomez, H.; Kellum, J.A.; Peng, Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.-W.; He, S.-P.; Lan, J.-G.; Zhu, W.-Z. Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br. J. Pharmacol. 2022, 179, 3886–3904. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Gurumani, M.Z.; Merscher, S.; Fornoni, A. Glucose- and Non-Glucose-Induced Mitochondrial Dysfunction in Diabetic Kidney Disease. Biomolecules 2022, 12, 351. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Hua, K.-F.; Chen, A.; Wei, C.-W.; Chen, W.-S.; Wu, C.-Y.; Chu, C.-L.; Yu, Y.-L.; Lo, C.-W.; Ka, S.-M. NLRP3 inflammasome: Pathogenic role and potential therapeutic target for IgA nephropathy. Sci. Rep. 2017, 7, 41123. [Google Scholar] [CrossRef]
- Liu, D.; Xu, M.; Ding, L.-H.; Lv, L.-L.; Liu, H.; Ma, K.-L.; Zhang, A.-H.; Crowley, S.D.; Liu, B.-C. Activation of the Nlrp3 inflammasome by mitochondrial reactive oxygen species: A novel mechanism of albumin-induced tubulointerstitial inflammation. Int. J. Biochem. Cell. Biol. 2014, 57, 7–19. [Google Scholar] [CrossRef]
- Yang, S.-M.; Ka, S.-M.; Hua, K.-F.; Wu, T.-H.; Chuang, Y.-P.; Lin, Y.-W.; Yang, F.-L.; Wu, S.-H.; Yang, S.-S.; Lin, S.-H.; et al. Antroquinonol mitigates an accelerated and progressive IgA nephropathy model in mice by activating the Nrf2 pathway and inhibiting T cells and NLRP3 inflammasome. Free Radic. Biol. Med. 2013, 61, 285–297. [Google Scholar] [CrossRef]
- Li, M.; Zeringer, E.; Barta, T.; Schageman, J.; Cheng, A.; Vlassov, A.V. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130502. [Google Scholar] [CrossRef] [PubMed]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Lebleu, V.S. The biology function and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Thongboonkerd, V. Roles for Exosome in Various Kidney Diseases and Disorders. Front. Pharmacol. 2019, 10, 1655. [Google Scholar] [CrossRef]
- Pan, T.; Jia, P.; Chen, N.; Fang, Y.; Liang, Y.; Guo, M.; Ding, X. Delayed Remote Ischemic Preconditioning ConfersRenoprotection against Septic Acute Kidney Injury via Exosomal miR-21. Theranostics 2019, 9, 405–423. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, A.; Wang, H.; Klein, J.D.; Tan, L.; Wang, Z.-M.; Du, J.; Naqvi, N.; Liu, B.-C.; Wang, X.H. miR-26a Limits Muscle Wasting and Cardiac Fibrosis through Exosome-Mediated microRNA Transfer in Chronic Kidney Disease. Theranostics 2019, 9, 1864–1877. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Forner, M.J.; Pinto, C.; Chaves, F.J.; Cortes, R.; Redon, J. Increased Urinary Exosomal MicroRNAs in Patients with Systemic Lupus Erythematosus. PloS ONE 2015, 10, e0138618. [Google Scholar] [CrossRef]
- Feng, Y.; Lv, L.-L.; Wu, W.-J.; Li, Z.-L.; Chen, J.; Ni, H.-F.; Zhou, L.-T.; Tang, T.-T.; Wang, F.-M.; Wang, B.; et al. Urinary Exosomes and Exosomal CCL2 mRNA as Biomarkers of Active Histologic Injury in IgA Nephropathy. Am. J. Pathol. 2018, 188, 2542–2552. [Google Scholar] [CrossRef]
- Jiang, Z.-Z.; Liu, Y.-M.; Niu, X.; Yin, J.-Y.; Hu, B.; Guo, S.-C.; Fan, Y.; Wang, Y.; Wang, N.-S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Tao, J.; Shi, A.; Zhang, J.; Yu, P. Exosomes Regulate NLRP3 Inflammasome in Diseases. Front. Cell Dev. Biol. 2021, 9, 802509. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Y.; Liang, C.; Liu, B.; Ding, F.; Wang, Y.; Zhu, B.; Zhao, R.; Yu, X.Y.; Li, Y. Stem cell-derived exosomes prevent pyroptosis and repair ischemic muscle injury through a novel exosome/circHIPK3/FOXO3a pathway. Theranostics 2020, 10, 6728–6742. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, S.; Chang, S.; Ren, D.; Shali, S.; Li, C.; Yang, H.; Huang, Z.; Ge, J. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway. J. Mol. Cell. Cardiol. 2020, 142, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.-X.; Mao, Y.; Cao, Q.; Chen, Y.; Zhou, L.-B.; Li, S.; Chen, H.; Chen, J.-H.; Zhou, G.-P.; Jin, R. Exosome-mediated pyroptosis of miR-93-TXNIP-NLRP3 leads to functional difference between M1 and M2 macrophages in sepsis-induced acute kidney injury. J. Cell. Mol. Med. 2021, 25, 4786–4799. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jin, L.; Liu, Y.; Li, L.; Ma, Y.; Lu, L.; Ma, J.; Ding, P.; Yang, X.; Liu, J.; et al. Exosomes Derived from Mesenchymal Stem Cells Protect the Myocardium Against Ischemia/Reperfusion Injury Through Inhibiting Pyroptosis. Drug Des. Devel. Ther. 2020, 14, 3765–3775. [Google Scholar] [CrossRef]
- Tan, J.; Dong, L.; Jiang, Z.; Tan, L.; Luo, X.; Pei, G.; Qin, A.; Zhong, Z.; Liu, X.; Tang, Y.; et al. Probiotics ameliorate IgA nephropathy by improving gut dysbiosis and blunting NLRP3 signaling. J. Transl. Med. 2022, 20, 382. [Google Scholar] [CrossRef]
- Ke, R.; Wang, Y.; Hong, S.; Xiao, L. Endoplasmic reticulum stress related factor IRE1α regulates TXNIP/NLRP3-mediated pyroptosis in diabetic nephropathy. Exp. Cell Res. 2020, 396, 112293. [Google Scholar] [CrossRef]
- Lv, S.; Li, X.; Wang, H. The Role of the Effects of Endoplasmic Reticulum Stress on NLRP3 Inflammasome in Diabetes. Front. Cell Dev. Biol. 2021, 9, 663528. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, Y.; Chen, C.; Wang, C. Anisodamine inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in rhabdomyolysis-induced acute kidney injury. Apoptosis 2017, 22, 1524–1531. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Bi, X.; Hu, C.; Ding, W. NLRP3 Deletion Attenuated Angiotensin II-Induced Renal Fibrosis by Improving Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. Nephron 2021, 145, 518–527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).